The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
2017-01-06MohamedShahbaRedaElSheshtawyAbdelSalamElAzabAlaaAbdelGhaffarMahaZiadaAdelZaky
Mohamed I Shahba, Reda I El-Sheshtawy*, Abdel-Salam I El-Azab, Alaa E Abdel-Ghaffar, Maha S Ziada, Adel A Zaky
1Animal Reproduction and AI Department, Veterinary Division, National Research Centre, Dokki, Giza, Egypt.
2Theriogenology Department, Faculty of Veterinary Medicine, Benha University, Egypt.
3Artificial Insemination and Embryo Transfer Department, Animal Reproduction Research Institute, Al-Haram, Egypt.
The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
Mohamed I Shahba1, Reda I El-Sheshtawy1*, Abdel-Salam I El-Azab2, Alaa E Abdel-Ghaffar2, Maha S Ziada3, Adel A Zaky3
1Animal Reproduction and AI Department, Veterinary Division, National Research Centre, Dokki, Giza, Egypt.
2Theriogenology Department, Faculty of Veterinary Medicine, Benha University, Egypt.
3Artificial Insemination and Embryo Transfer Department, Animal Reproduction Research Institute, Al-Haram, Egypt.
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Buffalo
Semen
Freeze-drying
Sperm preservation
Comet assay
Objective:To display the effect of different freeze-drying media and temperature of storage on the ultrastructure and DNA of freeze-dried buffalo bull spermatozoa.
1. Introduction
Application of artificial insemination (AI) with frozen-thawed semen has been stated on a restricted level in buffalo due to poor preservability and fertility of buffalo bull spermatozoa. Also, more than 40%-50% of sperms are sensitive to damage during the freezing process, in addition to species diversity in susceptibility to cryopreservation methods. For the aforementioned information, the sperm DNA outputs as a result of freeze-drying process (lyophilization) may differ from one species to another. Our study on freeze-drying of buffalo bull spermatozoa suppliedthe available literature. Sperm freeze-drying is considered as an alternative technique to cryopreservation. Development of freezedrying was aimed to preserve biologically active materials such as enzymes, pharmaceutical materials (e.g. antibiotics) and others [1,2]. Furthermore, it has been used to conserve cells, awing to its capability to hinder water via ice sublimation[3]. Nowadays, a great deal of research attention has paid to freeze-drying of sperm. Compare to ordinary cryopreservation, freeze-drying needs lower cost, no liquid nitrogen, little space for sperm storage, and it is a more reliable method of sperm shipping. Even though sperm freezedrying in different species has been documented, there are scattered reports for buffalo bull sperm. The first trial to conserve sperm using dehydration was documented by Polge et al.[3] using fowl sperm; although sperm appeared motile after rehydration, their fertilizing capacity was not assessed. After then, trials to freeze-dry mammalian sperm exhibited unsatisfactory results[4,5]. The first recorded birth following AI with freeze-dried sperm was reported in rabbit[6]. There’s obvious success in production of offspring with freezedried sperm following the application of intracytoplasmic sperm injection (ICSI)[7,8]. Freeze-drying provided new potentials for storage and transportation of freeze-dried sperm at room temperature or at 4 ℃, with many benefits for preservation of spermatozoa from animals [9]. One of the important challenges with any preservation method is the degree of cellular damage. Regardless of the protocol applied, cryopreservation has a damaging effect on sperm, resulting in reduction of both motility and fertilizing capacity[10]. Therefore, in spite of apparent reduction in motility, cells still viable and characterized by normal nucleus and centrosome integrity which are essential for the success of ICSI[11]. Although freeze-drying was focused on proper preservation of structural and functional sperm characteristics, an intact sperm nucleus is a necessary part for success of embryo production[7,12]. Nuclei of sperm are highly stable and concentrated with DNA organization[13]; 6-time more compact and 40-time lower than somatic cells[14,15]. This DNA packing is important to protect the cell and reduce injuries caused by external factors before fertilization. DNA of sperm can injure during freezedrying and particularly during storage when inadequate protection is given. It is established that DNA injures could be due to activation of endogenous nucleases, oxidative stresses and storage conditions which takes place after freeze-drying[16,17]. Many trials were carried out to protect sperm structures during cryopreservation via various protecting substances, albumin[7,18], EGTA[12,19], EDTA[20] and trehalose[21]. The main target of the current study is to investigate the effect of various freeze-drying media and different storage temperatures on the ultrastructural components and DNA of freezedried buffalo bull sperm.
2. Materials and methods
2.1. Semen collection and evaluation
