Effects of selenium on human sperm parameters after freezing and thawing procedures
2017-01-06ZahraRezaeianHosseinYazdekhastiSimaNasriZahraRajabiParvinFallahiFardinAmidi
Zahra Rezaeian, Hossein Yazdekhasti, Sima Nasri, , Zahra Rajabi, Parvin Fallahi, Fardin Amidi
1Department of Anatomy, Tehran University of medical sciences, Tehran, Iran
2Payame-Noor University, Tehran, Iran
3Department of Infertility, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
Effects of selenium on human sperm parameters after freezing and thawing procedures
Zahra Rezaeian3, Hossein Yazdekhasti1, Sima Nasri2, , Zahra Rajabi1, Parvin Fallahi3, Fardin Amidi1*
1Department of Anatomy, Tehran University of medical sciences, Tehran, Iran
2Payame-Noor University, Tehran, Iran
3Department of Infertility, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Sperm parameters
Selenium
Oxidative stress
Freezing
Thawing
Infertility
Objective:To evaluate the effects of pre-freezing treatment of human semen samples with selenium on semen parameters after thawing procedure.Methods:Each sample was divided into four groups: two groups were washed and other two remained unwashed. 5 µg/mL of selenium was added to one of the washed and unwashed group.Results:The results showed that sperm motility in unwashed seleniumtreated samples was higher compared to washed untreated samples (P<0.05). DNA damage percentage in unwashed treated samples compared to both untreated samples showed no difference (P>0.05) but in washed sperm samples, DNA damaged percentage decreased in treated samples compared to untreated ones (P<0.05). Moreover, the effect of selenium on morphology of washed treated sperms was higher than unwashed untreated samples after thawing procedure. Normal morphology percentage in unwashed treated samples was significantly higher than both unwashed samples after thawing (P<0.001). In the washed treated samples a higher number of sperms with normal morphology compared to washed untreated samples (P<0.001) were observed. Conclusion: Based on the results, we recommend 5 µg/mL of selenium should be used ininfertility clinics to increase sperm quality after freezing and thawing procedures.
1. Introduction
Causes of infertility may include male, female or both and other unexplained factors. About 50% of infertility is believed that result from male factors associated with abnormal semen parameters. Some forms of abnormalities that may be observed in these men include oligospermia, asthenospermia, teratospermia (oligoasthenozoospermia) and azoospermia. Human sperm freezing is used extensively in assisted reproductive technology especially before chemotherapy, radiotherapy and some surgical procedures [1]. Oxidative stress and reactive oxygen radicals (ROS) can be produced in semen, and it has been shown to have some harmful effects on spermatozoa[2]. In semen abnormal spermatozoa, germinal cell precursors and leukocytes have potential to produce ROS[3]. On the other hand, ROS generated by spermatozoa plays an important role in normal physiological processes such as sperm capacitation, acrosome reaction and maintenance of fertilization ability[4]. Due to high susceptibility of spermatozoa to oxidative stress and ROS, several damages on spermatozoa such as lipid peroxidation, DNA damage, apoptosis and sperm motility reduction can be induced[5].
Antioxidants are molecules that have ability to inhibit or reduce oxidative process in other molecules by scavenging released free radicals[6]. Antioxidants are divided into two groups, enzymatic and non-enzymatic. Enzymatic antioxidants include superoxide dismutase, glutathione peroxidase, catalaseand glutathionereductase, whereas non-enzymatic antioxidants include vitamins A, C, E, pyruvate, glutathione, and coenzyme Q. Antioxidant function of selenium (Se) is mediated through glutathione peroxidase enzyme activity. There are several antioxidants in semen that are known to improve sperm quality such as vitamin E and C, as well as Se and Zn which are components of antioxidant systems. However, presumably these antioxidant agents are insufficient in preventing lipid peroxidation and sperm plasma membrane injury during the freezing-thawing process because the protective antioxidant systems in sperms originate from cytoplasm and sperms discard their huge part of cytoplasm as residual body during final stages of differentiation. Freezing and thawing procedures cause significant reduction in motility and metabolic activity in sperms, however, vitamin E and Se have been found to improve freezing induced damages and reduced sperm motility[7]. During cryopreservation, semen is exposed to cold shock and atmospheric oxygen which in turn increases the susceptibility to lipid peroxidation due to higher production of ROS. Sperm plasma membrane is one of the important structures affected by cryopreservation which then can affect the viability and fertility of the sperm cells[8].
