Seminal plasma hormonal profile of Arabian stallions that are classified‘good’or‘poor’for semen freezing
2017-01-06DAElBadryGamalElSisyAmalAboElMaaty
DA El-Badry, Gamal A El Sisy, Amal M Abo El-Maaty*
1Department of Artificial Insemination and Embryo Transfer, Animal Reproduction Research Institute, Agriculture Research center, Giza, Egypt
2Department of Animal Reproduction and A.I., National Research Center, Dokki, Giza, Egypt
Seminal plasma hormonal profile of Arabian stallions that are classified‘good’or‘poor’for semen freezing
DA El-Badry1, Gamal A El Sisy2, Amal M Abo El-Maaty2*
1Department of Artificial Insemination and Embryo Transfer, Animal Reproduction Research Institute, Agriculture Research center, Giza, Egypt
2Department of Animal Reproduction and A.I., National Research Center, Dokki, Giza, Egypt
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Leptin
IGF-1
Thyroid hormones
Semen freezing
Stallion
Objective: To relate seminal plasma reproductive and metabolic hormone profile to both semen freezability and post-thaw semen characteristics. Methods: Semen was collected from each Arabian stallion (N=16) for five consecutive weeks and evaluated before conducting this study. Seminal plasma was collected during semen processing for measuring leptin, insulin, Insulin like growth factor-I (IGF-1), cortisol, testosterone, estradiol and thyroid hormones. Stallions were classified into good (N=10) and poor freezer (N=6). Semen post-thaw motility (%), viability index, membrane and acrosome integrity (%) were also evaluated. Results: Good freezer stallions had significantly (P=0.0 001) high cortisol, estradiol, insulin, IGF-1, T3and T4, but significantly low leptin (P=0.003). Post-thaw sperm viability index (P=0.0 001), membrane integrity (%) (P=0.002) and motility (%) (P=0.0 001) at 0, 1, 2 and 3 h of good freezer stallions was significantly high compared to poor freezers. Testosterone, insulin and IGF-1 in seminal plasma of good freezer stallions had significant (P<0.01) positive correlation with sperm post-thaw viability index but estradiol and insulin in seminal plasma of poor freezers correlated with sperm post-thaw viability index. Within good freezer stallions, testosterone in seminal plasma correlated positively with both viability and acrosome integrity but within poor freezer stallions it had a negative correlation with both of them. Individual variation existed between animals and significantly affected seminal plasma hormones and semen post-thaw characteristics. Conclusion: Good freezer stallions could be selected depending on assaying seminal plasma hormones. Both estradiol and IGF-1 could be used as semen extender supplementation to improve sperm post-thaw motility, viability and acrosome integrity.
1. Introduction
Stallion selection depended largely on performance and phenotype, rather than semen quality and freezability. A wide inter-individual variability in equine sperm quality became the main challenge affecting the efficiency of semen cryopreservation[1]. According to individual variability, horses had been classified into either good or bad freezers[2]. The predictive value of several markers for semen quality parameters related to successful freezability of horse semen was investigated[2,3]. The intra-individual freezability variations was related to sperm membrane protein, fatty acid or lipid content[3], to seminal plasma constituents of the accessory glands that controlled by reproductive hormones[4] and to differences in circulating hormone concentrations[5].
Seminal plasma is a cocktail of secretions produced by the testes, epididymis and accessory sex glands acting as a vehicle and containing factors that modulate the fertilizing ability of the ejaculated spermatozoa[6]. Leptin[7,8] and insulin[9,10] hormones exist in seminiferous and in seminal plasma, expressed in and secreted by spermatozoa to regulate sperm motility and acrosome reaction[7-10]. Insulin-like growth factor-I (IGF-1) of equine testicular fluid, during all spermatogenic stages and spermatozoa and seminal plasma had been associated with sperm characteristics and fertility[11]. Identification of IGF-1 in seminal plasma and spermatozoa influenced sperm motility[12], membrane integrity, lipid peroxidation and fructose uptake[13]. Testosterone is an essential hormone for spermatogenesis[14], and during its absence spermatogenesis is being arrested[15]. Both testosterone and estrogen levels in the semen reflexes reproductive health status and testicular estradiol plays a fundamental role in gamete survival, proliferation, differentiation, and maturation of spermatids[16]. Thyroid hormones affects testis development, spermatogenesis, semen quality and malefertility[17,18].
The objective of the present study was to investigate the associations between both seminal plasma metabolic, reproductive hormones and quality of frozen-thawed semen of good and bad freezer Arabian stallions.