Five mature bulls, kept at Animal Reproduction Research Institution, Agriculture Research Center, Ministry Agriculture, were implemented in this study. Semen was collected by using the artificial vagina once a week. Immediately after collection, semen was evaluated. Only semen samples with >80% motility and <10% morphologically abnormal sperm were used for this study.
2.2. Freeze-drying media
Medium1: 10 mmol/L Tris-HCl buffered supplemented with 50 mmol/L of each of NaCl and EGTA [ethyleneglycol-bis (b-aminoethyl ether)-N, N, N’ N’-tetraacetic acid] and pH of final solution adjusted to 8.2.
Medium 2: 10 mmol/L Tris and 1 mmol/L EDTA and pH of final solution adjusted to 8.0.
Medium 3: TCM 199 with Hank’s salts (Gibco Life Technologies Inc., Grand Island, NY, USA) supplemented with 10% (v/v) FCS (Gibco Life Technologies Inc.).
Medium 4: TCM 199 with Hanks salts supplemented with 10% (v/v) fetal calf serum and 0.2 mol/L trehalose.
All the media were designed according to Martins et al.[22] except for medium 2 was designed according to Kaneko et al [20].
2.3. Experimental design
Ejaculates collected weekly were pooled, and processed in two portions: First portion was cryopreserved with Tris-Fructose-Egg yolk-Glycerol extender as described by Foote[23] with a total concentration of 30 106 sperm/0.5 mL to be freeze-dried with the different media used in this study. Second portion was freeze-dried immediately with the different media used in this study. According to Abdalla et al.[24] semen samples were centrifuged in a percoll gradient (45%-90%) for 20 min at 700 g to remove seminal plasma. Subsequently, sperms were washed twice in Tyrode’s albumen lactate pyruvate (TALP) [25] to remove percoll remains, and allocated into the four freeze-drying media (1, 2, 3 and 4 respectively).
2.4. Sperm freeze-drying
For all the media used samples were diluted, placed in tubes of 1.5 mL and kept at room temperature for 30 min. Then sperm cell suspensions were cooled in liquid nitrogen vapor (approximately -80 ℃ for 1 h), by keeping the tubes at a distance of 5 cm from liquid nitrogen surface before plunged into it. Frozen samples were immediately inserted into a programmable freeze-dryer stabilized at (-55 ℃) and 0.001 mbar pressure. After 24 h of freeze-drying, thetubes containing the samples were covered with aluminum foil and stored for 3 months at different temperatures: 4 ℃, -20 ℃ and -80 ℃.
2.5. Rehydration
Freeze-dried sperm samples were rehydrated by adding 100 µL of milli-Q water at room temperature.
2.6. Ultrastructural assessment
To evaluate sperm ultrastructure, transmission electron microscopy was done on samples from all freeze-drying media[22]. Sperms of each freeze drying media were fixed for 3 h at room temperature in a solution containing 2% glutaraldehyde, 2% paraformaldehyde, 5% sucrose and 5 mM, CaCl2in 0.1 M sodiumcacodylate buffer, pH 7.2. After fixation, the specimens were rinsed in buffer, and post-fixed (1 h) in 1% osmium tetroxide, 0.8% potassium ferricyanide, 5 mM CaCl2in 0.1 M sodium cacodylate buffer. Dehydration was carried out in acetone and embedding in Epon 812 resin. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined in a JEOL 1011 (JEOL,Tokyo, Japan) transmission electron microscope, operating at 80 kV. All electron microscopy reagents were purchased from Electron Microscopy Sciences (Ft. Washington, PA, USA).
2.7. Detection of DNA fragmentation by comet assay
According to Singh et al. [26], freeze-dried spermatozoa from all media (10 µL of 1 106 cells/mL suspension) were mixed with 1% (w/v) low-melting agarose gel (90 µL), added onto agarosecovered slides, treated with lysis solution for 3 h (including 10 mM dithiothreitol for 0.5 h and 4 mM lithium diiodosalicylate for 1.5 h in the latter two-thirds) and then processed with electrophoresis under a pH >13 alkaline condition (10 V, 20 min). Half of the sperm suspension was treated with 10 mM H2O2for 20 min at 4 ℃ before being mixed with low melting agarose gel. Sperm DNA were stained with SYBR Green, and the captured BMP images of the comet by fluorescence microscope (E600; Nikon, Tokyo, Japan) were analyzed by the Comet Score software (>100 comets per sample). The DNA fragmentation index (tail moment) was calculated as the length of comet tail (pixel)×the % DNA librated.
3. Results
Regardless to semen status and storage temperature, results of transmission electron microscopy showed that in the sperm cell components, the most affected part by freeze-drying was the plasma membrane (Figure 1-8; A-H). Microtubules organization was also disordered in the majority of sperms freeze-dried in medium 2 and 3 (Figure 2; b, 3; c, 6; f, 7; g), diverging from freeze-drying media 1 and 4 (Figure 1; a, 4; d, 5; e, 8; h), in which microtubules were intact. Conversely, the acrosome and mitochondria were well protected in all media.
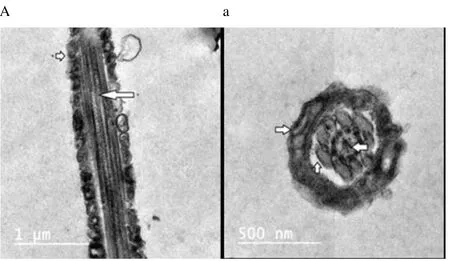
Figure 1. Electron micrographs showing raw buffalo bull spermatozoa after freeze-drying in medium 1.