Se is an essential metal-like trace element in mammalian diet and its importance has been well established in humans[9]. Foods such as sea foods, liver, grain, egg yolk, milk, water and soil are major natural sources of Se with its levels generally depending on soil[10]. Typical dietary intake of Se in the US is 80-120 µg/ d, and the recommended daily allowance is 70 µg in men and 50 µg in women[11]. Se is a component of the glutathione peroxidase enzyme which is known as an important antioxidant and a marker of oxidative stress[12]. Experimental evidences confirmed the positive effects of Se on human health[13]: reduction of disease symptoms and improvement of some disorders such as infertility, viral infections, cancers, cardiovascular disease and so on[14]. Also Se is an essential element in spermatogenesis and male fertility as well[15]. In a study that was performed by Moslemi et al[16]. Effect of Se-vitamin E supplementation on different parameters of 690 infertile men and pregnancy rate was evaluated. Their results showed that supplemental Se and vitamin E may improve semen quality and pregnancy rate in idiopathic male infertility diagnosed with asthenoteratospermia or asthenospermia in semen analysis, and also have beneficial and protective effects especially on sperm motility[16]. Other studies have also shown that administration of Se to subfertile patients induced a statistically significant rise in sperm motility[17]. Various other studies have revealed the effects of cryopreservation on human sperms function and fertility whereas those conducted on animal model using Se as the most potent component of antioxidant system improved sperm quality and quantity[18]. For example, Seremak et al. demonstrated that addition of Se in the quantity of 1 µg/mL to cryomedium of ram semen improved survival rate following freezing-thawing process[19]. Unfortunately, there are few or no literature publications on the effects of post treatment of human semen with Se on semen parameters. As a first study, this research aimed to evaluate the effect of pre-freezing treatment of human semen with Se on different parameters of human sperm after thawing process.
2. Materials and methods
This study was approved by the Ethical Committee of the Faculty of Basic Sciences of Payame Noor University (Tehran, Iran). This experimental study was performed on 42 semen samples referred to Shariati infertility center in Tehran during February and March 2013, because of infertility problems of their wives but did not show any indications of infertility. All the 42 donors who participated had an informed written consent and the mean age of these individuals was (32.1±3.9) years. In all participants, after 3 d of sexual abstinence, semen samples were collected by masturbation in sterile containers and were transferred to laboratory immediately after ejaculation. The semen samples were allowed to complete liquefaction at 37 ℃ for 30 min. After liquefaction, a small aliquot was removed from each specimen and the sperm parameters were determined according to World Health Organization (WHO) guidelines[20]. Each sample was then divided into two groups: one group unwashed and the other washed. Washed samples were processed by swim-up technique. In swim-up technique, semen be overlaid directly with culture medium and the sperm allowed to swim from the seminal plasma into the culture medium. Washed and unwashed samples were also divided into two subgroups: one without Se treatment as control and the other with 5 µg/mL of sodium selenite (Na2SeO3). Control and treated specimens were then cryopreserved by liquid nitrogen vapor method[21].
An aliquot of the freezing medium equaling 25% of the original specimen volume was added to each of the samples. This process was repeated until the ratio of freezing medium to ejaculum (semen) became 1:1. Cryovials were loaded with 1 mL freezing medium/ semen mixture and kept at -20 ℃ for 8 min. The samples were then exposed to liquid nitrogen vapor at -79 ℃ for 2 h before plunging into liquid nitrogen for storage at -196 ℃. After 2 week interval, the samples were thawed at room temperature for 5 min and then transferred to 37 ℃ water bath for 20 min. To remove the freezing medium, the thawed samples were diluted with Hams F10(Biochrom. K.G) medium at a ratio of 1 volume of freezing medium/semen to 3 volumes of freezing medium and then washed by centrifugation at 300 g for 7 min. The supernatant was removed and the pellet resuspended in 0.7 mL Hams F10medium. A small aliquot was removed from each sample for assessment of different parameters. Light microscope (Nikon, Tokyo, Japan) with 40 magnification was used for motility evaluation. Sperms morphology was evaluated usingPapanicolaoustaining method[20]. In this method the staining was as follows; anterior area of acrosome stained light blue, posteriorarea dark blue, sperm neck slightly red, tail blue and excess residual cytoplasm pink.