2. Materials and methods
2.1. Animals and semen collection
At once weekly collection schedule, five ejaculates per stallion were obtained from 16 Arabian stallions, aged 7-12 years, and individually housed at Police Academy stud, Cairo, Egypt. Ten stallions were previously classified as good freezers and the other 6 stallions were poor freezers. Classification as so-called ‘good’ or‘poor’ freezers is based upon post-thaw motility. Stallions with postthaw spermatozoal motility greater than 35% were considered good freezers[19]. At the time of collection, early in the morning, a mare in estrus was used as a mount animal. Semen was collected using a lubricated and pre-warmed (45-50 ℃) Colorado model artificial vagina with an inline filter to separate the gel fraction. Immediately following collection, the gel-free portion of the ejaculate was evaluated for progressive motility and only ejaculates with at least 60% progressively motile sperm were used during this study.
2.2. Seminal plasma samples
Part of the raw semen was centrifuged at 400×g for 10 min to separate sperm from seminal plasma. The seminal plasma was removed and filtered through 1.2 µm filters to remove any residual sperm and stored at -80 ℃ until assay[20].
2.3. Hormonal assay
Commercial kits for leptin and IGF-1 were purchased from DRG Instruments, GmbH, Germany. Testosterone, estradiol 17-β, cortisol, insulin, T3and T4were measured using commercial ELISA (Chemux BioScience, Inc. San Francisco, USA). Sensitivity of the assay, intra and inter-assay precisions were 10 pg/mL, 9.1% and 9.8% for estradiol; 0.022 ng/mL, 6.6% and 7.3% for testosterone; 0.2 ng/mL, 4.1% and 9.0% for T3; 0.6 µg/dL, 6.4% and 9.9% for T4; 1.29 ng/mL, 6.62% and 7.79% for IGF-I; 1.0 ng/mL, 3.1% and 9.7% for leptin; 0.4 µg/dL, 2.9% and 3.8%, respectively for cortisol; and Sensitivity for insulin was 2.0 µIU/m.
2.4. Semen processing
The semen was extended 1:1 (semen: extender) in modified INRA-82 extender that had been warmed to 38 ℃[21]. The diluted samples were placed into 15-mL conical tubes and centrifuged for 10 min at 400×g[22]. Pellets were diluted with modified INRA-82 to a final sperm concentration of 100×106motile sperm/mL. Each aliquot was cooled slowly to 5 ℃ over 1 h under aerobic conditions, and then incubated at 5 ℃ for 30 min[23]. The extended semen was drawn into 0.5 mL straws (Minitube, Germany) and sealed thermally and placed 4 cm above liquid nitrogen in the vapor phase in foam box for 10 min before being plunged into the liquid phase[24]. The straws were then stored in goblets and kept immersed in liquid nitrogen. For thawing, two straws per treatment were warmed in a water bath at 37 ℃ for 30 s[25].
2.5. Evaluation of frozen-thawed semen
Spermatozoa motility was examined and recorded using a prewarmed stage of phase contrast microscope (200×) just after thawing (0 h) and at 1, 2 and 3 h post-thawing. In each semen sample, the percentage of plasmatic membrane integrity in sperm cells using hypo-osmotic stress (HOS) test was determined[26]. A 100 µL aliquot of each semen sample was mixed in 1.0 mL of a prewarmed 100 mOsm sucrose solution (1.712 g sucrose dissolved in 50 mL of sterile, de-ionized water). The mixture was incubated for 60 min at 37 ℃ in a 1.5 mL micro-centrifuge tube. Following incubation, a small drop of the sample was placed on a microscope slide and cover-slipped for examination using phase contrast microscopy (400×) to evaluate 100 spermatozoa for evidence of swelling and curling changes. Acrosomal status was evaluated by a dual staining procedure[27]. Briefly, spermatozoa were incubated with an equal volume of 0.2% trypan blue for 10 min and washed twice (centrifugation at 700×g for 6 min) with BO medium. Smears were made on glass slides and dried quickly on a warm stage. Slides were stained with 10% giemsa stain for 40 min, then were rinsed under a stream of distilled water, air-dried, and covered with coverslips. Spermatozoa were classified as acrosome intact (light purple-dark pink acrosome), and damaged/lost acrosome (unstained or blue acrosome).
2.6. Statistical analysis
Data are presented as mean±standard error of mean (SEM). Data are subjected to independent sample t-test using computer software SPSS[28]. Simple one way ANOVA was used to study the effect of animal within each animal class. Correlations between seminal plasma hormones and frozen-thawed semen parameters were also performed within each animal class.