Figure 2. Electron micrographs showing raw buffalo bull spermatozoa after freeze-drying in medium 2.

Figure 3. Electron micrographs showing raw buffalo bull spermatozoa after freeze-drying in medium 3.

Figure 4. Electron micrographs showing raw buffalo bull spermatozoa after freeze-drying in medium 4.
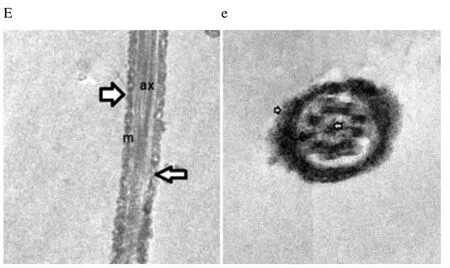
Figure 5. Electron micrographs showing frozen-thawed buffalo bull spermatozoa after freeze-drying in medium 1.

Figure 6. Electron micrographs showing frozen-thawed buffalo bull spermatozoa after freeze-drying in medium 2.

Figure 7. Electron micrographs showing frozen-thawed buffalo bull spermatozoa after freeze-drying in medium 3.

Figure 8. Electron micrographs showing frozen-thawed buffalo bull spermatozoa after freeze-drying in medium 4.
The present results of (Table 1) showed that the freeze-drying medium (EDTA solution) exhibited and the lowest percent of DNA damage (9.3%) while the freeze drying medium (TCM with trehalose) exhibited the highest percent of DNA damage (13.6%) at temperature of storage (4 ℃) (see Figure 9 and Figure 10, respectively). In contrast, the freeze-drying medium (EGTA solution) exhibited the lowest percent of DNA damage (15.3%), while the freeze-drying medium (TCM with trehalose) exhibited the highest percent of DNA damage (18%) at temperature of storage (-20 ℃) (see Figure 11 and Figure 12, respectively). On the other hand, the freeze-drying medium (EDTA solution) exhibited the lowest % of DNA damage (11.3), while the freeze-drying medium (TCM with trehalose) exhibited the highest percent of DNA damage (20%)at temperature of storage (-80 ℃) (see Figure 13 and Figure 14, respectively).
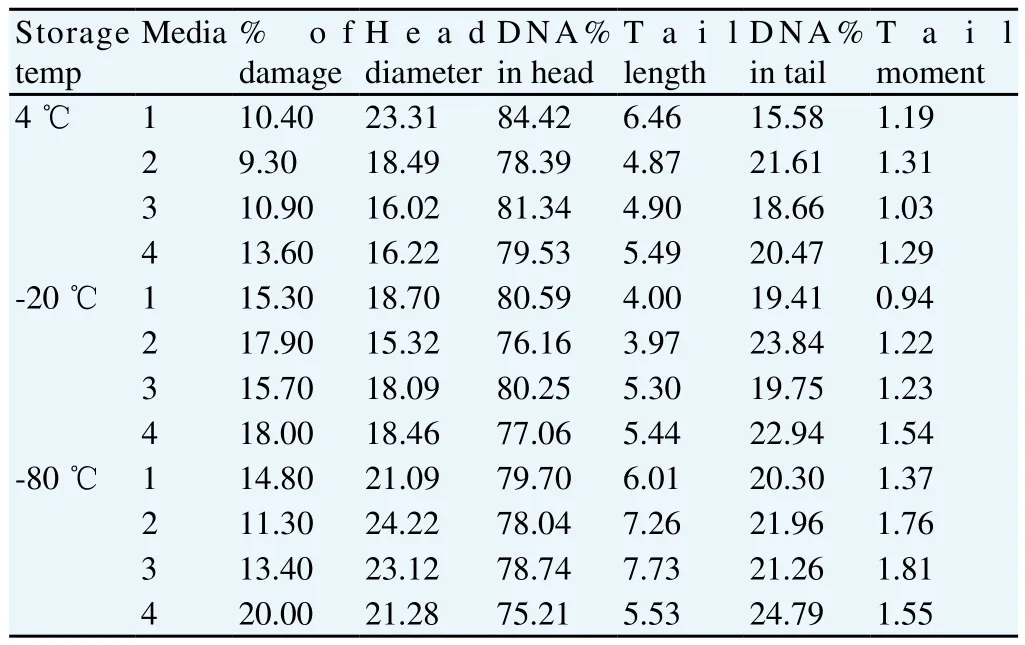
Table 1 Comet results of raw buffalo bull spermatozoa after freeze-drying.

Figure 9. Comet assay of raw buffalo bull spermatozoa after freeze-drying in medium 2 and storage at (4 ℃).

Figure 10. Comet assay of raw buffalo bull spermatozoa after freezedrying in medium 4 and storage at (4 ℃). The presence of comet tails indicates fragmented DNA in these sperms.
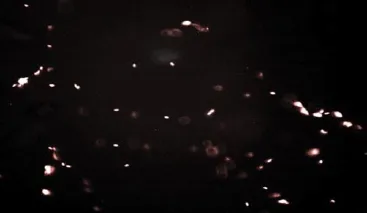
Figure 11. Comet assay of raw buffalo bull spermatozoa after freezedrying in medium 1 and storage at (-20 ℃).

Figure 12. Comet assay of raw buffalo bull spermatozoa after freezedrying in medium 4 and storage at (-20 ℃). The presence of comet tails indicates fragmented DNA in these sperms.

Figure 13. Comet assay of raw buffalo bull spermatozoa after freeze-drying in medium 2 and storage at (-80 ℃).

