Oral dydrogesterone for luteal support in frozen-thawed embryo transfer artificial cycles: A pilot randomized controlled trial
2017-01-06BatoolHosseinRashidiMahyaGhazizadehEnsiehShahrokhTehraniNejadMaryamBagheriMansourehGorginzadeh
Batool Hossein Rashidi, Mahya Ghazizadeh, Ensieh Shahrokh Tehrani Nejad, Maryam Bagheri, Mansoureh Gorginzadeh
1Obstetrics and Gynecology at Tehran University of Medical Sciences, Vali-e-Asr Reproductive Health Research Center
2Tehran University of Medical Sciences, Tehran, Iran
3Tehran University of Medical Science, Vali-e-Asr Reproductive Health Research Center
4Tehran University of Medical Sciences, Vali-e-Asr Reproductive Health Research Center, Keshavarz Boulevard, Tehran, Iran
Oral dydrogesterone for luteal support in frozen-thawed embryo transfer artificial cycles: A pilot randomized controlled trial
Batool Hossein Rashidi1, Mahya Ghazizadeh2, Ensieh Shahrokh Tehrani Nejad1, Maryam Bagheri3, Mansoureh Gorginzadeh4*
1Obstetrics and Gynecology at Tehran University of Medical Sciences, Vali-e-Asr Reproductive Health Research Center
2Tehran University of Medical Sciences, Tehran, Iran
3Tehran University of Medical Science, Vali-e-Asr Reproductive Health Research Center
4Tehran University of Medical Sciences, Vali-e-Asr Reproductive Health Research Center, Keshavarz Boulevard, Tehran, Iran
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Frozen-thawed embryo transfer
Objective:To compare the clinical efficacy of oral dydrogesterone with both vaginal progesterone suppository and intramuscularly injected progesterone for luteal phase support in frozen-thawed embryo transfer (FET) artificial cycles.Methods:In this pilot single-blind randomized controlled trial, 180 infertile women undergoing FET cycles were recruited and allocated into three equal groups named as group A receiving 50 mg intramuscular progesterone ampules twice daily, group B receiving oral dydrogesterone 20 mg twice daily, and group C receiving 400 mg intravaginal progesterone suppository twice daily. Clinical pregnancy rates were the primary outcome. Abortion, ectopic pregnancy and live birth rates were the secondary outcome.Results:Pregnancy and live birth rates were comparable for all the three groups (P=0.466 and 0.367 respectively). Miscarriage rates were not significantly different among groups (P=0.487). All the resulting pregnancies for each group were intrauterine with none of them associated with ectopic origin.Conclusion:Given that oral dydrogesterone seems to be more accepted by patients in terms of ease of use, lower cost and satisfaction, it could be prescribed for luteal phase support in artificial FET cycles as effective as either intramuscular or vaginal supplements.
1. Introduction
Defective secretory transformation of the endometrium or luteal phase deficiency, is still a challenging concept in reproductive endocrinology[1,2]. Beyond its significance in the management of infertility and recurrent abortions, luteal phase deficiency is also a major concern in in-vitro-fertilization (IVF) cycles[3,4]. Following ovulation in a natural cycle, the mature ovarian follicle transforms to the corpus luteum which will become the major source of progesterone production before the placenta takes over this function for about seven weeks. This progesterone which results from the pulsatile secretion of the luteinizing hormone (LH), prepares the endometrium for implantation and maintenance of pregnancy[1-6]. Historically, pregnancies resulting from assisted reproductive technology (ART) have been threatened with implantation failure or miscarriage and either the quantitative or the qualitative defects of corpus luteum are to be blamed for that[3-6]. Given a wide array of manipulations done in artificial cycles such as pituitary down regulation with gonadotropin releasing hormone agonists, administration of human chorionic gonadotropin (HCG) for final oocyte maturation, the pulsatile pattern of LH secretion will be lost. Furthermore, retrieving oocytes during oocyte pick-up would diminish the number of granulosa cells undergoing later luteinization. These changes along with decreased endometrial receptivity will subsequently result in implantation failure[2,6,7]. Therefore, in order to improve fertility outcomes and maintain pregnancies, appropriate supplementation of luteal phase is absolutely crucial[1-8]. Luteal support has been associated with improvements in IVF outcomes[7,8]. In recent years, frozen-thawed embryo transfer (FET) has gained increasing popularity thanks to advancements in laboratory technology[9,10]. Contrary to the sophisticated protocols of fresh IVF cycles, FET cycles make it possible to transfer fewer embryos per