Nicotine effect toward the oocyte level of rats(Rattus novergicus)
2017-01-06YayukDwirahayuSugengMashudi
Yayuk Dwirahayu, Sugeng Mashudi
1Department of Nursing, Faculty of Health Sciences, Muhammadiyah University of Ponorogo, Ponorogo, East Java, Indonesia
2Department of Midwifery, Faculty of Health Sciences, Muhammadiyah University of Ponorogo, Ponorogo, East Java, Indonesia
Nicotine effect toward the oocyte level of rats(Rattus novergicus)
Yayuk Dwirahayu1, Sugeng Mashudi2*
1Department of Nursing, Faculty of Health Sciences, Muhammadiyah University of Ponorogo, Ponorogo, East Java, Indonesia
2Department of Midwifery, Faculty of Health Sciences, Muhammadiyah University of Ponorogo, Ponorogo, East Java, Indonesia
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Germinal vesicle
Germinal vesicle breakdown
Metaphase I
Metaphase II
Nicotine
Objective:To discover the effect of some nicotine’s doses to the maturity of rat’s oocyte in reproductive age.Methods:This study was using an experimental design with 3 treatment groups (subcutaneous injection of nicotine 35, 52.5 and 70 mg/kgBW/d for 7 d) and 1 control group. After nicotine exposure treatment, the oocytes of rats were taken for the histological purpose to observe the oocyte’s maturity grade in germinal vesicle (GV), Germinal Vesicle Breakdown (GVBD), Metaphase I (M I), or Metaphase II (M II). All of the oocytes were stained using Aceto orcein 1 %. The data was analyzed based on One-Way Analysis of Variance (α=0.05).Results:The significant result got further analyzed using LSD test. The result obtained that there were no significant differences of GV and GVBD oocytes in all groups. Otherwise, the result of One-Way ANOVA showed that there were significant differences of M-II oocytes in all groups. LSD test obtained that there was significant differences between control group and 3 treatment groups, and nicotine’s treatment 52.5 mg/kg body weight (BW) with 35 mg/kg BW. This research obtained that the number of GV and GVBD oocytes were lower than the control group. Conclusion: there was no M I and M II oocytes, because nicotine treatment were less than the control group.
1. Introduction
Nicotine is one of the components in a cigarette that considered as addiction substance that cause smoking habit. Infertility is a determination of the existence and cause of death of the products of conception or fertility disorders. Compounds that contained in cigarette smoke consist of 90% gas and 20% different kinds of particles. The gaseous substance in a cigarette is CO, CO2, cyanide, various hydrocarbons, and organic acids[1]. Women who smoke, their reproductive system will be affected, because every cigarette contains 4 000 chemicals. Chemicals particles in a cigarette are divided into 3 groups: nicotine, tar, and carbon monoxide. One of the impacts on women’s reproduction is infertility[1].
Some researches that have been conducted shows that the pair of men and women who are infertile after consuming cigarettes for more than 5 years. The cigarettes that are consumed contain 0.8-1.8 mg nicotine per cigarette; but it depends on the brand and size. One milligram of nicotine can be absorbed into the body during smoking one cigarette[2]. The same point was described by Mai et al. [3], and he stated that the risk of infertility of active smoker women is 1.6 times more than non-smoker women[3].
Research about cigarettes that have been conducted indicates the concentration of cotinine (nicotine metabolic compounds) got in follicular fluid, which can affect the maturation of oocytes for in vitro fertilization[4]. The assumption is proved by the presence of oocyte maturation reduction up to 50% in women over 40 years[4]. Other study conducted to hamsters that are treated with nicotine exposure for 7 d whose folliculogenesis process can be interrupted [5]. The folliculogenesis will be issued oocyte, so it is assumed that the duration of nicotine exposure for 7 d will affect oocyte maturation process. The research of Noor et al. [6] toward mice by providing nicotine subcutaneous injection with dosage 5, 7.5, and 10 mg/kg have been able to influence the meiosis of oocytes by the acquisition of oocyte aneuploidy[6]. Based on Mailhess et al. (2000), the dosage of nicotine exposure of this study was set into 35, 52.5, and 70 mg/kg[6]. That dosage came from Noor et al. [6] toward mice dosage then converted to rats dosage by multiplied it with 7.0[6,7].