Figure 14. Comet assay of raw buffalo bull spermatozoa after freeze-drying in medium 4 and storage at (-80 ℃). The presence of comet tails indicates fragmented DNA in these sperms.
Consequently, the present results of (Table 2) revealed that the freeze-drying medium (TCM without trehalose) exhibited the lowest percent of DNA damage (6.5%) while the freeze-drying medium (EDTA solution) exhibited the highest percent of DNA damage (13.9%) at temperature of storage (4 ℃) (see Figure 15 and Figure 16, respectively). On the contrary, the freeze-drying medium (EGTA solution) exhibited the lowest percent of DNA damage (13.2%) while the freeze drying medium (TCM without trehalose) exhibited the highest percent of DNA damage (20.8%) at temperature of storage (-20 ℃) (see Figure 17 and Figure 18, respectively). Conversely, the freeze-drying medium (EDTA solution) exhibited the lowest percent of DNA damage (6%) while the freeze drying medium (TCM with trehalose) exhibited the highest percent of DNA damage (12%) at temperature of storage (-80 ℃) (see Figure 19 and Figure 20, respectively).

Table 2 Comet results of frozen-thawed buffalo bull spermatozoa after freezedrying.

Figure 15. Comet assay of frozen-thawed buffalo bull spermatozoa after freeze-drying in medium 3 and storage at (4 ℃).

Figure 16. Comet assay of frozen-thawed buffalo bull spermatozoa after freeze-drying in medium 3 and storage at (4 ℃).

Figure 17. Comet assay of frozen-thawed buffalo bull spermatozoa after freeze-drying in medium1 and storage at (-20 ℃).

Figure 18. Comet assay of frozen-thawed buffalo bull spermatozoa after freeze drying in medium 3and storage at (-20 ℃).

Figure 19. Comet assay of frozen-thawed buffalo bull spermatozoa after freeze-drying in medium 2 and storage at (-80 ℃).