cycle. In FET cycles whether natural or artificial where there is already a lack of functional corpus luteum, endometrial preparation before embryo transfer is mainly dependent on exogenous progesterone products and thispriming schedule exerts a significant impact on the success rates of these cycles[11-13]. Utilization of the progesterone supplements as the superior agents for endometrial preparation have been well established[14-17]. Given the higher risk of ovarian hyper stimulation syndrome with HCG and premature endogenous LH surge with gonadotropin releasing hormone analogs, progesterone remains the supplement of choice[7,15,18]. No agreement has yet been made regarding the optimal scheme including the route, the dosage and the duration of progesterone supplementation[9-11,19]. Of the available routes of administration for luteal support in IVF cycles including intramuscular (IM), intravaginal and oral, none has been associated with better outcomes[10,11,15,19]. Although both IM and vaginal paths are being widely used, each has certain drawbacks with pain at the site of injection, risk of cellulitis and sterile abscess formation for the IM route and vaginal discharge along with irritation for the vaginal route[11,16-21]. Apart from efficacy, therefore, patient satisfaction and tolerability should be highly contemplated[20]. Poor bioavailability resulting from rapid hepatic metabolism had made oral means of progesterone prescription unpropitious for years[17,20-22]. However, with the introduction of dydrogesterone in ART, an optical isomer of progesterone which has a rather good bioavailability[17,23], this route appeared justifiable. Likewise, with regards to pregnancy rates, side-effects and safety profile, its application has been promising according to the recent studies[23-31]. Since its marketing in 1961, dydrogesterone has been attributed to quite a large number of implications. Nevertheless, concerning its usage for the support of luteal phase in FET cycles, still more robust evidence is required particularly in the form of randomized controlled trials (RCT). Hence, as the dydrogesterone tablets are readily available in our country with a reasonable cost, this study was designed to compare this synthetic product with the conventional IM and intravaginal progesterone supplements for luteal support in FET cycles.
2. Material and methods
2.1. Patients preparation
This was a pilot single-blind randomized controlled trial conducted at the tertiary infertility center of Vali-e-Asr during a one-year period from January 2015 to May 2016. It was approved by the Institutional Review Board and the Ethics Committee of the medical university. There gistration number of the trial was IRCT201406255181N15. A total of 185 infertile women were enrolled in the study. Written informed consent was obtained from all of them. The inclusion criteria were patients undergoing FET, because of leftover embryos from past fresh or frozen cycles, canceled previous cycles, because of bad endometrium or ovarian hyper stimulation syndrome or candidates for embryo donation. Women with other indications and methods of ART were excluded from the study.
2.2. Endometrial preparation
For endometrial preparation at the first step, 6 mg of oral estradiol was prescribed on the second day of the cycle until the endometrial thickness of 8 mm was reached when then the participants were randomized into three equal groups to receive the presumed progesterone protocol for further luteal phase support. Sequentially numbered sealed envelopes were prepared and provided by the study coordinator, according to random-number tables. Single blinding was done by keeping the person enrolling the participants and the study investigators uninformed of the type of the treatment protocol. Only the statistician had access to the data. For group A (n=60) 50 mg intramuscular progesterone ampules were injected twice daily (Aboureyhan Co. Iran). Group B received 20 mg dydrogesterone twice daily (Duphaston ; Abbot Co. USA). Group C (n=60) received 400 mg progesterone suppositories two times per day vaginally (Cyclogest; Actavis Co. UK). Three to five days after the commencement of the progesterone protocol embryo transfer was carried out followed by measuring the serum β-HCG level 12 d later. About 95% of the embryos were in the cleavage stage and a few were in the blastocyst stage. The treatment protocol was continued until 12 wk of pregnancy. The final outcome was assessed in terms of clinical pregnancy as the primary outcome and abortion or ectopic pregnancy rates as the secondary outcome. Clinical pregnancy was confirmed by ultrasound showing a viable fetus performed 6 weeks after ET.
