不同来源吸附载体大豆糖蜜对奶牛瘤胃内环境的影响
2017-01-11韩奇鹏张佩华揭红东周传社孔志伟
韩奇鹏,张佩华,罗 玲,揭红东,周传社,陈 亮,孔志伟
(1.中国科学院亚热带农业生态研究所亚热带农业生态过程重点实验室,湖南省畜禽健康养殖工程技术中心,农业部中南动物营养与饲料科学观测实验站,湖南 长沙 410125;2.湖南农业大学动物科学技术学院畜禽遗传改良湖南省重点实验室,湖南 长沙 410128)
动物生产层
不同来源吸附载体大豆糖蜜对奶牛瘤胃内环境的影响
韩奇鹏1,2,张佩华2,罗 玲2,揭红东2,周传社1,陈 亮1,孔志伟1
(1.中国科学院亚热带农业生态研究所亚热带农业生态过程重点实验室,湖南省畜禽健康养殖工程技术中心,农业部中南动物营养与饲料科学观测实验站,湖南 长沙 410125;2.湖南农业大学动物科学技术学院畜禽遗传改良湖南省重点实验室,湖南 长沙 410128)
为考察不同来源吸附载体大豆糖蜜对奶牛瘤胃内环境的影响,采用3×3拉丁方试验设计,选用3头年龄相同(3胎次)、体重相近[(500±50) kg]且安装永久性瘤胃瘘管的中国荷斯坦奶牛为试验动物,分别饲喂基础日粮、豆皮糖蜜和麸皮糖蜜日粮。结果表明,豆皮糖蜜组和麸皮糖蜜组瘤胃pH均极显著高于对照组(P<0.01),而豆皮糖蜜组和麸皮糖蜜组之间瘤胃pH没有显著差异(P>0.05);麸皮糖蜜组瘤胃NH3-N浓度显著低于对照组(P<0.05),但与豆皮糖蜜组没有显著差异(P>0.05);各处理间奶牛瘤胃各单个挥发性脂肪酸(volatile fatty acid,VFA,包括乙酸、丙酸、异丁酸、丁酸、异戊酸和戊酸)浓度、总VFA浓度和乙丙比均没有显著差异(P>0.05);但与对照组相比,豆皮糖蜜组瘤胃总VFA浓度有降低趋势;而麸皮糖蜜组瘤胃总VFA浓度有提高趋势。结果显示,在奶牛饲料中可用豆皮糖蜜替代15%玉米以及用麸皮糖蜜替代6%玉米和10%麸皮,均具有调控瘤胃稳衡的作用,且麸皮糖蜜替代效果优于豆皮糖蜜。
大豆糖蜜;瘤胃内环境;奶牛
