Studies on the protamine 1 gene in the sperm DNA of male albino rats treated with local gin using BseR I endonuclease
2017-01-06MinariJBSalauOY
Minari JB, Salau OY
1Department of Cell Biology and Genetics, University of Lagos
Studies on the protamine 1 gene in the sperm DNA of male albino rats treated with local gin using BseR I endonuclease
Minari JB1*, Salau OY1
1Department of Cell Biology and Genetics, University of Lagos
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Male infertility
Objective:To investigate the impact of local brewed gin (ogogoro) on the integrity of sperm DNA through the evaluation of the protamine 1 (PRM1) gene.Methods:Twenty four 9-week-old male albino rats were divided into five groups; the first group which served as the control were fed with normal fish feed and water ad libitum while the rest were fed with different volumes of the local gin (1, 2, 3 and 4 mL respectively) in conjunction with the normal fish feed and water for 24 d. The caudal epididymis was macerated in saline water and the sperm solution collected in an Eppendorf tube. The extraction of DNA was done using Jena Bioscience DNA preparation kit and the protocol was based on the spin column based genomic DNA purification from blood, animal and plant cells. The primer was designed to detect mutation in Protamine1 gene associated with male infertility. BseR I, a restriction endonuclease was used to digest the amplicon at 37 ℃ for 10 min and then inactivated at 80 ℃ for 20 min. The digest was separated on 2% agarose gel.Results:There was variation in the thickness of bands and the thinnest band was observed in the group that consumed the highest volume of local gin. There was also a close similarity between the amplicons from the group fed with 1mL of ogogoro and the control group that were given distilled water. There was no visible digestion by BseR I. There was a significant increase in the body weight across the entire experimental group and the highest weight was recorded in the 4 mL group. Multiple tumors were also seen in the livers of all the experimental groups. Conclusion: This study has shown that local gin can affect the sperm DNA by making it susceptible to damage because of the reduced viability of the PRM1 gene whose product i.e., PRM1 protein is needed for the proper compaction of the sperm DNA.
1. Introduction
Local gin is a clear, colorless, mobile, volatile, alcoholic drink that is brewed locally in western Africa. It is commonly called“Sapele water”, “Ogogoro”, “Paraga”, “Kaikai”, “Etonto”, “wuru”,“robirobi” in Nigeria though other countries have their own home brew such as “umkomboti” in South Africa, Ghana’s “Nsafufuo” or“muratina” and “chang’aa” in Kenya.
In Africa, alcoholic beverages were consumed for pleasure after brewing or tapping[1] and they were rarely traded in the market. This drink is of historical significance in Ghana and Nigeria because, as a local gin, colonial administrators barred it in an attempt to control the West African liquor trade in the early part of the last century[2]. Nigeria is one of the world’s leading consumers of alcoholic beverages with an average of about 15 L of pure alcohol per capita per annum[3]. It is an essential part of numerous religious and social ceremonies thus it carries a substantial cultural and economic significance in Nigeria. It is also used during traditional ceremonies such as pouring libation, weddings and funerals.
Little attention is placed on male infertility in developing countries because of the widely erroneous belief that infertility is a female problem[4]. The male contribution to infertility among couples worldwide has been estimated to be about 33% and in Nigeria, the male partners’ contribution to sub fertility is estimated to be about54% based on semen analysis alone[5]. Moreover, approximately 15% of patients with male factor infertility have a normal semen analysis[6] whereas in 8% of men with normal sperm parameters, different forms of sperm DNA damage are found[7].
Testicular germ cells are highly susceptible to damage from a number of toxic chemicals in comparison to somatic cells because they have higher amount of polyunsaturated fatty acids that are vulnerable to oxidation by free radicals arising from phagocytic Sertoli cells. These reproductive toxins are encountered in many ways; industrially and environmentally, therapeutically and selfadministered as recreational drugs[8]. Studies have reported increased levels of gonadotropins and a decreased level of testosterone in infertile males when compared with the fertile ones[9]. Many studies have been undertaken to determine the temporal relationship between alcohol and fertility. In human, high alcohol consumption is associated with serious disorders of spermatogenesis. Among chronic alcoholics, there is impairment of spermatogenesis and reductions in sperm counts and testosterone levels[10].