Eosin-nigrosinstaining was used for vitality evaluation. Stained sperms indicated permeability of sperm to dye and membrane damage whereas unstained sperms had healthy membrane which did not permit the dye to enter into the sperm cells. If sperm head stains red or dark pink it was considered dead whilst those whose head stained light pink or white was considered alive.
In order to evaluate sperm motility, we used light microscope with 40×magnification and based on WHO protocol, sperm motility was classified into three classes: progressive, non-progressive and immotile.
Ultimately, DNA damage was analyzed by toluidine blue staining based on WHO protocol. Toluidine blue staining is a metachromatic staining procedure used for chromatin structure evaluation. The basis of this procedure is the highly incorporation of Toluidine blue dye into damaged and compacted chromatins. When this dye is incorporated into histone and arginine-rich chromatin, the nucleus stains dark purple whereas its incorporation into protaminerich chromatin stains the nucleus light blue[22]. To determine the significant differences between the studied groups, statistical analysis including mean±SD, correlation coefficients (R2) and two way Anova tests were performed in three independent experiments using Prism (version 5) software. All tests were performed at a confidence level of 95%.
3. Results
Results of effects of Se on different sperm parameters are shown in Figure 1.
3.1. Percentage of sperm cells with normal morphology in treated and control samples after freezing-thawing procedure
In this respect, our results showed that the percentage of sperms with normal morphology in washed treated samples was higher than both unwashed samples after thawing (P<0.001). In washed treated sperms, a higher number of sperms with normal morphology compared to untreated samples (P<0.001) were also observed. The effect of Se on morphology of washed treated sperms was higher than unwashed treated samples after thawing procedure, however, washed or unwashed status of sample did not affect morphology of sperms after thawing process (P>0.05) (Figure 1a).
3.2. Percentage of dead sperm cells in treated and control samples after freezing-thawing procedure
In this research, Eosin-nigrosinstaining procedure was employed for the evaluation of sperm cells viability. The results of this staining method was as follows: the head of dead sperm cells stained red or dark pink and fresh sperms head showed white or light pink color. The percentage of dead sperm cells in both treated samples was lower compared to both untreated samples (P<0.001). Our results also showed that the percentage of dead sperm cells in both washed samples was lower than both unwashed (P<0.05) (Figure 1b).
3.3. Percentage of sperm cells with DNA damage in treated and control samples after freezing-thawing procedure
The percentage frequency of sperm cells with DNA damage in treated samples compared to both untreated samples showed a lower value (P<0.01) but this difference was higher in washed sperm samples (P<0.05)(Figure 1c).
3.4. Sperm cells motility alteration in treated and control samples after freezing-thawing procedure
Results of the evaluation of sperm cells motility showed that the percentage of motile sperms in unwashed treated samples was higher than washed untreated samples (P<0.05). A significant difference was also found in the percentage of motile sperms between treated and untreated washed samples, however, in both treated sperms, motile sperms were higher than both untreated samples (P<0.001). Our results also showed that Se has no significant effect on sperm motility in unwashed group (P>0.05). These results showed that Se is more effective on washed sperm cellsthanon unwashed samples (P<0.001) (Figure 1d).
4. Discussion
Previous results showed that freezing procedure affects the different sperm parameters[23]. Our results showed that using Se as a component of antioxidant system optimizes sperm parameters after freezing-thawing procedure. Previous studies have also shown that freezing-thawing procedure decreases sperms motility, normal morphology, viability, and increases frequency of sperms with DNA damage[24]. In this regard, Meryman et al. [25] stated that when sperm’s environmental temperature reduces to zero, adenosinetriphosphate production stops, sperm cells hibernate and all other activities come to a halt and/or sperm cells die afterwards. Cell damage occurs as the state of sperm cells changes from normal to freeze condition and vice versa, and the production of ROS has a very important role in this phenomena[25]. Different studies have indicated the effect of several antioxidants in the optimization of sperm quantity and quality after freezing-thawing process[26].