2.3. Statistical analysis
Data was analyzed with SPSS version 20 using student t-test, Chi2, Fisher’s exact and one-way ANOVA tests. P<0.05 and confidence interval (CI) of 95% were considered significant. Categorical data are presented as numbers or percent and continuous data as Mean±SD.
3. Results
The demographic, clinical and para clinical characteristics of the participants at the baseline are demonstrated in Table 1 and Table 2. Of 185 patients enrolled in the study, 180 entered the trial as the consort flow chart (Diagram 1) depicts. Finally, 59 subjects from group A, 60 subjects from group B and 58 patients from group C were entered the analysis. As shown in Table 2, the mean duration of both estradiol and progesterone prescription and the endometrial thickness before progesterone commencement were comparable for all the three groups (P=0.876, 0.065 and 0.447 respectively). Also, the mean numbers of frozen and transferred embryos were not different significantly between the groups (Table 2). The dominant cause of infertility for all the three arms of the trial (more than 65%) was the male factor with the female factor in the second order. There were 4 indications for performing FET including hyper stimulation syndrome, inappropriate endometrium, donation and leftovers with the leftovers and hyper stimulation to be the most common reasons respectively for each group. The transvaginal sonographic findings of the patients were also assessed. The majority of the subjects in each arm had normal pelvic ultrasound (51 subjects in IM group, 54 in oral group and 50 in suppository group). The most common abnormal finding was myoma (4 in IM group, 6 in oral group and 8 in suppository group). Overall, four patients had endometriosis based on TVS who were all in the IM group, of which, one became pregnant. The primary and secondary outcomes including pregnancy rates, abortion rates, ectopic pregnancy rates and live birth rates are presented in Table 3. According to our results, the pregnancy rates were comparable for all the three groups (P=0.466). No case of ectopic pregnancy occurred. Abortion and live birth rates, likewise, were not different significantly among groups (Table 3).
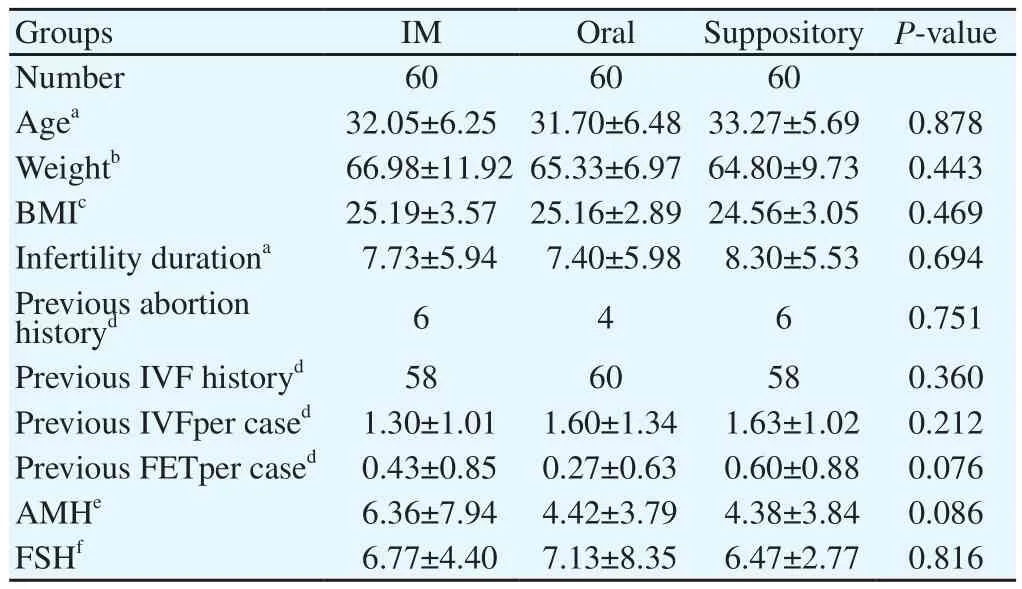
Table 1 Baseline characteristics of the patients.