The process of meiosis in oocytes is influenced by endogenous and exogenous factors through biochemical reactions during oocyte maturation[6]. Exogenous factor that is considerably influenced is the cigarettes, the largest of its component is nicotine. The differences between nicotine effects on oocyte maturation at age less than 40 years old and over 40 years old support this study to determine the effects of nicotine on oocyte maturation with different doses of reproductive age and consideration maturation of important oocyte in the reproductive age.
2. Materials and methods
This research was conducted using experimental design by providing a treatment in mice given a subcutaneous injection of nicotine with a dosage of 35, 52.5, and 70 mg/kg per day. The type of design used was a post-test only control group design with a schematic design of the study (Figure 1).
A sample of 36 rats (Rattus norvegicus) was divided into a control group, P1, P2and P3. Independent variable was nicotine dosage, while the dependent variables were the level of germinal vesicle (GV), germinal vesicle breakdown (GVBD), metaphase I (M I), or metaphase II (M II). After nicotine exposure treatment, the oocytes of rats were taken for the histological purpose to observe the oocyte’s maturity grade in GV, GVBD, M I and M II. All of the oocytes were stained using Aceto orcein 1%. The data was analyzed based on One-Way Analysis of Variance (ANOVA) (α=0.05). GV and GVBD oocyte was tested using Kruskal-Wallis comparison test because the data were not normally distributed, meanwhile, the data of M II oocyte were normally distributed. Otherwise, M I data could not be analyzed because it has zero data. The significant result got further analyzed using LSD test.
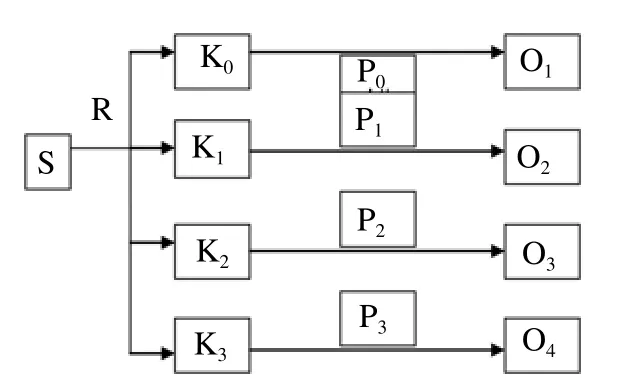
Figure 1. Research plan flowchart.
3. Results
This study provided nicotine exposure in rats (Rattus novergicus). The group consisted of negative control treatment (P0) for the control group (K0) as the comparison result, P1(nicotine 35 mg/kg) for K1, P2 (52.5 mg/kg) for K2, and P3(70 mg/kg) for K3(Figure 2-Figure 6).
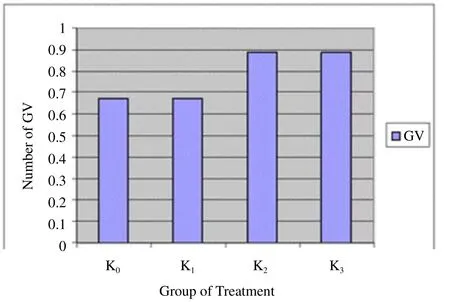
Figure 2. The number of GV in various groups.
Figure 2 shows that the greater the dose of nicotine makes the increasing number of GV. It shows the number of immature oocytes is also increased. Figure 3 shows that GVBD does not describe a similar pattern to GV. K0and K1show the same results but K2and K3do not produce data GVBD. Figure 4 shows that there is not found oocytes in the phase of M I. Figure 5 shows that the greater the nicotine dose given to the rats, the less number of M II oocytes produced. This illustrates that the decrease of mature oocytes due to nicotine exposure. The description of oocytes at different maturation levels obtained in this study can be seen in Figure 6. The level of maturation that found only GV, GVBD, and M II.