大豆糖蜜是以低温脱脂豆粕为原料,采用酸提和醇提等方法生产大豆浓缩蛋白过程中,经萃取、浓缩而成的纯天然副产物[1-3]。大豆糖蜜中一般含有固形物50%、蛋白质6%、灰分3%、脂肪5%和碳水化合物36%,成分较为复杂,是多种植物性化合物的集合体[4],可用于提取大豆异黄酮、大豆低聚糖、皂甙、大豆胰蛋白酶抑制素等活性成分[5-8];同时还可以利用微生物发酵生产丁醇、槐脂、乳酸等[8-11]。目前,对大豆糖蜜及其多活性成分在动物营养领域的研究处于起步阶段。随着大豆糖蜜添加量的增加,肉羊瘤胃菌体蛋白浓度不同程度升高;同时,大豆糖蜜可提高肉羊瘤胃液丙酸浓度和血液总蛋白浓度,降低丙酸转氨酶浓度;提高肉羊日增重和饲料转化率[12-15]。添加6%大豆糖蜜可促进鲁山牛腿山羊日增重和饲料转化率[16]。梁丽莉和赵海明[17]在奶牛日粮中添加3 kg大豆糖蜜,泌乳高峰期奶牛体重、产奶量和乳脂率均有所增加,在玉米较缺乏的地区向奶牛日粮中添加大豆糖蜜可替代部分玉米。上述研究均是在动物日粮中直接添加液态大豆糖蜜,而本研究利用具有吸附和固定作用的豆皮和麸皮作为大豆糖蜜的吸附载体,对大豆糖蜜进行吸附后再添加至奶牛日粮中,考察其对奶牛瘤胃内环境的影响,以期为实施反刍家畜瘤胃稳衡状态调控和促进奶牛养殖产业健康可持续发展提供理论依据和技术支撑。
1 材料与方法
1.1 试验动物及饲养管理
选择3头体况良好、年龄相同(3胎次)、体重相近[(500±50)kg]、安装永久性瘤胃瘘管的中国荷斯坦奶牛为试验动物。分别于06:00和15:00等量饲喂,自由饮水。
1.2 试验日粮
试验日粮参照NRC(2004)中国奶牛饲养标准配制[18]。试验日粮中分别添加的豆皮糖蜜和麸皮糖蜜每千克均能吸附300 g的大豆糖蜜(干物质计),豆皮糖蜜和麸皮糖蜜均由丰益(上海)生物技术研发中心有限公司提供。各组日粮精饲料组成及营养成分如表1所示。
1.3 试验设计
采用3×3拉丁方试验设计,进行3期动物试验。每期试验15 d,包括14 d预饲期和1 d样品采集。
1.4 样品采集与分析测定
分别于每期试验第15天的05:00、06:00、07:00、08:00、10:00、12:00、15:00、16:00、18:00、21:00和23:00采集瘤胃内容物50 mL。用pH计(REX PHS-3C,上海仪器设备厂)测定其pH值后,4层纱布过滤[20]。再5 000 r·min-1离心10 min去除原虫和饲料大颗粒,取上清液,采用比色法(UV2450紫外可见分光光度计,Shimadzu Corporation,Japan)测定氨氮(NH3-N)浓度[21-22]。