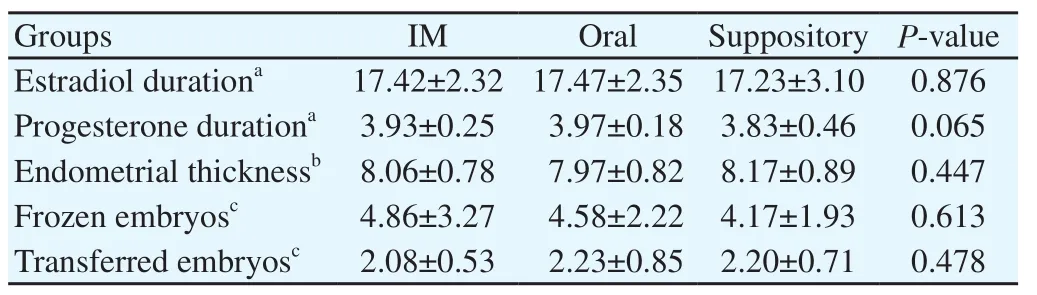
Table 2 Characteristics of FET cycles.
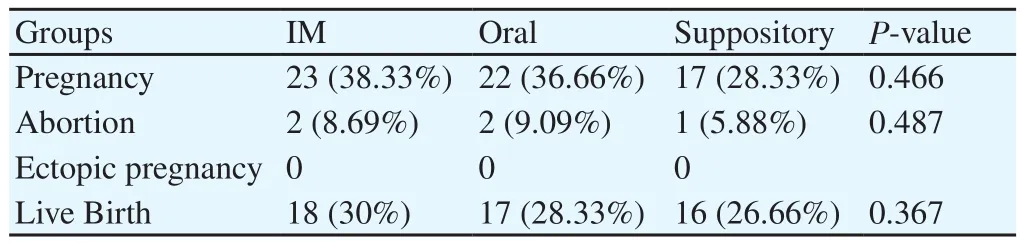
Table 3 Clinical outcomes of the three groups.
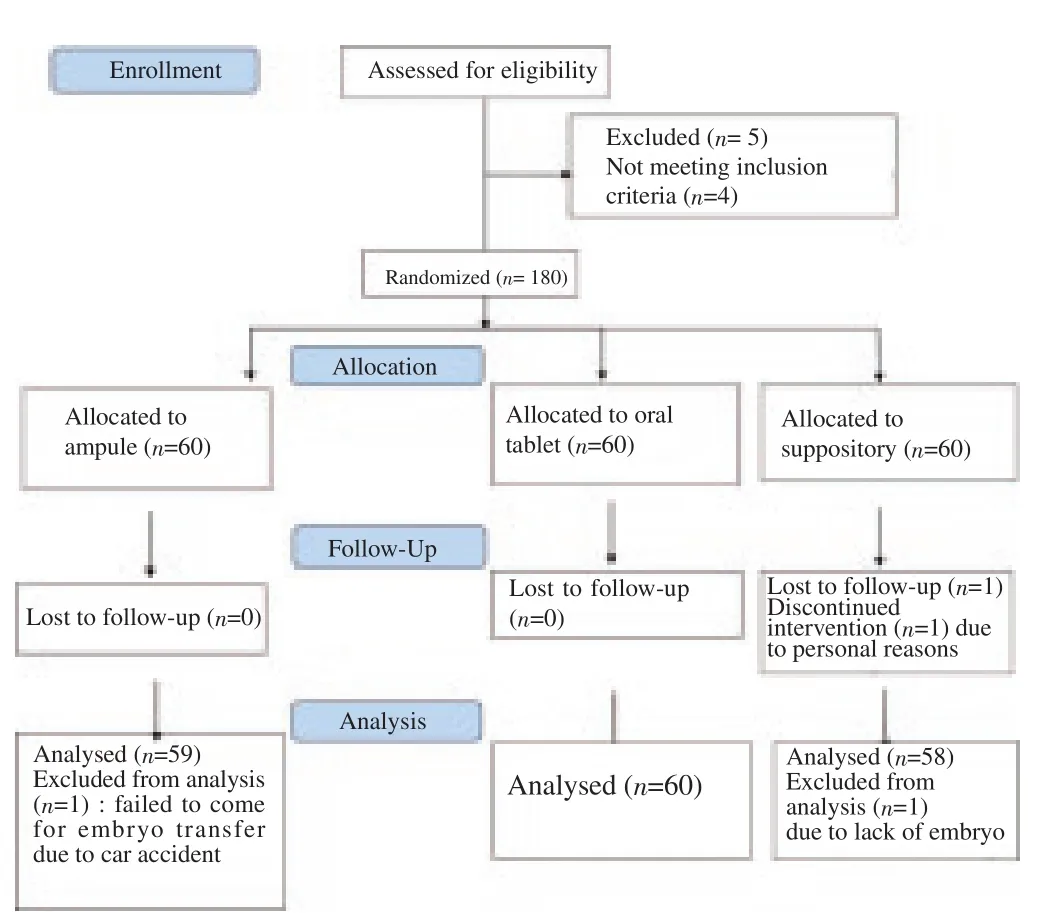
Diagram 1. Consort flow diagram.