Spermatogenesis is a tightly regulated and complex biological process of cellular differentiation that results in the production of haploid male germ cells. During spermatogenesis, a complex and dynamic process of proliferation and differentiation occur as spermatogonia are transformed into mature spermatozoa. This unique process involves a series of meioses and mitoses, changes in cytoplasmic architecture, replacement of somatic cell-like histones with transition proteins, and the final addition of protamines, leading to a highly packaged chromatin.
The presence of DNA damage in the male germ line has been linked with a variety of adverse outcomes such as low fertilization rates, decrease in embryo implantation, miscarriage, cancer and other diseases in the offspring[11]. It is also known that some paternal genes are crucial for early embryo development. Most genes in spermatozoa are hypermethylated and those regions hypomethylated are usually related to development regulators, biosynthetic or metabolism loci[12]. The organization of sperm nuclear DNA takes place in the haploid stage of spermatogenesis called spermiogenesis. In the testicular phase, his tones are replaced at first by lysinerich transition proteins (TPs) and then by protamines. Two theories have been proposed to explain the phenomenon of why ejaculated human spermatozoa possess anomalies in their nuclear DNA. The first theory arises from studies performed in animal models and is linked to the unique manner in which mammalian sperm chromatin is packaged. Endogenous nicks in DNA have been shown to be normally present at specific stages of spermiogenesis in rats and mice[13].
The second theory of why DNA damage is present in ejaculated human spermatozoa arises from the use of the TUNEL assay as a marker of apoptosis. A number of studies have therefore stated that the presence of TUNEL-positive sperm indicates that these sperm are indeed apoptotic[14].
This study was designed to investigate the implication of locally brewed drink (ogogoro) on the sperm nuclear DNA integrity analyzed through molecular technique of polymerase chain reaction (PCR). This is due to the fact that some tests such as semen analysis, sperm chromatin dispersion (SCD) test, The terminal deoxynucleotidyl transferase-mediated (TdT) deoxyuridine triphosphate (dUTP) nick end labeling assay (TUNEL) are limited to sperm counts and fragmentation analysis which may not be informative enough about the integrity of the sperm DNA as researchers have discovered that even in the absence of fragmentation, some damage may be detected in some genomic regions[15].
In the human, it has been known for many years that the chromatin of the mature sperm nucleus can be abnormally packaged. In addition, abnormal chromatin packaging and nuclear DNA damage appear to be linked[16]. There is also a strong association between the presence of nuclear DNA damage in the mature spermatozoa of men and poor semen parameters[17].
The aim of the research was to investigate the impact of local brewed gin (ogogoro) on the quality and integrity of sperm DNA.
2. Materials and methods
2.1. Animal and experimental design
Twenty-four 9-week-old albino rats with an average weight of 166.6 g were kept in clean cages and acclimatized for 1 week. Animals were randomly divided into five groups; the first group which served as the control were fed with normal fish feed and water ad libitum while the rest were fed with different volumes of the local gin (1, 2, 3 and 4 mL respectively) in conjunction with the normal fish feed and water for 18 d. Experiment was carried out in the animal house of the University of Lagos, Lagos, Nigeria in accordance with the rules in Nigeria governing the use of laboratory animals as acceptable internationally[18]. During the induction period, eight rats died. The feeding was halted for 2 weeks and the animals were finally fed for 6 d with the gin and sacrificed.
2.2. Epididymal sperm preparation
The caudal epididymis was macerated in saline water and the sperm solution collected in an Eppendorf tube. The molecular analysis was carried out at the molecular laboratory of FowM biotechnology company, Jibowu, Lagos state.