另取1.5 mL上清液分装于预先装有25%偏磷酸的离心管内摇匀,采用气相色谱法测定乙酸、丙酸、丁酸等挥发性脂肪酸(VFA)含量[23]。色谱条件:色谱柱为Agilent TC-C18柱(250 nm×4.6 nm,i. d. 5 μm),流速为0.6 mL·min-1,进样体积20 μL,紫外检测波长210 nm;柱温为室温。流动相经0.22 μm纤维膜过滤,超声波脱气[24]。
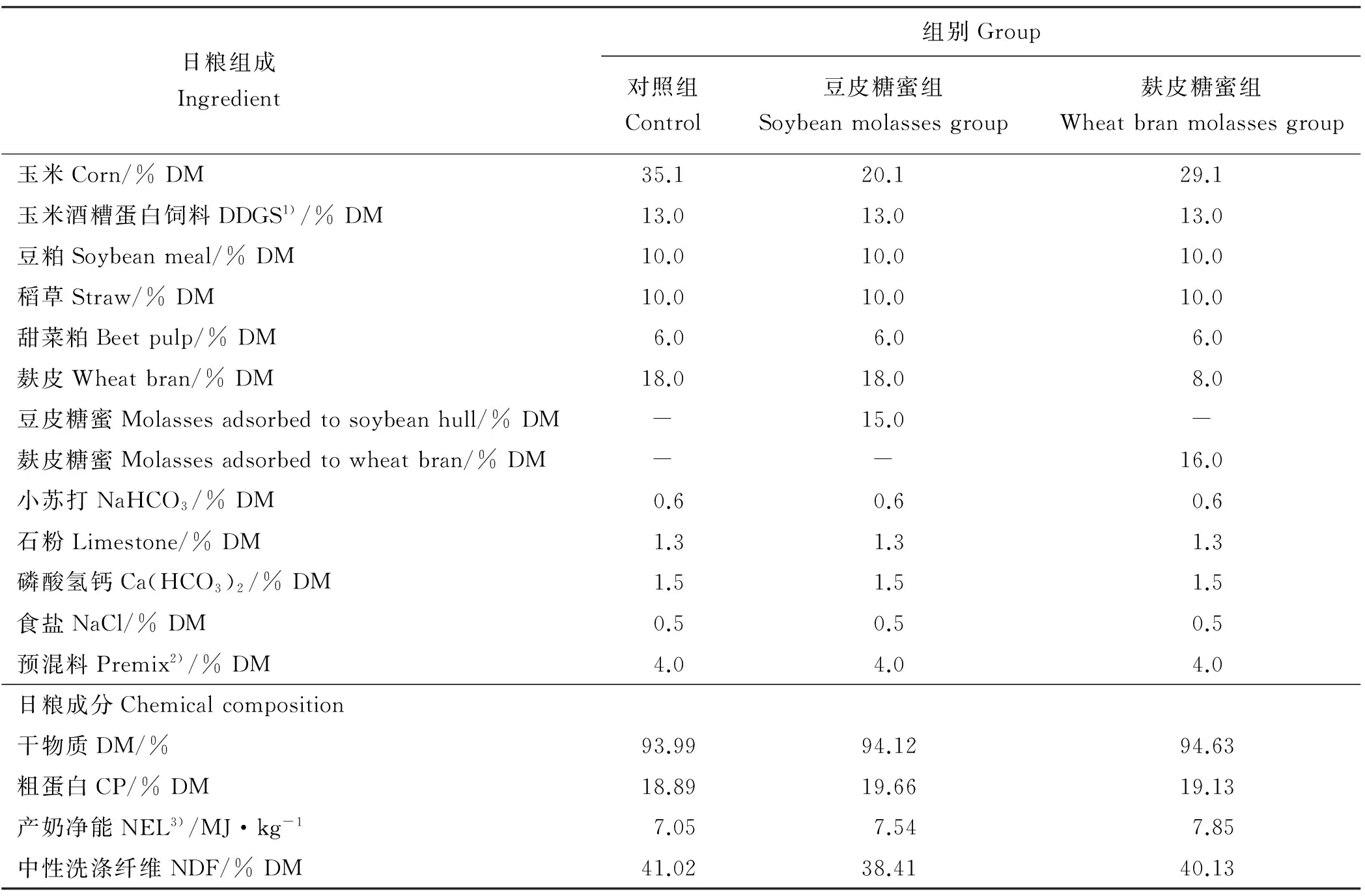
表1 试验奶牛日粮精饲料组成及其营养成分Table 1 Ingredients and chemical composition of concentrate of the experimental diets
注:2)预混料成分组成如下:MgSO4·H2O 113.87 g·kg-1,MnSO4·H2O 9.54 g·kg-1,ZnSO4·H2O 9.61 g·kg-1,FeSO4·7H2O 2.69 g·kg-1,CuSO4·5H2O 2.55 g·kg-1,CoCl2·6H2O 181.68 mg·kg-1,Na2SeO3112.80 mg·kg-1,KI 58.86 mg·kg-1,VA 500 000 IU·kg-1,VD 60 000 IU·kg-1,VE 2 000 IU·kg-1。 3)产奶净能为估测值[19]。
Note: 2)DDGS means distillers dried grains with soluble. 2)Premix components were as follows: MgSO4·H2O 113.87 g·kg-1, MnSO4·H2O 9.54 g·kg-1, ZnSO4·H2O 9.61 g·kg-1, FeSO4·7H2O 2.69 g·kg-1, CuSO4·5H2O 2.55 g·kg-1, CoCl2·6H2O 181.68 mg·kg-1, Na2SeO3112.80 mg·kg-1, KI 58.86 mg·kg-1, vitamin A (VA) 500 000 IU·kg-1, vitaminD (VD) 60 000 IU·kg-1, vitaminE (VE) 2 000 IU·kg-1. 3)NEL was estimated value[19].