4. Discussion
The preferred method of luteal phase support with progestogenic supplements is through IM injection in the United States and intravaginal suppository or gel in Europe[15,24,25]. Due to initial better pregnancy outcomes following IM progesterone, this method is still the desired route in many centers despite the lower patient compliance and comfort[2,24]. Soon, vaginal route which has some advantages over IM injection became popular especially when the success rates came out to be comparable or even better in women with endometriosis[24]. These advantages include lower local complications, more patient satisfaction and higher uterine concentrations of progesterone[6,16,24] although, side effects like vaginal irritation, discharge and interference with intercourse have made this method unfavorable and annoying too[6,9-12,15-18].Vaginal administration is associated with less systemic absorption compared to IM route and is, therefore, claimed to have less suppressive effects on the hypothalamus-pituitary-ovarian axis. This is why advocates of vaginal route consider it to be more physiologic which does not interfere with the endogenous corpus luteum function. However, respecting FET cycles where we have no such functional corpus luteum like fresh embryo transfer cycles, less circulating progesterone concentrations could be a drawback. In this line, oral compounds like dydrogesterone which has far less complications in comparison with the other two conventional products could be a reasonable substitute[18,20,21,26]. Dydrogesterone has good oral bioavailability and excellent patient compliance[23,28,30]. Furthermore, its effectiveness has been already confirmed[23-33]. Therefore, considering the fact that this oral agent is not yet regarded as a standard mode for luteal support, this study was carried out. Moreover, there is also lack of randomized controlled trials at least at the national level for evaluating its efficacy and tolerability in FET cycles. The novelty of our study was that dydrogesterone was compared with both IM and vaginal routes simultaneously in the format of a triple-armed RCT and for the FET cycles. According to our results, clinical pregnancy live birth rates were not significantly different in the dydrogesterone group compared to the vaginal route. These findings were consistent with that of Salehpour et al., Tomic et al. and Ganesh et al.[28,29,32]. The pregnancy and live birth rates were not statistically different from the intramuscular injection group either, which were in agreement with Guo et al., trial results[33]. All of the subjects in our study received both estradiol and progesterone in a sequential pattern as they all underwent artificial FET cycles in order to simulate the endogenous endocrine milieu of a natural cycle. Except for duration, the estradiol protocol was the same for all the patients which were in accordance with most other similar studies with only the endometrial thickness of 8 mm as the criteria for progesterone commencement[10]. We had no cancelled cycles. In other words, embryo transfer was done for every participant 3-5 d after progesterone administration based on embryo age and ultrasound findings. Thereby, this could be regarded as one limitation of our study as we did not assume the natural ovulation and luteinization that may occur in 5% of cycles[34]. The reason for delaying the embryo transfer until a couple of days after progesterone commencement was to decrease uterine contractility as a result of estrogen prescription[25]. According to Casper study, progesterone injection can better reduce the endometrial contractile activity compared to vaginal suppositories and was hence, the preferred progesterone supplement at least for the first couple of days following transfer. However, the final results which were clinical pregnancies were comparable for all the three groups in our study[10]. Of course, in that study the effects of oral type of progesterone on sub endometrial wave activity were not assessed. Concerning the fact that dydrogesterone can result in continuous andstable serum concentrations of progesterone just like IM injection, it could be an appropriate surrogate for progesterone ampules which are not much user-friendly.