3. Results
Good freezer stallions had a significantly (P<0.05) low leptin but high cortisol, estradiol, insulin, IGF-1, T3and T4(Table 1). Leptin, cortisol, estradiol, insulin, IGF-1, and T4(P<0.05) showed significant individual variation (Table 1).
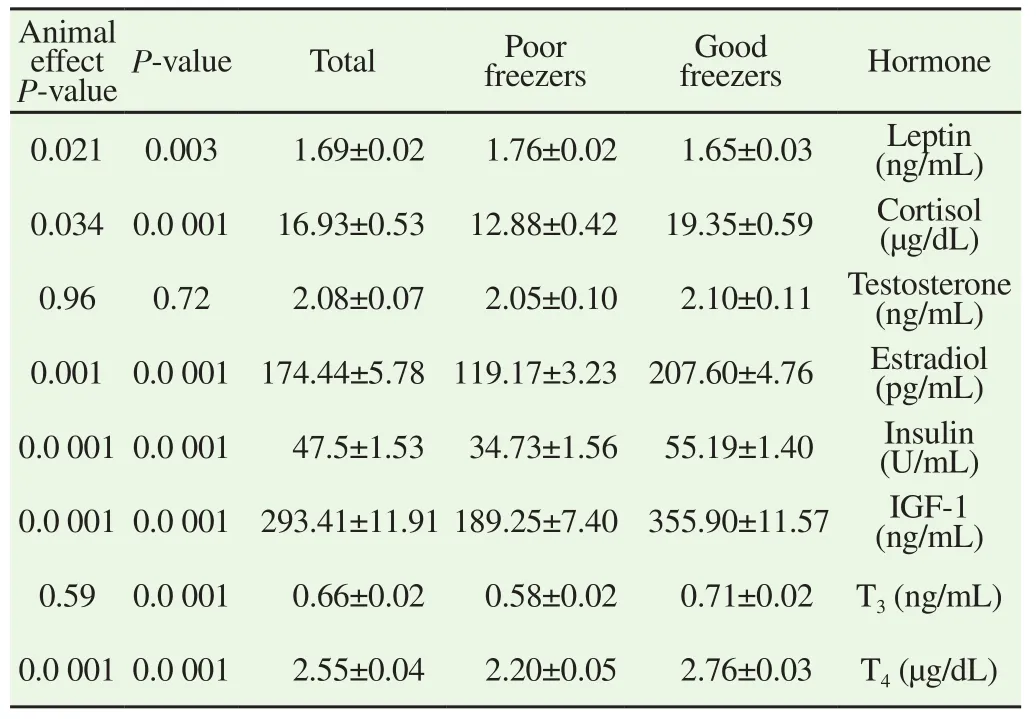
Table 1 Mean ± S.E.M of hormones concentration in seminal plasma collected from stallions classified according to their semen freezability.
Good freezer group showed highest ability to preserve sperm motility over 3 h period than bad freezer one (Table 2). Also individual variation between animals significantly (P=0.0 001) affected sperm post-thaw motility at all examination times (Table 2). The viability index (P=0.0 001), post-thaw sperm membrane integrity percent (HOS; P=0.002) were significantly high for good freezer stallions compared to bad freezer ones. Variations between animals were only significant (P=0.0 001) for viability index (Table 3).
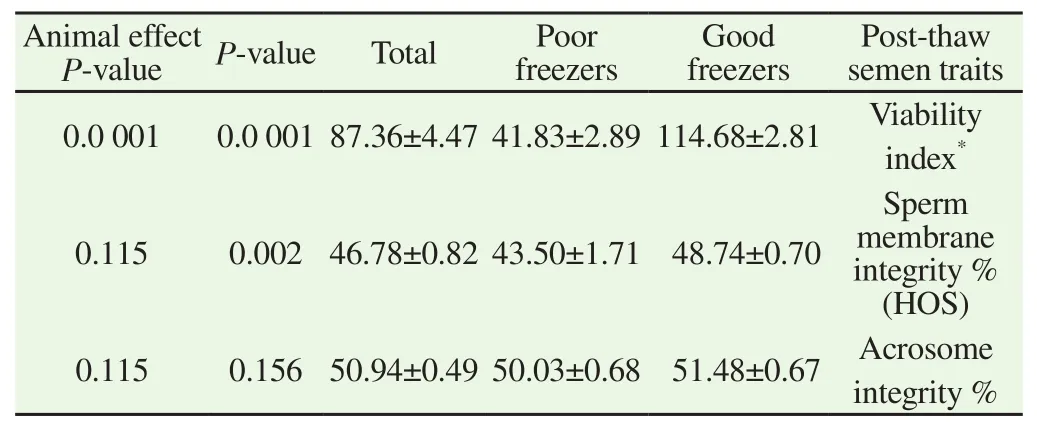
Table 3 Mean ± SEM of sperm post-thaw viability index, sperm membrane integrity % (HOST) and sperm acrosome intergrity % of good and poor freezer stallions.