1.5 试验数据统计及分析
试验数据采用SAS(8.2版)的MIXED过程统计,不同瘤胃发酵物的差异采用CONTRAST语句进行比较。各时间点作为重复,统计差异显著性定义为P<0.05,P>0.05为差异不显著。
2 结果与分析
2.1 大豆糖蜜对奶牛瘤胃pH值和NH3-N浓度的影响
豆皮糖蜜组和麸皮糖蜜组瘤胃pH值均极显著高于对照组(P<0.01),而豆皮糖蜜组和麸皮糖蜜组之间瘤胃pH值没有显著差异(P>0.05)(表2)。麸皮糖蜜组瘤胃NH3-N浓度显著低于对照组(P<0.05),但与豆皮糖蜜组没有显著差异(P>0.05),且豆皮糖蜜组与对照组之间瘤胃NH3-N浓度亦没有显著差异(P>0.05)。
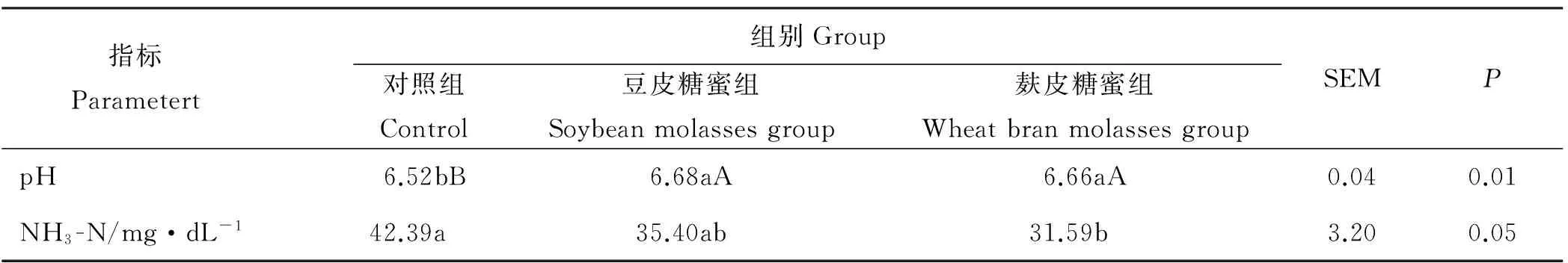
表2 大豆糖蜜对奶牛瘤胃pH值和NH3-N浓度的影响Table 2 Effects of soybean molasses on pH value in the rumen and NH3-N concentration in dairy cows
注:同行数据不同小写字母表示差异显著(P<0.05),不同大写字母表示差异极显著(P<0.01)。下表同。
Note: Different lower case or capital letters within the same row indicate a significant difference at the 0.05 or 0.01 levels, respectively.
2.2 大豆糖蜜对奶牛瘤胃VFA浓度的影响
各处理间奶牛瘤胃各单个VFA(乙酸、丙酸、异丁酸、丁酸、异戊酸和戊酸)浓度、总VFA浓度和乙丙比(A∶P)均没有显著差异(P>0.05)(表3)。但与对照组相比,豆皮糖蜜组瘤胃总VFA浓度有降低趋势,较对照组降低了5.04%;而麸皮糖蜜组瘤胃总VFA浓度有提高趋势,较对照组提高了10.34%。
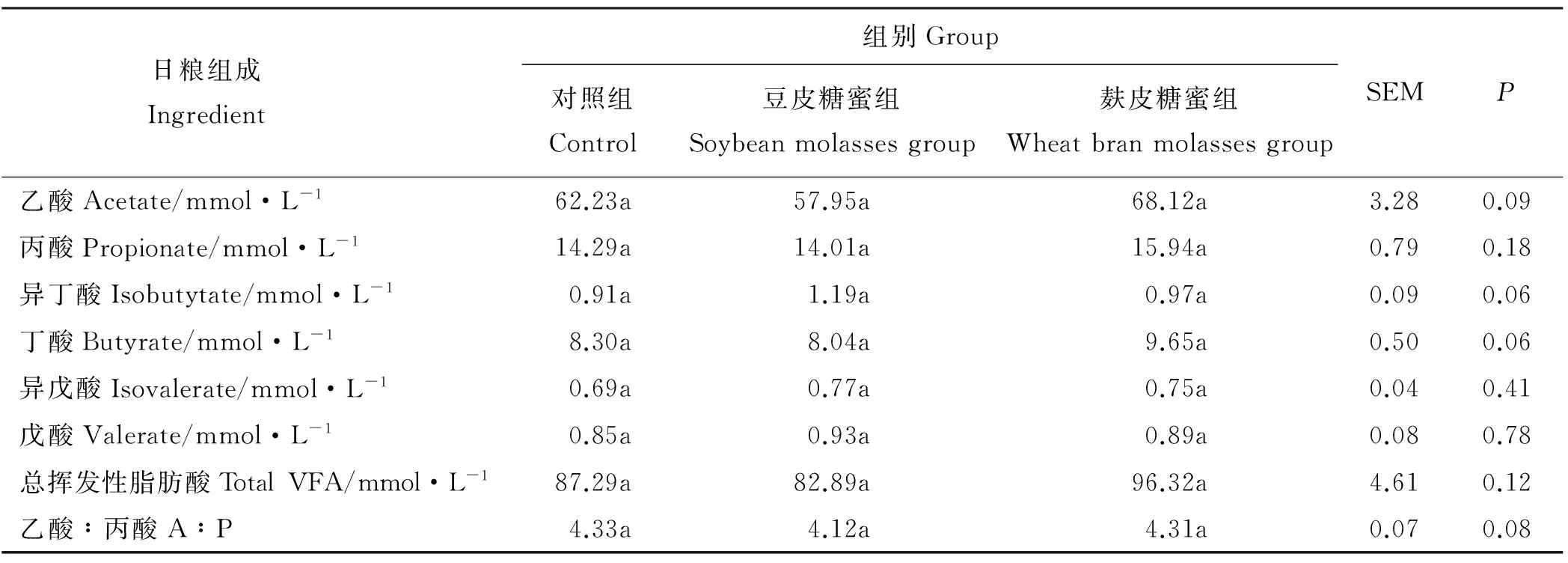
表3 大豆糖蜜对奶牛瘤胃VFA浓度的影响Table 3 Effects of soybean molasses on ruminal VFA concentration in dairy cows
3 讨论
3.1 大豆糖蜜对瘤胃pH的影响