In this study, the effect of Se as a component of antioxidant system on different parameters of sperm after cryopreservation was studied. Kefer et al. [27] using X-ray fluorescence microscopy showed that Se is involved in spermatogenesis[27]. Moreover, Keskes-Ammar et al. [28] confirmed the protective and beneficial effects of Se on semenquantity and suggested its application in male infertility treatment [28].
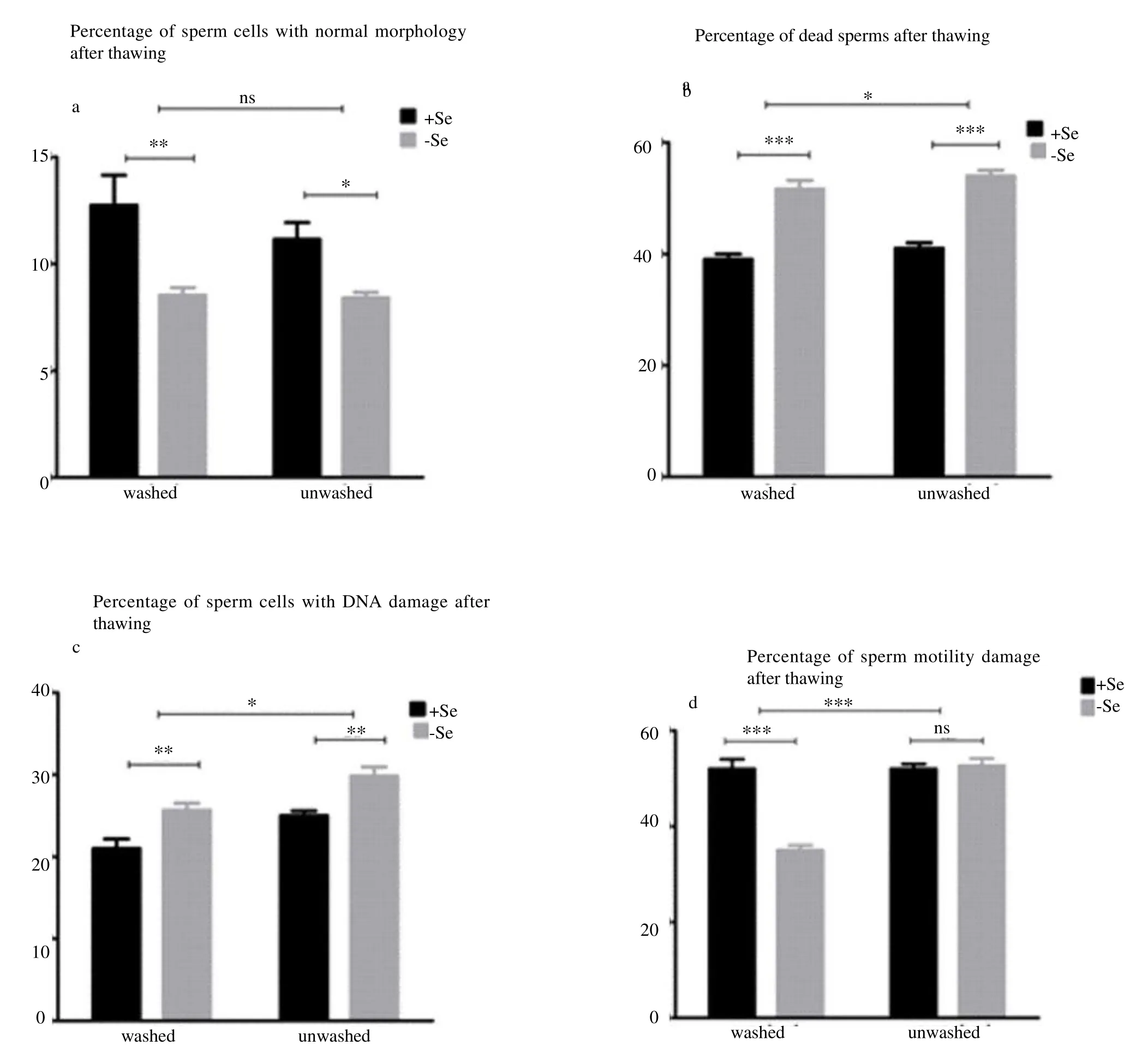
Figure 1. Effect of Se on different semen parameters, on morphology (a), on sperm vitality (b), on percentage of sperm cells with DNA damage (c) and on sperm motility (d).