Figure 20. Comet assay of frozen-thawed buffalo bull spermatozoa after freeze-drying in medium 4 and storage at (- 80 ℃).
4. Discussion
In the current study, we found out new data concerning on the degree that various freeze-drying media protected the structural and functional characteristics of the buffalo bull sperm. We investigated that EGTA solution sufficiently protected the nucleus, acrosome and mitochondria of buffalo bull sperm. This come in agreement with Olaciregui et al. [27] in stallion and Kusakabe et al. [12] in mice, who stated that freeze-drying medium with EGTA, better maintains chromosome integrity of spermatozoa during freeze-drying than does ordinary cell culture medium. In addition, we found that presence of EDTA exhibited the lowest percent of DNA damage in both raw at (4 and -80 ℃) and frozen semen at (-80 ℃). These findings are in accordance with Kaneko et al. [20] who stated that integrity of chromosome could be maintained well by adding a small amount of EDTA into the solution during freeze-drying. Our results also, revealed that TCM without trehalose gave the lowest percent of DNA damage in frozen-thawed semen (4 ℃). However, TCM with trehalose gave the highest percent of DNA damage in raw and frozen-thawed semen. It is well known that both EGTA and EDTA, chelate calcium (inhibits or reduces the activity of calciumdependent endonucleases[12] through reducing the availability of the circulating calcium; therefore, EGTA is advised to minimize chromosome injuries[28]. The main objective of investigations on sperm freeze-drying is to conserve its motility and maintain fertilizing capacity, enabling the sperm to be used AI or in vitro fertilization. However, this objective has not been achieved in any species. In the present exploration, sperms were immotile after freeze-drying in all media. The sperm plasma membrane injuries were established by electron microscopy. In this respect, the plasma membrane is highly susceptible to injuries, due to loss of water during dehydration[4,6,12,21,29]. Water loss from phospholipid head groups in cell membranes could be due to lateral phase separation resulting in extravasation of intracellular contents[30]. Acrosome is the main second part of sperm which is highly affected by freezedrying. In spite of >70% of the sperm had an intact acrosome (Figure 1-8; A-H), in all media tested regardless to semen status or storage temperature. In this regard, Martins et al. [22] recorded that freeze-drying media conserved acrosome integrity. In spite of the slight differences among media used, in general we demonstrated a significant disorganization in microtubules with media containing EDTA and TCM without trehalose (Figure 2; b, 3; c, 6; f, 7; g), Media containing EGTA and trehalose had the lowest rate of tail separation. Martins et al. [22] in bovine and Hirabayashi et al. [31] in rats obtained similar results. These results are in disagreement with those reported in pigs[9], mice[7,12,19] and rabbits[8]. However, Men et al. [32] suggested that freeze-drying medium supplied with considerable amounts of trehalose could maintain DNA integrity but not fertilizing ability before ICSI. Maybe bovine sperm has better steadiness in the connection region than other species, which could give an explanation for the low rate of loss of tail after freezedrying when compared to data from other species[22]. According toelectron microscopy, in all media the mitochondria were conserved after freeze-drying. Therefore, Martins et al. [22] hypothesized that the medium with EDTA, due to its hypertonicity, could affect microtubule integrity. In conclusion, we demonstrated that the freeze-drying medium containing EGTA and EDTA solution were more sufficient in avoiding injuries to constituents of buffalo bull sperm, especially the nuclei. Also, the storage temperature of freezedried sperm affects sperm nuclear integrity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgements
The authors are greatly indebted to the National Research Centre and Animal Reproduction Research Institute for sponsoring this work and also greatly indebted to RSTDG for their.
[1] Keskintepe L, Pacholczyk G, Machnicka A, Norris K, Curuk MA, Khan I. Bovine blastocyst development from oocytes injected with freeze-dried spermatozoa. Biol Reprod 2002; 67: 409-415.
[2] Kusakabe H, Kamiguchi Y. Chromosomal integrity of freeze dried mouse spermatozoa after 137Cs gamma-ray irradiation. Mutat Res 2004; 556: 163-168.
[3] Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperature. Nature 1949; 164: 666-667.
[4] Sherman JK. Freezing and freeze-drying of human spermatozoa. Fertil Steril 1954; 5: 357-71.
[5] Bialy G, Smith VR. Freeze-drying of bovine spermatozoa. J Dairy Sci 1957; 40: 739-745.
[6] Yushchenko NP. Proof of the possibility of preserving mammalian spermatozoa in a dried state. Proc Lenin Acad Agr Sci 1957; 22: 37-40.
[7] Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechno 1998; 16: 639-641.
[8] Liu JL, Kusakabe H, Chang CC, Suzuki H, Schmidt DW, Julian M, et al. Freeze-dried sperm fertilization leads to full-term development in rabbits. Biol Reprod 2004; 70: 1776-1781.
[9] Kwon IK, Park KE, Niwa K. Activation, pronuclear formation, and development in vitro of pig oocytes following intracytoplasmic injection of freeze-dried spermatozoa.Biol Reprod 2004; 71: 1430-1436.
[10] Critser JK, Huse-Benda AR, Aaker D, Arneson BW, Ball GD. Cryopreservation of human spermatozoa. I. Effects of holding procedure and seeding on motility, fertilizability, and acrosome reaction. Fertil Steril 1987; 47: 656-663.
[11] Choi YH, Varner DD, Love CC, Hartman DL, Hinrichs K. Production of live foals via intracytoplasmic injection of lyophilized sperm and sperm extract in the horse. Reproduction 2011; 142: 529-538.
[12] Kusakabe H, Szczygiel MA, Whittingham DG, Yanagimachi R. Maintenance of genetic integrity in frozen and freeze-dried mouse spermatozoa. Proc Natl Acad Sci USA 2001; 98: 13501-13506.
[13] Yanagimachi R. Stability of the mammalian sperm nucleus.Zygote1994; 2: 383-384.
[14] Ward WS. The structure of the sleeping genome: implication of sperm DNA organization for somatic cells. J Cell Biochem 1994; 55: 77-82.
[15] Ward WS, Zalensky AO. The unique complex organization of the transcriptionally silent sperm chromatin. Crit Rev Eukaryot Gene Expr 1996; 6: 139-147.
[16] Olaciregui M, LuñoV,Gonzalez N, De Blas I, Gil L. Freeze-dried dog sperm: Dynamics of DNA integrity. Cryobiology 2015; 71(2): 286-290.
[17] Hara H, Tagiri M, Hwang IS, Takahashi M, Hirabayashi M, Hochi S. Adverse effect of cake collapse on the functional integrity of freeze-dried bull spermatozoa. Cryobiology 2014; 68(3): 354-360.
[18] Wakayama T, Whittingham DG, Yanagimachi R. Production of normal offspring from mouse oocytes injected with spermatozoa cryopreserved with or without cryoprotection. J Reprod Fertil 1998; 112: 11-17.
[19] Kaneko T, Whittingham DG, Yanagimachi R. Effect of pH value of freeze-drying solution on the chromosome integrity and developmental ability of mouse spermatozoa. Biol Reprod 2003; 68: 136-139.