The correlations among seminal plasma hormones (leptin, cortisol, testosterone, estradiol, insulin, IGF, T3, T4) and frozenthawed semen characteristics of good and poor freezer stallions were showed in Table 4. Leptin in seminal plasma of good freezer stallions had positive correlation with semen post-thaw motility at hour 2 (r=0.28; P=0.046) (HOS) and 3 (r=0.36; P=0.011), and sperm membrane integrity (r=0.42; P=0.008), but tended to have significant correlation with viability index (r=0.24; P=0.095), and acrosome integrity (r=0.25; P=0.084). Cortisol in seminal plasma of good freezers had significant positive correlation with post-thaw motility at 1 h (r=0.31; P=0.029), and sperm membrane integrity percent (r=0.31; P=0.028), but tended to have the same correlation with both post-thaw motility at 2 h and viability index (r=0.24; P=0.09). Testosterone in seminal plasma of good freezers has significant positive correlation with semen post-thaw motility at 2 h (r=0.42; P=0.003), and 3 h (r=0.38; P=0.007), viability index (r=0.37; P=0.008), and acrosome integrity (r=0.28; P=0.049) but in seminal plasma of bad freezers had negative correlation with sperm post-thaw motility, viability index, sperm membrane integrity and acrosome integrity. Estradiol of seminal plasma of good freezers tended to have a correlation with sperm post-thaw motility at 1 h (r=0.25; P=0.085), but that of bad freezers had a strong positive and highly significant correlations with sperm post-thaw motility, viability index and acrosome integrity, Insulin of good freezers has significant correlation with sperm post-thaw motility at 0 (r=0.45; P=0.001), 1 h (r=0.43; P=0.002), and viability index (r=0.35; P=0.014). However, bad freezers insulin correlated significantly with sperm post-thaw motility and viability index.
IGF-1 in seminal plasma of good freezers had positive significant correlations with sperm post-thaw motility at 0 h (r=0.51; P=0.0 001), 1 h (r=0.44; P=0.002), 2 h (r=0.34; P=0.015), and viability index (r=0.43; P=0.002). T4 in seminal plasma of only good freezers correlated with sperm post-thaw motility at 0 h (r=0.34; P=0.017).
4. Discussion
Stallions’ intra-individual variability in the acceptability for semen freezing is a major constraint for the equine industry that influences the commercial availability of frozen semen. Recently, there is great research attention to disclose the mechanisms and theories the individual variations associated with sperm damage during cryopreservation and to assess the freezability of the ejaculates before the freezing process. Recognition of most suitable stallions or ejaculates for cryopreservation will enable the use of stallion frozen sperm by avoiding the production of inferior-quality semen. However, a technique to recognize good ejaculates prior to freezing is still indefinable. Markers of freezability depended on sperm shape[29,30], and the chemical structure of the sperm plasma membrane[31].
Results of the current study indicated that good freezer stallion had a significant increase of cortisol, estradiol, insulin, IGF-1, T3and T4but a decrease of leptin than poor freezer one. Seminal plasma level of leptin varies between different species[32]. Spermatozoa of different species secrete and have receptors leptin[9,33-35]. The presence of leptin levels in seminal plasma of both intact and vasectomized men, indicates that testes are not the sole origin of seminal leptin and either seminal vesicles or prostate glands secrete it[36].