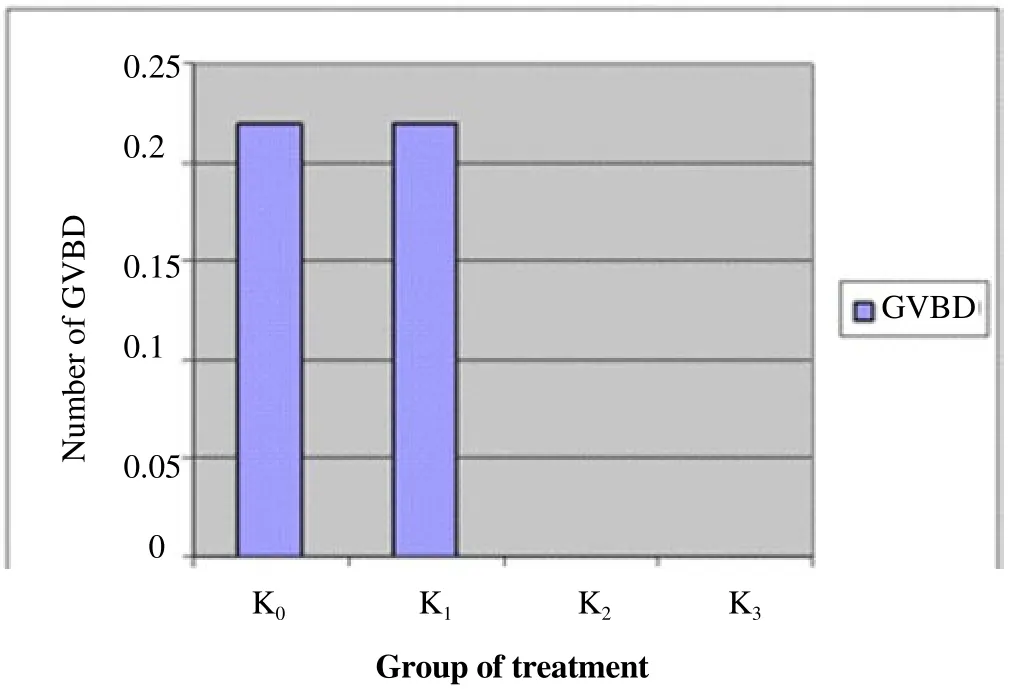
Figure 3. The number of GVBD on different groups.
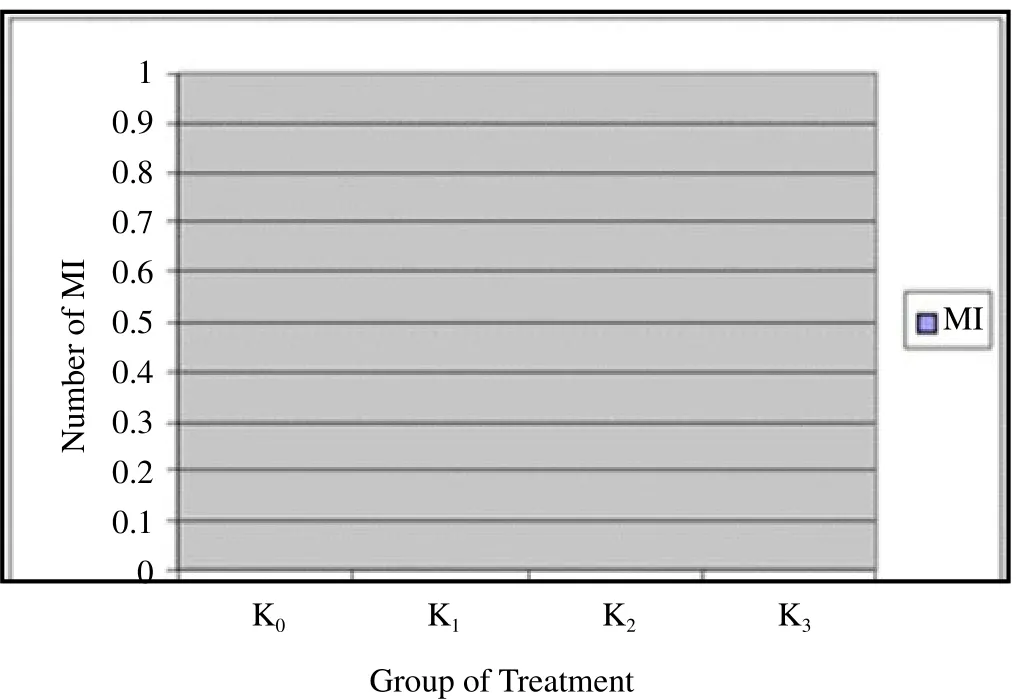
Figure 4. The number of M I in various groups.