[20] Kaneko T, Serikawa T. Long-term preservation of freeze-dried mouse spermatozoa. Cryobiology 2012; 64: 211-214.
[21] McGinnis LK, Zhu L, Lawitts JA, Bhowmick S, Toner M, Biggers JD. Mouse sperm desiccated and stored in trehalose medium without freezing. Biol Reprod 2005; 73: 627-633.
[22] Martins CF, Báo SN, Dode MN, Correa GA, Rumpf R. Effects of freezedrying on cytology, ultrastructure, DNA fragmentation, and fertilizing ability of bovine sperm. Theriogenology 2007; 67: 1307-1315.
[23] Foote RH. Fertility of bull semen at high extension rates in Tris buffered extenders. J Dairy Sci 1970; 53:1475-1477.
[24] Abdalla H, Hirabayashi M, Hochi S. The ability of freeze-dried bull spermatozoa to induce calcium oscillations and resumption of meiosis. Theriogenology 2009; 71: 543-552.
[25] Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up and percoll method on success of in vitro fertilization and early embryonic development.Theriogenology1995; 44: 859-869.
[26] Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988; 175: 184-191.
[27] Olaciregui M, Lu o V, Marti JI, Aramayona J, Gil L. Freeze-dried stallion spermatozoa: evaluation of two chelating agents and comparative analysis of three sperm DNA damage assays.Andrologia 2016; 48(9): 900-906
[28] Szczygiel MA, Moisyadi S, Ward WS. Expression of foreign DNA is associated with paternal chromosome degradation in intracytoplasmic sperm injection-mediated transgenesis in the mouse. Biol Reprod 2002; 68: 1903-1910.
[29] Kawase Y, Suzuki H. A study on freeze drying as a method for preserving mouse sperm. J Reprod Dev 2011; 57: 176-182.
[30] Crowe JH, Crowe LM, Carpenter JF, Prestelski SJ, Hoekstra FA. Anhydrobiosis: cellular adaptations to extreme dehydration. In: Dantzler WH, editor. ComparativePhysiologyHandbook of Physiology, vol. II. Oxford: Oxford University Press; 1997, p.1445-1477.
[31] Hirabayashi M, Kato M, Ito J, Hochi S. Viable rat offspring derived from oocytes intracytoplasmically injected with freezedried sperm heads. Zygote 2005; 13: 79-85.
[32] Men N, Kikuchi K, Nakai M, Fukuda A, Tanihara F, Noguchi J, et al. Effect of trehalose on DNA integrity of freeze dried boar sperm, fertilization, and embryo development after intracytoplasmic sperm injection. Theriogenology 2013; 80: 1033-1044.
ment heading
10.1016/j.apjr.2016.11.002
*Corresponding author: *Corresponding author: Reda I. El-Sheshtawy, Animal Reproduction and AI department, Veterinary Research Division, National Research Centre, Dokki, Giza, Egypt.
Tel: +202-01222695899
E-mail: rielsheshtawy@gmail.com
Financial support:Through the project entitled: "Trials for freeze-drying of bull semen " ID 12655,
Methods:The semen samples were collected once weekly from five mature buffalo bulls maintained in Animal Reproduction Research Institute, Ministry of Agriculture, Al-Haram, Giza, Egypt. Samples were allocated into two portions; the first one was cryopreserved in Tris-Fructose-Egg yolk-Glycerol, while the second portion was immediately freeze-dried. Semen samples were centrifuged in a percoll gradient (45%-90%) for 20 min at 700 g to remove seminal plasma. Subsequently, sperms were washed twice in Tyrode’s albumen lactate pyruvate to remove percoll remains, and allocated into the freeze-drying media. The media tested were: medium1 (EGTA solution), medium 2 (EDTA solution), medium 3 (TCM199 with Hanks salts and 10% FCS) and medium 4 (TCM199 with Hanks salts and 10% FCS and trehalose). For all the media used, samples were diluted, placed in