As reported in the current study, the correlation of seminal plasma leptin to sperm motility after thawing of poor freezers and to 2 h, 3 h after thawing of good freezers was positive and significant. In contrast, seminal plasma leptin had a negative correlation with fresh sperm cell motility and the 1st h of post-thaw motility[37,38], but correlated to oligozoospermic in infertile men[39], varicocelerelated spermatogenesis dysfunction in animal experiment[40], semen parameters[41]. The absence of significant correlation between seminal leptin sperm post-thaw motility of good freezers at 0 h, 1 h and of poor freezers at 1 h, 2 h, and 3 h were also confirmed by absence of correlation between seminal plasma leptin and any of semen characteristics[36,42,43]. Seminal plasma leptin tended to correlate with stallion post-thaw sperm membrane integrity supplementation (P>0.05) but in viro its supplementation significantly improved sperm motility and membrane integrity[10,44]. In the current study, seminal plasma cortisol of good freezer stallions are significantly higher than poor freezers and is nearly similar to the blood serum cortisol level of Arabian stallion[45] that is increased by natural mating[46] and sexual stimulation[47]. However, the increased cortisol did not exert any effects on testosterone levels and any of semen quality parameters of pony stallions[48], because testicular function in stallions may be well protected against temporarily increased cortisol levels. Moreover, mature stallions are able to convert conversion of active cortisol to inactive cortisone[49]. Seminal plasma cortisol of good freezer stallions correlated positively with post-thaw motility at 1 h and with oh of poor freezer stallions. Simlarly, seminal cortisol levels correlated with sperm motility[50]. In the current study, no significant different between seminal plasma testosterone levels of good or poor freezer stallions was observed, surprisingly, correlation between seminal plasma testosterone with all post-thawing semen parameter of good freezer stallions was positive but was negative with those of poor freezer stallions. Testosterone level in Arabian stallions appeared to be affected by age, seasonal variation, sexual stimulation and social environment of the horse[45,51,52]. Similarly, no differences in plasma testosterone concentrations were recorded between both normal andpoor semen quality stallions[53] and rams[54] but seminal plasma testosterone concentrations were significantly higher in fertile bulls[55].
Good freezer stallions of the current stallions had higher estradiol compared to poor freezers. Plasma levels of estrogen conjugates were associated with sperm concentrations in subfertile stallions and could be an indicator of altered sperm quality[56], sexual behavior[5], and resting condition[57]. Leydig cells are the synthesizers of estradiol from testosterone by aromatase in equine gonads[5,58]. Estrogens play an important role in sperm maturation, capacitation, and penetration[59].
The positive correlation between estradiol and post-thaw motility at 1 h, 2 h and 3 h of poor freezer stallions is similar to that reported with frozen-thawed sperm cell motility and sperm viability of Arab stallion[37].
Good freezer stallions of this study had high seminal plasma insulin concentration and were significantly similarly correlated with frozenthawed sperm cell motility at 1 h, 2 h and sperm viability index of good and poor freezers. Similar correlations had been previously reported in stallion semen[37]. Supplementation of freezing extender with insulin increased motility, kinematics and acrosome integrity of frozen semen[60], by promoting better glucose uptake by sperm providing more substrate for maintenance of flagellar activity[61].
Insulin-like growth factor I (IGF-I) in seminal plasma of good freezer stallions correlated significantly with sperm post-thaw motility at 0 h, 1 h, 2 h and viability index. IGF-I was essential for germ cell development, maturation and sperm motility[12,62]. IGF-I concentrations in the seminal plasma of stallions were positively correlated with fertility rates[11]. Similarly, a significant correlation existed between IGF-I level in seminal plasma and post-thaw motility of frozen-thawed spermatozoa as well as viability index[11,37]. Seminal plasma IGF-1 enhanced post-thawing sperm recovery after by promoting RNA, protein, and lipids synthesis, resulting in improving sperm plasma membrane function and survivability[63], decreased lipid peroxidation production during cryopreservation and increased total and progressive motility[13], improved sperm motility and plasma membrane integrity of frozen-thawed semen[61].
A significant increase of T4in good freezer stallions of the present study correlated with only postthaw motility at 0 h and tended to correlate with post-thaw motility at 1 h and sperm membarane integrity. Similarly, significant correlations between post-thaw motility and T4level[37], thyroid stimulating hormone level[18]. Thyroid hormones influenced testicular function directly by affecting on testis where its receptors are highly expressed and indirectly by affecting gonadotropins[64].
In conclusion, except for testosterone and thyroid hormones, all seminal plasma levels of cortisol, estradiol, insulin and IGF-1 were significantly higher in good freezer stallion than poor freezer one. Seminal plasma mean leptin concentration was significantly lower in good freezer stallion than poor freezer one indicated that hormones in seminal plasma and their association with sperm postthaw motility, viability and membrane integrity could be used as biomarkers to predict the acceptability of Arabian horse’s semen for cryopreservation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] Yeste M, Estrada E, Casas I, Bonet S, Enric J, Rodríguez-Gil JE. Good and bad freezability boar ejaculates differ in the integrity of nucleoprotein structure after freeze-thawing but not in ROS levels. Theriogenology 2013; 79 : 929-939.
[2] Ortega-Ferrusola C, Sotillo-Galán Y, Varela-Fernández E, Gallardo-Bolaños JM, González-Fernández L, Tapia JA, et al. Detection of apoptosis like changes during the cryopreservation process in equine sperm. J Androl 2008; 29: 213-221.