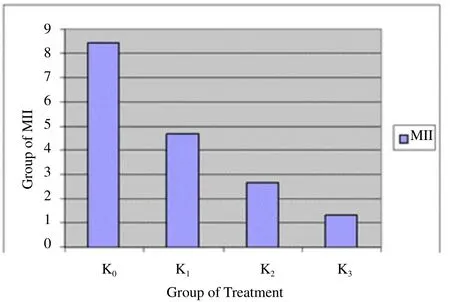
Figure 5. The number of M II in the various groups.
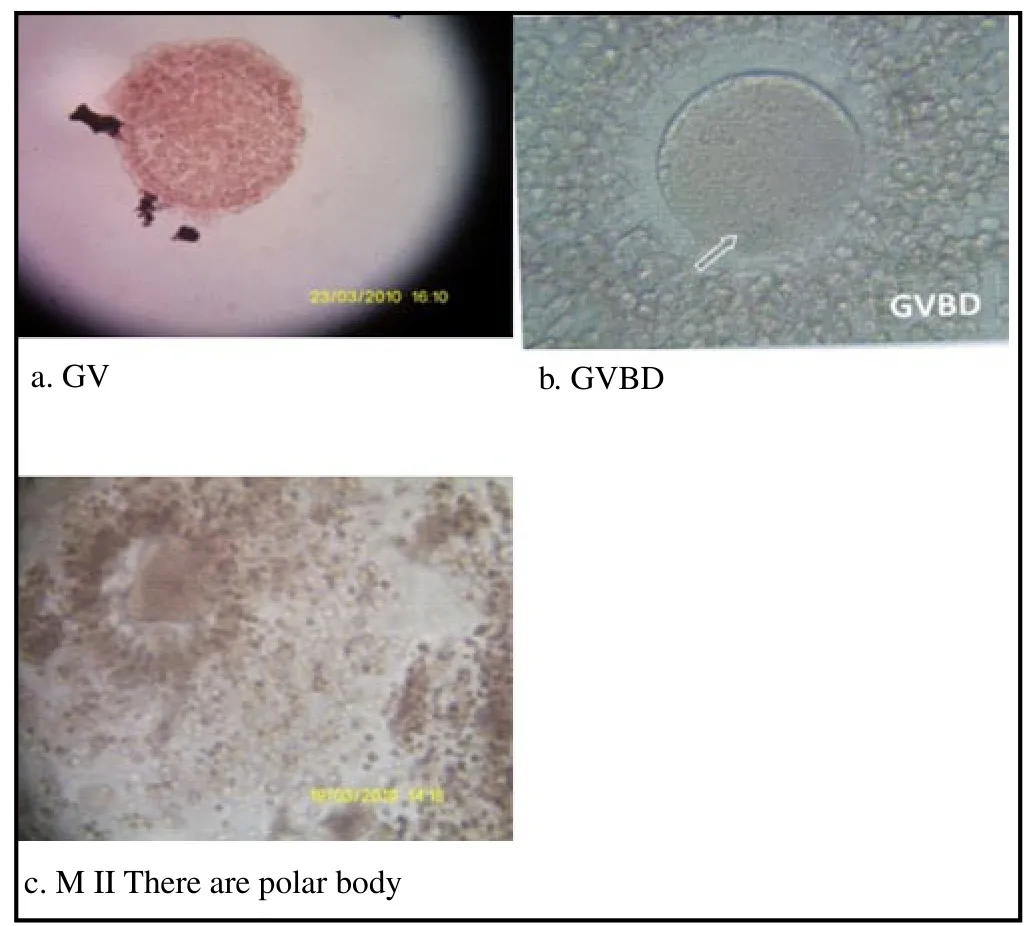
Figure 6. Different level of maturation of oocytes.
Oocytes amount in M II maturity is followed by BNT test to determine a treatment group was significantly different. BNT test of the maturation of M II showed that comparison of all groups are significant different, except between P2and P3.
4. Discussion
Oocytes maturity after GV is GVBD. The oocyte maturation process, GVBD phase, is begun because there is a response to hormone stimulus which is started by the outbreak of the core wall or GVBD. With the onset of GVBD, chromosomes condense into a compact form. The centrosome (a special area in the form of cytoplasm that solidifies) divide the two centrioles that look like cylinder shape. This cylinder shape moves apart and forms the spindle for cell proliferation purpose[8].