瘤胃pH是评价瘤胃发酵状况的综合性指标,它主要受日粮性质,唾液分泌及瘤胃内酸、碱性物质排除、吸收的影响,决定着瘤胃微生物对底物的发酵利用效率。大豆(Glycinemax)糖蜜中低聚糖的主要发酵产物是乙酸、丙酸和丁酸,且这几种是反刍动物最重要的挥发性脂肪酸,约占瘤胃发酵总挥发性脂肪酸的95%以上[25]。本研究显示,各组瘤胃pH值在6.52-6.68内变化,均处在正常生理范围内[14],且试验组间差异不显著,表明试验奶牛采食含大豆糖蜜的日粮后,瘤胃自身具有的缓冲能力仍能维持瘤胃内pH值处于正常范围之内。
3.2 大豆糖蜜对瘤胃NH3-N浓度的影响
NH3-N是由瘤胃代谢中外源蛋白质和内源含氮物质降解产物氨基酸脱氢基而产生,是瘤胃中主要微生物体系生长的氮源[26-27],其在瘤胃中的浓度也是体现微生物对氮源降解和利用的一个动态指标,受饲料蛋白的溶解度、瘤胃壁吸收和食糜排空速度的影响。本研究显示,与对照组相比,豆皮糖蜜组奶牛瘤胃内NH3-N浓度呈降低趋势,而麸皮糖蜜组奶牛瘤胃内NH3-N浓度显著降低(P<0.05),这可能是由于大豆糖蜜中存在的低聚糖加速了饲料蛋白质的分解,致使NH3-N浓度增加;但同时又由于促进了瘤胃微生物对所分解生成的NH3的利用率提高并合成菌体蛋白,从而导致瘤胃内NH3-N浓度下降[14];还可能是由于吸附后的豆皮糖蜜和麸皮糖蜜蛋白质含量(13.25%和19.81%,实测值)及其蛋白组分尤其是瘤胃可降解蛋白和非降解蛋白比值之间的差异所致。
3.3 大豆糖蜜对瘤胃挥发性脂肪酸的影响
挥发性脂肪酸是指含有1~6个碳链的短链脂肪酸,其提供的能量占反刍动物所需的70%~80%[28],是反刍动物生长发育的直接能量来源之一。挥发性脂肪酸的主要成分有乙酸、丙酸、异丁酸、丁酸、异戊酸、戊酸等。大豆糖蜜中含有59%~62%的大豆低聚糖,具有较多的可消化物质,而大豆低聚糖的主要发酵产物是乙酸、丙酸和丁酸。本研究表明,各试验组瘤胃挥发性脂肪酸浓度和乙丙比均没有显著差异,但与对照组相比,豆皮糖蜜组瘤胃总挥发性脂肪酸浓度有降低趋势,而麸皮糖蜜组瘤胃总VFA浓度有提高趋势。这可能是由于本研究应用的豆皮糖蜜和麸皮糖蜜中大豆糖蜜的吸附浓度均为30%所致。
4 小结
在奶牛饲料中可用豆皮糖蜜替代15%玉米以及用麸皮糖蜜替代6%玉米和10%麸皮,均具有调控瘤胃稳衡的作用,且麸皮糖蜜替代效果优于豆皮糖蜜。根据大豆糖蜜主要功能物质大豆异黄酮、大豆皂甙、低聚糖的作用,可以进一步对奶牛生产性能和乳品质等关键指标进行相应的研究。
References:
[1] 韩奇鹏,张佩华,罗玲,朱丹,周传社,Weiss W P.大豆糖蜜的主要活性成分及其对反刍动物的影响.中国饲料,2015(18):11-13.