[3] Ortega-Ferrusola C, Sotillo-Galán Y, Varela-Fernández E, Gallardo-Bolaños JM, González-Fernández L, Tapia JA, et al. Apoptotic markers can be used to forecast the freezeability of stallion spermatozoa. Anim Reprod Sci 2009; 114: 393-403.
[4] Holt WV, Medrano A, Thurston LM, Watson PF. The significance of cooling rates and animal variability for boar sperm cryopreservation: insights from the cryomicroscope. Theriogenology 2005; 63: 370-382.
[5] Cavinder CA, Zoller JL, Briers G, Sigler DH. Sexual behavior and blood hormone profiles around the time of ejaculation and subsequent sperm characteristics in stallions. Profess Animl Sci 2010; 2: 540-546.
[6] Manjunath P, Chandonnet L, Leblond E. Major proteins of bovine seminal vesicle bind to spermatozoa. Biol Reprod 1993; 50: 27-37.
[7] Abo El-Maaty AM, El Sisy GA, Shaker MH, Ezzo OH. Age-related rump fat, fat percent, body fat mass, leptin, androgens and semen parameters of Arab stallions. Asian Pacific J Reprod 2014; 3: 184-191.
[8] Glander HJ, Lammert A, Paasch U. Leptin exists in tubuli seminiferi and in seminal plasma. Andrology 2002; 34: 227-233.
[9] Andò S, Aquila S. Arguments raised by the recent discovery that insulin and leptin are expressed in and secreted by human ejaculated spermatozoa. Mol cell Endocrinol 2005; 245: 1-6.
[10] Lampiao F, du Plessis SS. Insulin and leptin enhance human sperm motility, acrosome reaction and nitric oxide production. Asian J Androl 2008; 10: 799-807.
[11] Macpherson ML, Simmen RCM, Simmen FA, Hernandez J, Sheerin BR, Varner DD. Insulin-like growth factor-I and insulin-like growth factor binding protein-2 and -5 in equine seminal plasma: association with sperm characteristics and fertility. Biol Reprod 2002; 67: 648-654.
[12] Henricks DM, Kouba AJ, Lackey BR, Boone WR, Gray SL. Identification of insulin-like growth factor I in bovine seminal plasma and its receptor on spermatozoa: Influence on sperm motility. Biol Reprod 1998; 59: 330-337.
[13] Selvaraju S, Reddy IJ, Nandi S, Rao SB, Ravindra JP. Influence of IGF-I on buffalo (Bubalus bubalis) spermatozoa motility, membrane integrity, lipid peroxidation and fructose uptake in vitro. Anim Reprod Sci 2009; 113: 60-70.
[14] Kerr JB, Millar M, Maddocks S, Sharpe RM. Stage-dependent changes in spermatogenesis and sertoli cells in relation to the onset of spermatogenic failure following withdrawal of testosterone. Anat Rec 1993; 235: 547-559.
[15] Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994, p.1363-1434.
[16] Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010 ; 365(1546): 1517-1535.
[17] Singh R, Hamada AJ, Agarwal A. Thyroid hormones in male reproduction and fertility. Open Reprod Sci J 2011; 3: 98-106.
[18] Mayahi S, Mamouei M, Tabatabaei S, Mirzadeh K. Reproductive characteristics and thyroidal function in relation with season in Khuzestan buffalo (Bubalus bubalis) bulls. Veterinary Research Forum 2014; 5: 201-205.
[19] Vidament M, Dupere AM, Julienne P, Evain A, Noue P, Palmer E. Equine frozen semen: Freezability and fertility field results. Theriogenology 1997; 48: 907-917.
[20] Brinsko SP, Love CC, Bauer JE, Macpherson ML, Varner DD. Cholesterol-to-phospholipid ratio in whole sperm and seminal plasmafrom fertile stallions and stallions with unexplained subfertility. Anim Reprod Sci 2007; 99: 65-71.
[21] El-Badry DA, Gabr FI. Biochemical and enzymatic studies on semen of Arabian stallions that are classified ‘good’ or ‘poor’ for freezing. J Egypt Vet Med Assoc 2013; 73: 749-768.
[22] Cochran JD, Amann RP, Froman DP, Pickett BW. Effects of centrifugation, glycerol level, cooling to 5 ℃, freezing rate and thawing rate on the post-thaw motility of equine sperm. Theriogenology 1984; 22: 25-38.
[23] Crockett EC, Graham JK, Bruemmer JE, Squires EL. Effect of cooling of equine spermatozoa before freezing on post-thaw motility: Preliminary results. Theriogenology 2001; 55: 793-803.