These nonstandard results probably caused by the deviation standard of data that was too wide and less specific, therefore, the small difference between the data will not create a significant difference. The SD value in this study was high as a consequence of trouble shooting during research method. Not all rats ovum is taken for the histopathological observation that caused the data varies from zero to the highest number of ova which was made the data have high SD value.
These results indicated that the impact of nicotine exposure toward oocyte maturation has not been able to suppress the level of GV/ GVBD. The factors that might be influenced were the number of experimental animals, the dose, and duration of nicotine exposure. In this study, the limited rats number used as the experimental animal has a weakness, if ovum were unavailable in one rat (zero) it would affect the whole data. Therefore, further studies need more rats number for better data obtained.
The nicotine exposure dosage determination was based on the study conducted by Noor et al. [6] which provides exposure to nicotine in mice oocytes. Furthermore, the nicotine mice dosage was had been converted to rats dose. The previous study observed the 4th level of maturation, while Noor et al. [6] observed the meiosis process occurred in ovum at M II phase.
In this study GV and GVB were not significantly different, and it was supported by a similar study conducted by Jennings et al. [4] that also provides nicotine exposure toward mice ovum. The results prove that nicotine does not affect the level of maturation to GV/ GVBD, but induce abnormal spindle and chromosomes of the oocyte maturation process. The exposure duration used in this study was 7 d that according to research conducted by Bordel et al. [5], which performed nicotine exposure for 7 d toward hamsters to observe folliculogenesis[5]. The previous studies were similar but they have different research purposes that make result obtained on GV and GVBD were different significantly.
The process of maturation after GV and GVBD is M I. This study obtained that all M I data were zero, indicated that there were not metaphase I process in all experimental animals. That was probably caused by a rapid process for M I before it turned into M II process and its growth stalled in the secondary oocyte[9] M I arose after GVBD process, where the nuclear membrane was folded and pores disappeared. The rest of the nuclear membrane disappeared quickly. Nuclei also disappear quickly after it contacts with the cytoplasm[9].M I was not found in this study, the same result was also obtained by Noor et al. [6] who examined the ovum maturation as a result of nicotine exposure found out that all ovum were had already being in the M II process. The rapid change of GVBD to M II is allegedly being a reason for the absence of M I in this study.
The last phase is the M II that occurred after the M I. The One-Way ANOVA test result obtained that there were significant differences among all groups. This shows that nicotine affects the maturation of the ovum by reducing the number of M II in nicotine-exposed rats. The little number of M II oocytes indicated that the number of mature oocytes was insignificant due to the number of M II oocytes show that they were already mature. Oocytes which have been developed that are already mature when they were taken from the follicle will have complete meiosis process after reaching metaphase II [10]. The mature oocytes number of nicotine-exposured rats group is low because of increased nicotine exposure is associated with estradiol reduction that followed by a decrease of ovarian stimulation and the number of oocytes[11].
Nicotine is an oxide compound that can increase oxidative stress in the body. Oxidative stress that occurs in follicular fluid thus causing follicular granulosa cell apoptosis and disrupt the folliculogenesis process makes the follicles shrink[5,12]. The size of the follicle affects the development of oocyte. Noor et al., [6] mentioned that nicotine can affect oocyte maturation process. Furthermore, the oocyte ability to complete meiosis and maturation depends on the size of the follicle. The number of mature oocytes from small follicles less than mature oocytes from large follicles, thus the presence of nicotine decrease the number of mature oocytes because it affected the follicles size[13,14].
The BNT test result shows that there was a significant difference between the control group and the treatment group. This means the group which was not treated with nicotine produced mature oocytes. Otherwise, the group treated with nicotine in any dose will inhibit oocyte maturation. The dosage differences showed that only doses in groups P2and P3that were not significantly different, but other groups were significantly different. This indicated that low dosage of nicotine (35 mg/kg body weight) can disturb the maturation of rats ovum, the same result was obtained by other higher dosages. Otherwise, the intermediate dose (52.5 mg/kg) and high dose (70 mg/kg) obtained they were not significantly different toward oocyte process disruption. It indicated that intermediate and high doses performed the same effect in this study. The dose that may be decreased the number of M II in this study was started from 35 mg/ kg body weight of rats (Rattus novergicus).