In this current study, normal morphology percentage in washed treated and unwashed groups showed an increase compared to both untreated groups (P<0.001). Seremak et al. [19] found that percentage of ram sperms with progressive movement in Se (1 µg Se/mL) treated group was identical to control group[19]. Watana be showed that in Se deficient mice, the proportion of abnormal sperm was high, ranging from 6.8% to 49.6%, and the most frequently occurring abnormalities in sperm shape were in the sperm head[34]. In another study Kaur et al. [29] indicated that 6 and 8 ppm of dietary Se caused dose time dependent reduction in body and reproductive organs’weights and increased the number of morphologically abnormal spermatozoa[29]. Additionally, Gadea et al.[30]showed that addition of Glu to thawed sperm samples had no effect on optimization of standard semen parameters such as morphology[30]. In the same line, our results indicated that the addition of 5 µg/mL of Se to human sperm before freezing-thawing procedures caused an increase in sperms with normal morphology. Sperm morphology is an important indication of seminiferous epithelial status, and degenerative changes in seminiferous epithelium have been found to have negative effects on sperm morphology[31].
On the effect of Se on sperm viability, Dorostkar et al. [31]showed that addition of 1 and 2 µg/mL Se to water buffalo semen samples significantly increased sperm motility of fresh and equilibrated semen compared to the control without affecting sperms viability, in freezed-thawed semen however, extenders containing 1 and 2 µg/mL of Se significantly improved sperm viability beside other parameters [32]. In some other studies, different doses of natural and synthetic antioxidants were shown to improve sperms viability[33].
Our investigation showed that 5 µg/mL Se has a potential effect on the reduction of DNA damage. Eghbali et al. [33] performed an investigation on buffalo bulls and they found that 1 and 2 mg/mL of Se can increase semen antioxidants activity and reduce DNA damage after freezing and thawing procedure[34]. In terms of protective effect of Se against sperm cells’ DNA damage, Xu et al. [34] determined the association between concentrations of cadmium and Se in seminal plasma and the degree of oxidative DNA damage in human spermatozoa. Their results showed that cadmium in seminal plasma can affect semen quality and oxidative DNA damage in human spermatozoa, and Se can protect against oxidative DNA damage in human sperm cells[35]. Similar to the study above, our results confirmed that more than semen Se concentration and treatment of sperm cells with Se before cryopreservation can have a protective effect against DNA damage. In different section of this study, we compared the effects of Se on different sperm parameters between washed and unwashed samples. Our results indicated that Se is more effective on different parameters of washed samples. Overall, semen has controlled generation of ROS and also involved in normal function and physiology of spermatozoa. From our results, it can be inferred that washing sperm samples can eliminate ROS and improve the effectiveness of antioxidants.
In this study, percentage of motile sperm in Se treatment groups (washed and unwashed groups) was significantly higher than control group. After thawing, class A (fast progressive) and B (progressive) sperm motility decreased to about 0%. However, class C (Non-progressive) and D (Non-motile) sperm motility did not decrease significantly. Freezing effect was so high that it could extremely increase class D sperm motility. In this study, motility mean was calculated from the summation of class C and D sperm motility after freezing and thawing procedure. Dorostkar et al. [31] evaluated different doses of Se (1, 2, 4 and 8 µg/mL) in buffaloes and they concluded that 1 and 2 µg/mL Se dosages significantly improved sperm motility in treatment groups compared to control group and 4 and 8 µg/mL Se dosages showed a significant decrease in sperm motility compared to control group. Florin et al. (2012) freezed Mangalita boar semen with the addition of vitamin E and C supplements and they observed that these supplements improved sperm motility after thawing[36]. Vitamin E can dissolve covalent linkages, and vitamin C can also inhibit free radicals production and thus can reduce harmful effects of freezing and thawing procedures. Ozkavukcu, et al. [36] evaluated effects of freezing and thawing procedures on human sperms and they found that after freezing and thawing procedures, class A, B and C sperm motility decreased but class D increased[37]. These results confirmed our findings, however, Muratori et al. [37] by adding glutathione peroxidase as antioxidant to domestic animal sperm samples found that sperm motility increased after freezing and thawing procedures compared to control group [38]. Effects of antioxidants on sperm quality could be as a results of very low usage of antioxidant doses or short period of treatment because supplements should reach a threshold concentration to have sufficient effects on oxidative stress[39]. In our investigation, we observed significant increase in sperm motility in Se treated groups, as well, that could have resulted from the effects of Se on sperm mitochondrial oxidative phosphorylation.