[2] 王校红,田娟娟,王丹.大豆糖蜜的综合利用.粮油食品科技,2010,22(1):22-24. Wang J H,Tian J J,Wang D.Comprehensive utilization of soy molasses.Science and Technology of Cereals,Oils and Food,2010,22(1):22-24.(in Chinese)
[3] 李大鹏,刘洋.大豆糖蜜的功能性成分及其应用.农产品加工:学刊,2010,10(7):37-39. Li D P,Liu Y.Functional ingredients in soy molasses and its application.Academic Periodical of Farm Products Processing,2010,10(7):37-39.(in Chinese)
[4] Daninel C.Topical application of soy molasses.US Patent:5871743,1999.
[5] Waggle.Recovery of isoflavones from soy molasses.US Patent:6706292,2004.
[6] Dobbins T.Process for isolating saponins from soybean-derived materials.US Patent:6355816,2002.
[7] Cegla.Process for production of soybean sugars and the product produced thereof.US Patent:6913771,2005.
[8] 董晓宁,赵海福,赵强,移瑞瑞,靳正娟.航天紫花苜蓿中黄酮的提取及抑菌活性.草业科学,2014,31(4):771-775. Dong X N,Zhao H F,Zhao Q,Yi R R,Jin Z J.Study on extraction technology and antibacterial of flavonoids from space breeding alfalfa.Pratacultural Science,2014,31(4):771-775.(in Chinese)
[9] Qurshi N,Lolas A,Blaschck H P.Soy molasses as fermentation substrate for production of butanol usingClostridiumbeijerinckiiBA.Journal of Industrial Microbiology & Biotechnology,2001,26(5):290-295.
[10] Daniel K,Solaiman Y,Ashby R D,Nunez A,Foglia T F.Production of sophorolipids by andida bombicola grown on soy molasses as subsrate.Biotechology Letters,2004,24(9):1241-1245.
[11] 任海伟,赵拓,李金平,李雪雁,李志忠,徐娜,王永刚,喻春来,高晓航,王晓力.玉米秸秆与废弃白菜混贮料的发酵特性及其乳酸菌分离鉴定.草业科学,2015,32(9):1508-1517. Ren H W,Zhao T,Li J P,Li X Y,Li Z Z,Xu N,Wang Y G,Yu C L,Gao X H,Wang X L.Fermentation characteristics of mixed silage of corn stalk and waste cabbage and isolation and identification of lactic acid bacteria.Pratacultural Science,2015,32(9):1508-1517.(in Chinese)
[12] Montelongo J L,Chassy B M,McCord J D.Lactobacillus salivarius for conversion of soy molasses into lactic acid.Journal of Food Science,1993,58(4):863-866.