The converting dose from rats to human is multiplied by 56.0[7]. The nicotine dose that assumed to reduce human oocytes during M II process is 1.960 mg or 1.96 g. During smoking, the human body can absorb nicotine ranged from 11.5 mg, thus it is assumed that mature oocytes of women will significantly decrease after exposed for about 1 960 cigarettes, either the woman is an active or passive smoker. The number of cigarettes is still influenced by the volume of smoke and air inhaled, surround air condition, and the intensity of smoking [15].
We conclude that nicotine exposure doses from 35, 52.5, and 70 mg/kg did not affect the maturation of oocytes process either at GV stage nor GVBD. M I oocytes phase was not found in this study (zero). Nicotine exposure in all doses could decrease the number of oocytes at M II phase. Furthermore, there is needed further research which can conduct a larger number of dose and exposure duration to obtain the better results.
Acknowledgement
The author thank to the Muhammadiyah University of Ponorogo for facilitating this research.
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] Schardein JL. Chemically Induced Birth Defect. New York: Marcel Dekker Inc; 2005, p. 764-768.
[2] Firns S, Cruzat VF, Keane KN, Joesbury KA, Lee AH, Newsholme P, et al. The effect of cigarette smoking, alcohol consumption and fruit and vegetable consumption on IVF outcomes: a review and presentation of original data. Reprod Biol Endocrinol 2015; 13: 134.
[3] Mai Z, Lei M, Yu B, Du H, Liu J. The effects of cigarette smoke extract on ovulation, oocyte morphology and ovarian gene expression in mice. PloS ONE 2014; 9(4): e95945.
[4] Jennings PC, Merriman JA, Beckett EL, Hansbro PM, Jones KT. Increased zona pellucida thickness and meiotic spindle disruption in oocytes from cigarette smoking mice. Hum Reprod 2011; 26(4): 1-7.
[5] Bordel R, Laschke MW, Menger MD, Volmar B. Nicotine does not effect vascularization but inhibit growth of freely transplanted ovarian follicles by inducing granulosa cell apoptosis. Hum Reprod 2006; 21(3): 610-616.
[6] Noor MS, Bahkriansyah HM, Widjiati W, Santoso B. Nicotine supplementation blocks oocyte maturation in Rattus norvegicus. Univ Med 2013; 32(2): 92-98.
[7] Kusumawati D. Pengaruh Pemberian Stevoisida terhadap Reproduksi dan Perkembangan Embrio Tikus Putih. PhD Thesis. Airlangga University. Surabaya 2003.
[8] Greenstein D. Control of oocyte meiotic maturation and fertilization. Wormbook. Nashville 2005. p. 1-12.
[9] Centola GM, Anderson LD, Channing CP. Oocyte maturation inhibitor (OMI) activity in protein granulosa cells. Gamet Res 2005; 4(5): 451-461.
[10] Beall S, Brenner C, Segars J. Oocyte maturation failure: a syndrome of bad eggs. Fertil Steril 2010; 94(7): 2507-2513.
[11] Freour T, Masson D, Dessolle L, Allaoua D, Dejoie T, Mirallie S, et al. Ovarian reserve and in vitro fertilization cycles outcome according to women smoking status and stimulation regimen. Arch Gynecol Obstet 2012; 285(4): 1177-1182.
[12] Paixão LLO, Gaspar-Reis RP, Gonzales GPL, Santos AS, Santana AC, Santos RMM, et al. Cigarette smoke impairs granulosa cell proliferation and oocyte growth after exposure cessation in young Swiss mice: an experimental study. J Ovarian Res 2012; 5: 25.
[13] Son W-Y, Lee S-Y, Lim J-H. Fertilization, cleavage and blastocyst development according to the maturation timing of oocytes in vitro maturation cycles. Hum Reprod 2005; 20(11): 3204-3207.
[14] Lim JH, West KL, Pubinstein Y, Bergel M, Postnikov YV, Bustin M. Chromosomal protein HMGN1 enhances the acetylation of lysine 14 in histone H3. EMBO J 2005; 24(7): 3038-3048.
[15]Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005; 57(1): 79-115.
ment heading
10.1016/j.apjr.2016.10.005
*Corresponding author: Sugeng Mashudi*, Department of Midwifery, Faculty of Health Sciences, Muhammadiyah University of Ponorogo, Ponorogo, East Java, Indonesia.
E-mail: nershudi@gmail.com
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
- Factors affecting length of gestation in artificially inseminated Marwari Mares of India
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
- Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
- The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