[24] Cristanelli MJ, Amann RP, Squires EL, Pickett BW. Effects of egg yolk and glycerol level in lactose-EDTA-egg yolk extender and of freezing rate on the motility of frozen-thawed stallion spermatozoa. Theriogenology 1985; 23: 25-38.
[25] Salazar JL, Teague SR, Love CC, Brinsko SP, Blanchard TL, Varner DD. Effect of cryopreservation protocol on post-thaw characteristics of stallion sperm. Theriogenology 2011; 76: 409-418.
[26] Nie GJ, Wenzel JGW. Adaptation of the hypo-osmotic swelling test to assess functional integrity of stallion spermatozoal plasma membranes. Theriogenology 2001; 55: 1005-1018.
[27] Didion BA, Dobrinsky JR, Giles JR, Graves CN. Staining procedure to detect viability and the true acrosome reaction in spermatozoa of various species. Molecular Reprod Develop 1989; 22: 51-57.
[28] SPSS: Statistical package for social science. Chicago, IL: PC software, Version16. Inc.; 2007. For Windows.
[29] Martinez IN, Moran JM, Pena FJ. Two-step cluster procedure after principal component analysis identifies sperm subpopulations in canine ejaculates and its relation to cryoresistance. J Androl 2006; 27: 596-603.
[30] Nunez-Martinez I, Moran JM, Pena FJ. Sperm indexes obtained using computer assisted morphometry provide a forecast of the freezability of canine sperm. Int J Androl 2007; 30: 182-189.
[31] Macias Garcia B, Gonzalez Fernandez L, Ortega Ferrusola C, Morillo Rodriguez A, Gallardo Bolanos JM, Rodriguez Martinez H, et al. Fatty acids and plasmalogens of the phospholipids of the sperm membranes and their relation with the post-thaw quality of stallion spermatozoa. Theriogenology 2011; 75: 811-818.
[32] Lackey BR, Gray SL, Henricks DM. Measurement of leptin and insulin-like growth factor-I in seminal plasma from different species. Physiol Res 2002; 51: 309-311.
[33] Aquila S, Gentile M, Middea E. Leptin secretion by human ejaculated spermatozoa. J Clin Endocrinol Metab 2005; 90: 4753-4761.
[34] Aquila S, Rago V, Guido C. Leptin and leptin receptor in pig spermatozoa: Evidence of their involvement in sperm capacitation and survival. Reprod 2008; 136: 23-32.
[35] Abavisani A, Baghbanzadeh A, Shayan P. Leptin mRNA in bovine spermatozoa. Res Vet Sci 2011; 90: 439-442.
[36] Camina JP, Lage M, Menendez C. Evidence of free leptin in human seminal plasma. Endocrine 2007; 17: 169-174.
[37] El-Badry DA, Mahmoud MA. Relationship between some seminal plasma hormones and quality of fresh, chilled and frozen-thawed semen in Arabian horses. J Egypt Vet Med Assoc 2015; 75: 23-44.
[38] Ishikawa T, Fujioka H, Ishimura T. Expression of leptin and leptin receptor in the testis of fertile and infertile patients. Andrologia 2007; 39: 22-27.
[39] Hanafy S, Halawa FA, Mostafa T. Serum leptin correlates in infertile oligozoospermic males. Andrologia 2007; 39: 177-180.
[40] Chen B, Guo JH, Lu YN. Leptin and varicocele-related spermatogenesis dysfunction: Animal experiment and clinical study. Int J Androl 2008; 32: 532-541.
[41] Jorsaraei SGA, Shibahara H, Yustawati A. The leptin concentrations in seminal plasma of men and its relationship to semen parameters. Iran J Reprod Med 2010; 8: 95-100.
[42] Tena-Sempere M, Barreiro ML. Leptin in male reproduction: the testis paradigm. Mol Cell Endocrinol 2002; 188: 9-13.
[43] Zorn B, Osredkar J, Meden-Vrtovec H. Leptin levels in infertile male patients are correlated with inhibin B, testosterone and SHBG but not with sperm characteristics. Int J Androl 2007; 30: 439-444.
[44] Khaki A, Batavani RA, Najafi G. The in vitro effect of leptin on semen quality of water buffalo (Bubalus bubalis) bulls. Vet Res Forum 2013; 4: 7-12.
[45] Abo El-Maaty. Stress and its effects on horses reproduction. Vet Sci Dev 2011; 1: 54-57.
[46] Tamanini C, Giordano N, Chiesa F, Seren E. Plasma cortisol variations induced in the stallion by mating. Acta Endocrinol 1983; 102: 447-450.