[13] 马群山,陈勇,唐德江.饲料中添加大豆糖蜜对肉羊理化指标的影响.黑龙江八一农垦大学学报,2009,23(3):59-61,66. Ma Q S,Chen Y,Tang D J.The effect of physical and chemical index on sheep with added soybean molassas in diet.Journal of Heilongjiang Bayi Agricultral University,2009,23(3):59-61,66.(in Chinese)
[14] 马群山,陈勇,张爱忠,邹芳,刘彩娟.饲料中添加大豆糖蜜对肉羊瘤胃发酵的影响.中国畜牧杂志,2009,45(15):33-36. Ma Q S,Chen Y,Zhang A Z,Zou F,Liu C J.Effect of soy molasses on mutton sheep rumen fermentation.Chinese Journal of Animal Science,2009,45(15):33-36.(in Chinese)
[15] 马群山,袁荣志,唐德江.饲料添加大豆糖蜜对绵羊生长发育的影响.黑龙江八一农垦大学学报,2008,23(1):63-65. Ma Q S,Yuan R Z,Tang D J.The effect of growth and development on sheep with added soybean molassas in diet.Journal of Heilongjiang Bayi Agricultral University,2008,23(1):63-65.(in Chinese)
[16] 毛朝阳.鲁山牛腿山羊饲喂大豆糖蜜粕试验研究.河南畜牧兽医:综合版,2009,30(6):8-9. Mao C Y.Experimental study on molasses meal Lushan Mountain corbel goats fed soybean.Henan Journal of Animal Husbandry and Veterinary Medicine,2009,30(6):8-9.(in Chinese)
[17] 梁丽莉,赵海明.日粮中添加大豆糖蜜对泌乳高峰期奶牛的影响.饲料研究,2007,34(7):51-52. Liang L L,Zhao H M.Effect of adding soybean molasses to peak lactation cows diets.Feed Research,2007,34(7):51-52.(in Chinese)
[18] NY/T 34-2004.奶牛饲养标准.北京:中国标准出版社,2004.
[19] 冯仰廉,陆治年.奶牛营养需要和饲料成分.北京:中国农业科技出版社,2007.
[20] 王旭哲,岳亚飞,张凡凡,马春晖.全株玉米青贮营养品质的紧实度效应.草业科学,2016,22(9):1893-1900. Wang X Z,Yue Y F,Zhang F F,Ma C H.Compaction effect of whole corn silage nutritional quality.Pratacultural Science,2016,22(9):1893-1900.(in Chinese)
[21] 冯宗慈,高民.通过比色测定瘤胃液氨氮含量方法的改进.内蒙古畜牧科学,1993,36(4):40-41. Feng Z C,Gao M.Improvement of the method of determining the content of ammonia nitrogen in rumen fluid by colorimetric assay.Inner Mongolian Journal of Animal Sciences and Production,1993,36(4):40-41.(in Chinese)
[22] 王祚,周传社,汤少勋,谭支良.两种酵母对奶牛瘤胃体外发酵特性的影响.农业现代化研究,2014,35(2):218-224. Wang Z,Zhou C S,Tang S X,Tan Z L.Effect of two kinds of yeast on rumen fermentation in dairy cows.Research of Agricultural Modernization,2014,35(2):218-224.(in Chinese)
[23] 王梦竹,刘艳丰,王文奇,侯广田,张文举.苜蓿黄酮对杂交绵羊瘤胃发酵功能和纤维降解酶活性的影响.中国畜牧兽医,2015,42(11):2969-2976. Wang M Z,Liu Y F,Wang W Q,Hou G T,Zhang W J.Effect of alfalfa flavonoids on rumen fermentation function and cellulolytic enzymatic activity of crossbreed sheep.Chinese Animal Husbandry and Veterinary Medicine,2015,42(11):2969-2976.(in Chinese)
[24] 苏利红,曹雨莉,李飞,孙小琴,刘瑞芳.高效液相色谱测定山羊瘤胃液挥发性脂肪酸条件优化的研究.动物医学进展,2013,34(8):61-65. Su L H,Cao Y L,Li F,Sun X Q,Liu R F.Optimizing condition of high performance liquid chromatography for detecting volatile fatty acids in goat rumen fluid.Progress in Veterinary Medicine,2013,34(8):61-65.(in Chinese)
[25] 王新峰,潘晓亮,向春和,吕雪峰,徐思情.添加甜菜糖蜜对绵羊瘤胃pH和NH3-N浓度的影响.中国饲料,2006,28(2):25-27.
[26] Bergman E N.Energy contributions of volatile fatty acids from the gastrointestinal tract in various species.Physiological Reviews,1990,70(6):567-590.
[27] Bandle S,Gupta B N.Rumen fermentation,bacterial and total volatile fatty acid (TVFA) production rates in cattle fed on urea-molasses-mineral block licks supplement.Animal Feed Science and Technology,1997,65(7):275-286.