Based on our results, we suggest that 5 µg/mL Se can improve sperm parameters and sperm vitality after freezing and thawing procedures and this antioxidant could be utilized in clinic for treatment of infertility of sub fertile men. Moreover, our results showed that washed sperms (with or without Se) have better quality than unwashed sperms.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
We are grateful to all couples who participated in the study for their kind cooperation.
[1] Anger JT, Gilbert BR,Goldstein M. Cryopreservation of sperm: indications, methods and results. J Urol 2003; 170(4): 1079-1084.
[2] Serviddio G, Bellanti F, Vendemiale G. Free radical biology for medicine: learning from nonalcoholic fatty liver disease. Free Radic Biol Med 2013; 65: 952-968.
[3] Mahfouz R, Sharma R, Thiyagarajan A, Kale V, Gupta S, Sabanegh E, et al. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil Steril 2010; 94(6): 2141-2146.
[4] Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res 2009; 129(4): 357-367
[5] Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 2008; 636: 1-15.
[6] Kalthur G, Adiga SK, Upadhya D, Rao S, Kumar P. Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil Steril 2008; 89(6): 1723-1727.
[7] Galatioto GP, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF, Pace G, et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol 2008; 26(1): 97-102.
[8] Watson P. The causes of reduced fertility with cryopreserved semen. Ani Reprod Sci 2000; 60: 481-492.
[9] Akinloye O, Arowojolu AO, Shittu OB, Adejuwon CA, Osotimehin B. Selenium status of idiopathic infertile Nigerian males. Biol Trace Elem Res 2005;104(1): 9-18.
[10] Brown DG, Burk RF. Selenium retention in tissues and sperm of rats fed torula yeast diet. J Nutr1973; 103(1): 102-108.
[11] Allowances NRCCoD, Food NRC, Board N. Recommended dietary allowances. Nat Acad 1980: 2941.
[12] Mizuno K, Hirata S, Hoshi K, Shinohara A, Chiba M. Analysis of the phospholipid hydroperoxide glutathione peroxidase mRNA in the rat spermatozoon and effect of selenium deficiency on the mRNA. Biol Trace Elem Res 2000; 74(1): 71-79.
[13] Rayman MP. The importance of selenium to human health. Lancet 2000; 356(9225): 233-241.
[14] Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr 2003; 133(5): 1463S-1467S.
[15] Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril 2010; 93(7): 2222-2231.
[16] Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med 2011; 4: 99.
[17] MacPherson A, Scott R, Yates R. The effect of selenium supplementation in subfertile males. In: Anke M, Meissner D, Mills CF (Editors). Trace elements in man and animals (TEMA8). Media Touristik Gersdorf 1993; 19(6): 566-570.
[18] Zubair M, Ali M, Ahmad M, Sajid SM, Ahmad I, Gul ST. Effect of selenium and vitamin E on cryopreservation of semen and reproductive performance of animals (a review). 2015.
[19] Seremak B, Udala J, Lasota B. Influence of selenium additive on ram semen freezing quality. Electron J Pol Agric Univ 1999; 2(1): 1.
[20] Cooper TG, Noonan E, Von Eckardstein S, Auger J, Baker HG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod 2009: 16(3): 231-245..
[21] Esteves SC, Sharma RK, Thomas A, Agarwal A. Cryopreservation of human spermatozoa with pentoxifylline improves the post-thaw agonist-induced acrosome reaction rate. Hum Reprod 1998 13(12): 3384-3389.