[47] Rabb MH, Thompson DL, Bany BE, Colbom DR, Garza F, Hehnke KE. Effects of sexual stimulation, with and without ejaculation, on serum concentrations of LH, FSH, testosterone, cortisol and prolactin in stallions. J Anim Sci 1989; 67: 2724-730.
[48] Deichsel K, Pasing S, Erber R, Ille N, Palmec R, Aurich J, et al. Increased cortisol release and transport stress do not influence semen quality and testosterone release in pony stallions. Theriogenology 2015; 84: 70-75.
[49] Draper N, Stewart PM. 11beta-Hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endocrinol 2005; 186 : 251-71.
[50] Rangari K, Shrivastav TG. A correlation study between steroid hormone levels and anti-sperm antibodies in serum and seminal plasma of men with or without reduced sperm motility. J Endocrinol Reprod 2007; 11: 31-35.
[51] Altinsaat C, Üner AG, Sulu N, Ergün A. Seasonal variations in serum concentrations of melatonin, testosterone, and progesterone in Arabian horse. Ankara Üniv Vet Fak Derg 2009; 56: 19-24.
[52] Burger D, Dolivo G, Wedekind C. Ejaculate characteristics depend on social environment in the Horse (Equus caballus). Plos One 2015; http:// dx.doi.org/10.1371/journal.pone.0143185.
[53] Motton DD, Roser JF. HCG binding to the testicular LH receptor is similar in fertile, subfertile and infertile stallions. J Androl 1997; 18: 411-416.
[54] Moghaddam G, Pourseif MM, Asadpour R, Rafat SA, Jafari-Jozani R. Relationship between levels of peripheral blood testosterone, sexual behavior, scrotal circumference and seminal parameters in crossbred rams. Acta Scientiae Veterinariae, 2012; 40: 1049-1056.
[55] Shore L, Yehuda R, Marcus S, Bartoov B, Shemesh M. Effect of hCG injection on prostaglandin E concentrations in ram seminal plasma. Prostaglandins Other Lipid Mediat 2003; 70: 291-301.
[56] Roser JF, Hughes JP. Seasonal effects on seminal quality, plasma hormone concentrations and GnRH-induced LH response in fertile and subfertile stallions. J Androl 1992; 13: 214-219.
[57] Medica P, Cravana C, Fazio E, Ferlazzo A. 24-hour endocrine profiles of quarter horses under resting conditions. J Equine Vet Sci 2011; 31: 35-40.
[58] Almadhidi J, Seralini GE, Fresnel J, Silberzhan P, Gaillard JL. Immunohistochemical localization of cytochrome P450 aromatase in equine gonads. J Histochem Cytochem 1995; 43: 571-577.
[59] Chian RC, Blondin P, Sirard A. Effect of progesterone and/or estradiol-17 beta on sperm penetration in vitro of bovine oocytes. Theriogenology 1996; 46: 459-464.
[60] Van Tilburg MF, Silva JFS, Dias AJB, Quirino CR, Fagundes B. Influência da insulina na congelabilidade do sêmen de ovino [influence of insulin in the freezing of the ovine semen]. Ciência Animals 2008; 9: 731-739..
[61] Padilha RT, Magalhães-Padilha DM, Cavalcante MM, Almeida AP, Haag KT, Gastal MO, et al. Effect of insulin-like growth factor-I on some quality traits and fertility of cryopreserved ovine semen. Theriogenology 2012; 78: 907-913.
[62] Vickers MH, Casey PJ, Champion ZJ, Gravance CG, Breier, BH. IGF-I treatment increases motility and improves morphology of immature spermatozoa in the GH-deficient dwarf (dw/dw) rat. Growth Horm IGF Res 1999; 9: 236-240.
[63] Zamiri MJ, Khodaei HR. Seasonal thyroidal activity and reproductive characteristics of Iranian fat-tailed rams. J Anim Sci 2005; 88: 245-255. [64] Younis AI, Rooks B, Khan S, Gould KG. The effects of antifreeze peptide III (AFP) and insulin transferrin selenium (ITS) on cryopreservation of chimpanzee (Pan troglodytes) spermatozoa. J Androl 1998; 19: 207-214.
ment heading
10.1016/j.apjr.2016.10.012
*Corresponding author: Amal M Abo El-Maaty, Department of Animal Reproduction and AI, National Research Centre, Dokki, Giza, Egypt.
Tel: +202-01221278132
E-mail: amalaboelmaaty1@yahoo.com
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
- Nicotine effect toward the oocyte level of rats(Rattus novergicus)
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
- Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
- The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