[28] Martin V H.Challenging the retinal for altering VFA ratios in growing ruminates.Feed Mix,1996,15(4):66-68.
(责任编辑 武艳培)
Effects of wheat bran and bean hull as adsorption substrates for soybean molasses on the ruminal environment of dairy cows
Han Qi-peng1,2, Zhang Pei-hua2, Luo Ling2, Jie Hong-dong2,Zhou Chuan-she1, Chen Liang1, Kong Zhi-wei1
(1.Key Laboratory for Agro-ecological Processes in Subtropical Region, Hunan Research Center of Livestock &Poultry Sciences, South-Central Experimental Station of Animal Nutrition and Feed Science of Ministry of Agriculture, Institute of Subtropical Agriculture, The Chinese Academy of Science, Changsha 410125, China;2. Hunan Provincial Key Laboratory for Genetic Improvement of Domestic Animal, College of Animal Science and Technology, Hunan Agricultural University, Changsha 410128, China)
We examined the effects of soybean molasses adsorbed to different substrates on the ruminal environment of dairy cows. Three Chinese Holstein dairy cows (all the same age, and three parity) with an average body weight of (500±50) kg were allocated three dietary treatments in a 3×3 Latin square design. The cows were fitted with the permanent ruminal fistula, the three experimental diets were similar except that some wheat bran (replaced 15% of corn meal by adsorbed soybean molasses with wheat bran) or soybean hull (replaced 10% of wheat bran and 5% corn meal by adsorbed soybean molasses with soybean hull). The results showed that the molasses that were adsorbed to soybean hull and wheat bran significantly increased the pH value of the rumen compared to the control group (P<0.01). However, there was no significant difference (P>0.05) between the pH values of the rumens of cows fed diets containing molasses adsorbed to soybean hull and wheat bran. The wheat bran diet resulted in significantly lower ruminal NH3-N concentrations than the control diet (P<0.05), however, no differences were observed between soybean hull and control diets (P>0.05). Furthermore, no significant differences (P>0.05) were observed in individual volatile fatty acid (VFA) (acetate, propionate, isobutyrate, butyrate, isovalerate, and valerate) concentrations or the acetate/propionate (A∶P) ratio between the control and treatment groups. However, the ruminal total VFA concentration in cows of the soybean hull molasses group did show a lower trend than that observed in the control group, whereas ruminal total VFA concentration in cows of the wheat bran molasses group had a tendency to increase. These results suggest that it would be efficient that feed soybean hull molasses to replace 15% of corn meal instead of 10% of what bran and 5% corn meal in dairy cattle. Molasses adsorbed to both wheat bran and soybean hull have the ability to maintain ruminal homeostasis, although wheat bran is a better adsorption substrate than soybean hull.
soybean molasses; dairy cows; ruminal environment
Zhou Chuan-she E-mail:zcs@isa.ac.cn Zhang Pei-hua E-mail:peiqin41-@163.com
2016-03-22接受日期:2016-08-20
国家自然科学基金面上项目(31372342);中国科学院科技服务网络计划课题(KFJ-EW-STS-071);中国科学院战略性先导科技专项(XDA05020700);中央驻湘科研机构技术创新发展专项(2013TF3006)
韩奇鹏(1988-),男,内蒙古巴林右旗人,在读硕士生,主要从事反刍动物营养与饲料研究。E-mail:18711069677@163.com
周传社(1980-),男,湖南汨罗人,研究员,博士,主要从事反刍动物营养研究。E-mail:zcs@isa.ac.cn 张佩华(1970-),女,湖南汨罗人,副教授,博士,主要从事反刍动物营养与饲料研究。E-mail:peiqin41-@163.com
10.11829/j.issn.1001-0629.2016-0143
S823.9+1;S816.11
A
1001-0629(2016)12-2559-06*
韩奇鹏,张佩华,罗玲,揭红东,周传社,陈亮,孔志伟.不同来源吸附载体大豆糖蜜对奶牛瘤胃内环境的影响.草业科学,2016,33(12):2559-2564.
Han Q P,Zhang P H,Luo L,Jie H D,Zhou C S,Chen L,Kong Z W.Effects of wheat bran and bean hull as adsorption substrates for soybean molasses on the ruminal environment of dairy cows.Pratacultural Science,2016,33(12):2559-2564.