[22] Krzanowska H. Toluidine blue staining reveals changes in chromatin stabilization of mouse spermatozoa during epididymal maturation and penetration of ova. J Reprod Fertil 1982; 64(1): 97-101.
[23] O’connell M, McClure N, Lewis S. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod 2002; 17(3): 704-709.
[24] Asturiano JF, Marco-Jiménez F, Peñaranda DS, Garzón DL, Pérez L, Vicente JS, et al. Effect of sperm cryopreservation on the European eel sperm viability and spermatozoa morphology. Reprod Domes Anim 2007; 42(2): 162-166.
[25] Meryman HT. Cryopreservation of living cells: principles and practice. Transfusion 2007; 47(5): 935-945.
[26] Topraggaleh TR, Shahverdi A, Rastegarnia A, Ebrahimi B, Shafiepour V, Sharbatoghli M, et al. Effect of cysteine and glutamine added to extender on post-thaw sperm functional parameters of buffalo bull. Andrologia 2014; 46(7): 777-783.
[27] Kefer JC, Agarwal A, Sabanegh E. Role of antioxidants in the treatment of male infertility. Inter J Urol 2009; 16(5): 449-457.
[28] Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Syst Biol Reprod Med 2003; 49(2): 83-94.
[29] Kaur R, Kaur K. Effects of dietary selenium (SE) on morphology of testis and cauda epididymis in rats. Indian J Physiol Pharmacol 2000; 44(3): 265-272.
[30] Gadea J, García-Vazquez F, Matás C, Gardón JC, Cánovas S, Gumbao D. Cooling and freezing of boar spermatozoa: supplementation of the freezing media with reduced glutathione preserves sperm function. J Androl 2005; 26(3): 396-404.
[31] World Health Organization. WHO laboratory manual for the examination and processing of human semen. Switzerland: WHO Press; 2010.
[32] Dorostkar K, Alavi-Shoushtari SM, Mokarizadeh A. Effects of in vitro selenium addition to the semen extender on the spermatozoa characteristics before and after freezing in water buffaloes (Bubalus bubalis).Veter Res Forum 2012; 3(4): 263-268.
[33] Breininger E, Beorlegui NB, O’Flaherty CM, Beconi MT. Alpha-tocopherol improves biochemical and dynamic parameters in cryopreserved boar semen. Theriogenology 2005; 63(8): 2126-2135.
[34] Eghbali M, Alavi-Shoushtari SM, Asri-Rezaei S, Khadem Ansari MH. Calcium, magnesium and total antioxidant capacity (TAC) in seminal plasma of water buffalo (Bubalus Bubalis) bulls and their relationships with semen characteristics.Veter Res Forum 2012; 1(1): 12-20.
[35] Xu DX, Shen HM, Zhu QX, Chua L, Wang QN, Chia SE, et al. The associations among semen quality, oxidative DNA damage in human spermatozoa and concentrations of cadmium, lead and selenium in seminal plasma. Mutat Res 2003; 534(1-2): 155-163.
[36] Ghiuru FV, Ioan L, Roman I, Hettig A, Marius Z, Miclea V. Antioxidant medium for Mangalita boar semen cryopreservation. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Anim Sci Biotechnol 2010; 67: 1-2.
[37] Ozkavukcu S, Erdemli E, Isik A, Oztuna D, Karahuseyinoglu S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assisted Reprod Gen 2008; 25(8): 403-411.
[38] Muratori M, Luconi M, Marchiani S, Forti G, Baldi E. Molecular markers of human sperm functions. Inter J Androl 2009: 32(1): 25-45.
[39] Agarwal A, Said TM. Antioxidant medium for Mangalita boar semen cryopreservation. BJU Inter 2005: 95(4): 503-507.
ment heading
10.1016/j.apjr.2016.11.001
*Corresponding author: FardinAmidi, Department of Anatomy, Tehran University of medical sciences, Tehran, Iran.
Fax: +98 21 66419072.
E-mail: famidi@sina.tums.ac.ir
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
- Nicotine effect toward the oocyte level of rats(Rattus novergicus)
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
- Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
- The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