tubes of 1.5 mL and kept at room temperature for 30 min. then cooled in liquid nitrogen vapor (approximately -80 ℃ for 1 h), by keeping the tubes at a distance of 5 cm from liquid nitrogen surface before plunged into it. Frozen samples were immediately inserted into the freeze-dryer set at -55 ℃ and 0.001 mbar pressure. After 12-16 h of freeze-drying, the tubes containing the samples were covered with aluminum foil and stored for 3 months at different temperatures; 4 ℃, -20 ℃ and -80 ℃. Freezedried sperm samples were re-hydrated by adding 100 µL of milli-Q water at room temperature. To evaluate sperm ultrastructure, transmission electron microscopy was done. For detection of DNA fragmentation, comet assay was performed. Results:Electron microscopy showed that the most affected component of sperm cell by freeze-drying was the plasma membrane, which was destroyed in all media either in raw or frozen thawed sperm. Microtubules organization was also disorganized in the majority of the sperm from freeze-drying medium 2 and 3, diverging from freeze-drying media 1 and 4, in which microtubules were intact. Conversely, the acrosome and mitochondria were well protected in all media. However, the storage temperature has no effect. The freeze-drying media with EDTA solution exhibited the lowest percent of DNA damage (9.3) while the freeze-drying medium (TCM with trehalose) exhibited the highest percent of DNA damage (13.6) at temperature of storage (4 ℃). In contrast, the freeze-drying media with EDTA solution exhibited the lowest percent of DNA damage (15.3) while the freeze-drying medium (TCM with trehalose) exhibited the highest percent of DNA damage (18) at temperature of storage ( 20 ℃). On the other hand, the freezedrying medium with EDTA solution exhibited the lowest percent of DNA damage (11.3) while the freeze-drying medium (TCM with trehalose) exhibited the highest percent of DNA damage (20) at temperature of storage ( 80 ℃). Consequently, the freeze-drying medium (TCM without trehalose) exhibited the lowest percent of DNA damage (6.5) while the freeze-drying medium with EDTA solution exhibited the highest percent of DNA damage (13.9) at temperature of storage (4 ℃). On the contrary, the freeze-drying medium with EGTA solution exhibited the lowest percent of DNA damage (13.2) while the freeze-drying medium (TCM without trehalose) exhibited the highest percent of DNA damage (20.8) at temperature of storage ( 20 ℃). Conversely, the freeze-drying medium with EDTA solution exhibited the lowest percent of DNA damage (6) while the freeze-drying medium (TCM with trehalose) exhibited the highest percent of DNA damage (12) at temperature of storage ( 80 ℃).
Conclusion:From the present study, we demonstrated that the freeze-drying medium containing EGTA or EDTA were more efficient in avoiding damage to components of buffalo bull sperm, especially the nuclei. Therefore, the medium used for freeze-drying process directly affected the integrity of sperm nuclear. Also, the storage temperature of freeze-dried sperm affects integrity of sperm nuclear.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
- Factors affecting length of gestation in artificially inseminated Marwari Mares of India
- Nicotine effect toward the oocyte level of rats(Rattus novergicus)
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
- Substitution of egg yolk with different concentrations of soybean lecithin in trisbased extender during bulls' semen preservability
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