With regards to patient satisfaction and compliance, according to Chacravartky et al., trial more patients in the dydrogesterone group were satisfied in comparison with intravaginal micronized progesterone. Whereas based on Saharkhiz et al., study this was not the case and patients’ satisfaction was similar for both oral and vaginal methods[26,31]. The main goal of our study was to evaluate the pregnancy outcomes of dydrogesterone and not the sideeffects; regardless of that, what at least can be concluded is that dydrogesterone, if not better, is not worse than vaginal or IM route in terms of both clinical efficacy and patient compliance.
This study had some other limitations too. Given that it was a pilot study, the sample size for each arm was not big enough to get us to an acceptable power.
Regarding the fact that oral dydrogesterone is more accepted by patients in terms of ease of use, lower cost and satisfaction, it seems that it could be used for luteal phase support in FET cycles as effective as either the intramuscular or the vaginal route of progesterone administration.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgment
The authors would like to thank the staff of Vali-e-Asr Reproductive Health Research Center of Tehran University of Medical Sciences for their commitment to the execution of the project.
[1] Jones GES. Some newer aspects of management of infertility. JAMA 1949; 141: 1123-1129.
[2] Pritts EA, Atwood AK. Luteal phase support in infertility treatment: A metaanalysis ofthe randomized trials. Hum Reprod 2002; 17: 2287-2299.
[3] Csapo AI, Pulkkinen MO, Ruttner B, Sauvage JP, Wiest WG. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am J Obstet Gynecol 1972; 112: 1061-1067.
[4] Ulbaldi F, Bourgain C, Tournaye H, Smitz J, Van Steirteghem A, Devoey P. Endometrial evaluation by aspiration biopsy on the day of oocyte retrieval in the embryo transfer cycles in patients with serum progesterone rise during the follicular phase. Fertil Steril 1997; 67: 521-526.
[5] Kolibianakis EM, Bourgain C, Platteau P, Albano C, Van Steirteghem AC, Devroey P. Abnormal endometrial development occurs during the luteal phase of non-supplemented donor cycles treated with recombinant follicle stimulating hormone and gonadotropin releasing hormone antagonists. Fertil Steril 2003; 80: 464-466.
[6] Penzias AS. Luteal phase support. Fertil Steril 2002; 77(2): 318-323.
[7] Daya S, Gunby J. Luteal phase support in assisted reproduction cycles.Cochrane Database Syst Rev 2004; 3(3): CD004830.
[8] Nosarka S, Kruger T, Siebert I, Grové D. Luteal phase support in in vitro fertilization: Meta-analysis of randomized trials. Gynecol Obstet Invest 2005; 60(2): 67-74.
[9] Ghobara T, Vandekerckhove P. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev 2008 ; 1: CD003414.
[10] Groenewoud ER, Cantineau AE, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update 2013 ; 19: 458-470.
[11] Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P. An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update 2007; 13(6): 581-590.
[12] Shapiro D, Boostanfar R, Silverberg K, Yanushpolsky EH. Examining the evidence: Progesterone supplementation during fresh and frozen embryo transfer. Reprod Biomed Online 2014; 29 Suppl 1:S1-14; quiz S15-16.
[13] Leonard PH, Hokenstad AN, Khan Z, Jensen JR, Stewart EA, Coddington CC. Progesterone support for frozen embryo transfer: Intramuscular versus vaginal suppository demonstrates no difference in a cohort. J Reprod Med 2015; 60(3-4): 103-108.
[14] Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Rombauts L, Devroey P. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update 2006 ; 12(4): 333-340.
[15] van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phasesupport for assisted reproduction cycles. Cochrane Database Syst Rev 2011; 5(10): CD009154.
[16] Tavaniotou A, Allbano C, Smitz J, Devroey P. Impact of ovarian stimulation on corpus luteum function and embryonic implantation. J Reprod Immunol 2002; 55: 123-130.
[17] Yanushpolsky EH. Luteal phase support in in vitro fertilization. Semin Reprod Med 2015; 33(2): 118-127.
[18] Vaisbuch E, Leong M, Shoham Z. Progesterone support in IVF: Is evidencebased medicine translated to clinical practice? A worldwide web-based survey. Reprod Biomed Online 2012; 25(2): 139-145.
[19] Palomba S, Santagni S, La Sala GB. Progesterone administration for luteal phase deficiency in human reproduction: an old or new issue? J Ovarian Res 2015; 8: 77.
[20] Tavaniotou A, Smitz J, Bourgain C, Devroey P. Comparison between different routes of progesterone administration as luteal phase support in infertility treatments. Hum Reprod Update 2000; 6: 139-148.
[21] Zarutskie PW, Phillips JA. A meta-analysis of the route of administration of luteal phase support in assisted reproductive technology: Vaginal versus intramuscular progesterone. Fertil Steril 2009; 92(1): 163-169.
[22] Iwase A, Ando H, Toda S, Ishimatsu S, Harata T, Kurotsuchi S, et al. Oral progestogen versus intramuscular progesterone for luteal support after assisted reproductive technology treatment: A prospective randomized study. Arch Gynecol Obstet 2008; 277(4): 319-324.
[23] Kalinka J, Szekeres-Bartho J. The impact of dydrogesterone supplementation on hormonal profile and progesterone-induced blocking factor concentrations in women with threatened abortion. Am J Reprod Immunol 2005; 53(4): 166-171.
[24] Mitwally MF, Diamond MD, Abuzeid M. Vaginal micronized progesterone versus intramuscular progesterone for luteal support in women undergoing in vitro fertilization-embryo transfer. Fertil Steril 2010; 93: 554-569.
[25] Casper RF. Luteal phase support for frozen embryo transfer cycles: Intramuscular or vaginal progesterone? Fertil Steril 2014; 101(3): 627-628.
[26] Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: Results of a randomized study. J Steroid Biochem Mol Biol 2005; 97(5): 416-420.
[27] Patki A, Pawar VC. Modulating fertility outcome in assisted reproductive technologies by the use of dydrogesterone. Gynecol Endocrinol 2007; 23 Suppl (1): 68-72.
[28] Ganesh A, Chakravorty N, Mukherjee R, Goswami S, Chaudhury K, Chakravarty B. Comparison of oral dydrogesterone with progesterone gel and micronized progesterone for luteal support in 1 373 women undergoing in vitro fertilization: A randomized clinical study. Fertil Steril 2011; 95(6): 1961-1965.
[29] Salehpour S, Tamimi M, Saharkhiz N. Comparison of oral dydrogesterone with suppository vaginal progesterone for luteal-phase support in in vitro fertilization (IVF): A randomized clinical trial. Iran J Reprod Med 2013; 11(11): 913-918.
[30] Barbosa MW, Silva LR, Navarro PA, Ferriani RA, Nastri CO, Martins WP. Dydrogesterone versus progesterone for luteal-phase support: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol 2015; 18.
[31] Saharkhiz N, Zamaniyan M, Salehpour S, Zadehmodarres S, Hoseini S, Cheraghi L, et al. A comparative study of dydrogesterone and micronized progesterone for luteal phase support during in vitro fertilization (IVF) cycles. Gynecol Endocrinol 2016; 32(3): 213-217.
[32] Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: Randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2015; 186: 49-53.
[33] Guo W, Chen X, Ye D, He Y, Li P, Niu J, et al. Effects of oral dydrogesterone on clinical outcomes of frozen-thawed embryo transfer cycles. Nan Fang Yi Ke Da Xue Xue Bao 2013; 33(6): 861-865.
[34] El-Toukhy T, Taylor A, Khalaf Y, Al-Darazi K, Rowell P, Seed P, et al. Pituitary suppression in ultrasound-monitored frozen embryo replacement cycles. A randomised study. Hum Reprod 2004; 19(4): 874-879.
ment heading
10.1016/j.apjr.2016.10.002
*Corresponding author: Mansoureh Gorginzadeh, Tehran University of Medical Sciences, Vali-e-Asr Reproductive Health Research Center, Keshavarz Boulevard, Tehran, Iran.
Tel: +98-9122897058, +98-21-61192449.
E-mail: m_g802002@yahoo.com
Luteal phase support
Dydrogesterone
Vaginal progesterone suppository Intramuscular progesterone
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
- Nicotine effect toward the oocyte level of rats(Rattus novergicus)
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
- Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
- The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