2.3. Sperm DNA extraction
The extraction of DNA was done using Jena Bioscience DNA preparation kit and the protocol was based on the spin column based genomic DNA purification from blood, animal and plant cells. Prior to the extraction, 500 µL dd-water was added to the proteinase Ktube, 150 µL of dd-water to RNaseA tube and 48 mL 96%-99% ethanol to the washing buffer bottle.
The sperm solution was centrifuged at 10 000 r/min for 1 min and the supernatant was discarded. 300 µL lysis buffer was added and 2 µL RNase A to the cell pellet which was then vortexed for 30 s. 8 µLproteinase K was added and incubated for 10 min at 60 ℃. It was then cooled for 5 min. 300 µL binding buffer was added and vortexed after which the tube was placed on ice for 5 min and then centrifuged for 5 min at 10 000 r/min.
Spin column was placed into a 2 mL collection tube and 100 µL of activation buffer was added into the spin column. This was centrifuged at 10 000 r/min for 30 s and the flow-through was discarded. Supernatant was transferred to this spin column and centrifuged for 1 min at 10 000 r/min. Flow-through was discarded. For the primary washing, 500 µL of washing buffer was added into the spin column and centrifuged for 30 s at 10 000 r/min with the flow-through discarded. Secondary washing was also done in which after the flow-through was discarded, it was centrifuged again at 10 000 r/min for 1min to remove residual washing buffer. The 2 mL wash tube was discarded and the column was placed into the elution tube.
The elution was done by adding 40-50 µL of elution buffer into the centre of the column and incubated at room temperature for 1min. It was then centrifuged at 10 000 r/min for 2 min and the DNA was stored at -20 ℃.
2.4. Primer
The primer sequences were obtained from the work of Ravel et al. [19]. The primer was designed to detect mutation in protamine1 gene associated with male infertility. The sequences of the forward and reverse primers are 5’-CCA CGG AGG AGT CAT CTT GT-3’ and 5’-ATT TAT TGA CAG GCG GCA TT-3’, respectively.
2.5. Polymerase chain reaction (PCR)
The amplification was performed using Peltier Thermal Cycler PTC-100 from MJ Research in a total reaction volume of 50 µL containing 10 µL master mix, 34.6 µL dd-water, 0.2 µL each of forward and reverse primer and 5 µL of DNA. Amplication of the PRM1F/PRM1R amplicon was performed by an initial denaturation step at 95 ℃ for 5 min followed by 35 cycles of 95 ℃ for 30 s, 60 ℃ for 30 s, 72 ℃ for 30 s followed by an elongation step of 72 ℃ for 5 min.
2.6. Electrophoretic analysis
About 1.5 g of agarose was weighed into 100 mL TBE buffer to prepare 1.5% agarose. It was then autoclaved for 3 min. After the solution had cooled, 20 µL of SYBR safe dye was added and then poured into the electrophoretic chamber after which it was allowed to solidify. DNA ladder was loaded in the first well to monitor the sizes of the fragments. The analysis was done at 100 V for 90 min.
2.7. Restriction enzyme digestion
BseRI, a restriction endonuclease from an E. coli strain that carries the BseR I gene from Bacillus species was used to digest the amplicon at 37 ℃ for 10 min and then inactivated at 80 ℃ for 20 min. The digest was separated on 2% agarose gel.
2.8. Statistical analysis
Comparison of weight of the rats with volume of local gin given weekly was done using Pearson correlation by SPSS for windows version 21 software. The level of significance was P<0.05.
3. Results
Figure 1 shows the average weights of the rats in different groups for the 8 weeks. It can be seen from this figure that the weights of the rats increased steadily throughout the experiment. There was no significant decrease in the body weights of local gin treated rats when compared with the control. Group C (administered 4 mL of local gin) had the highest weight while the control had the lowest.
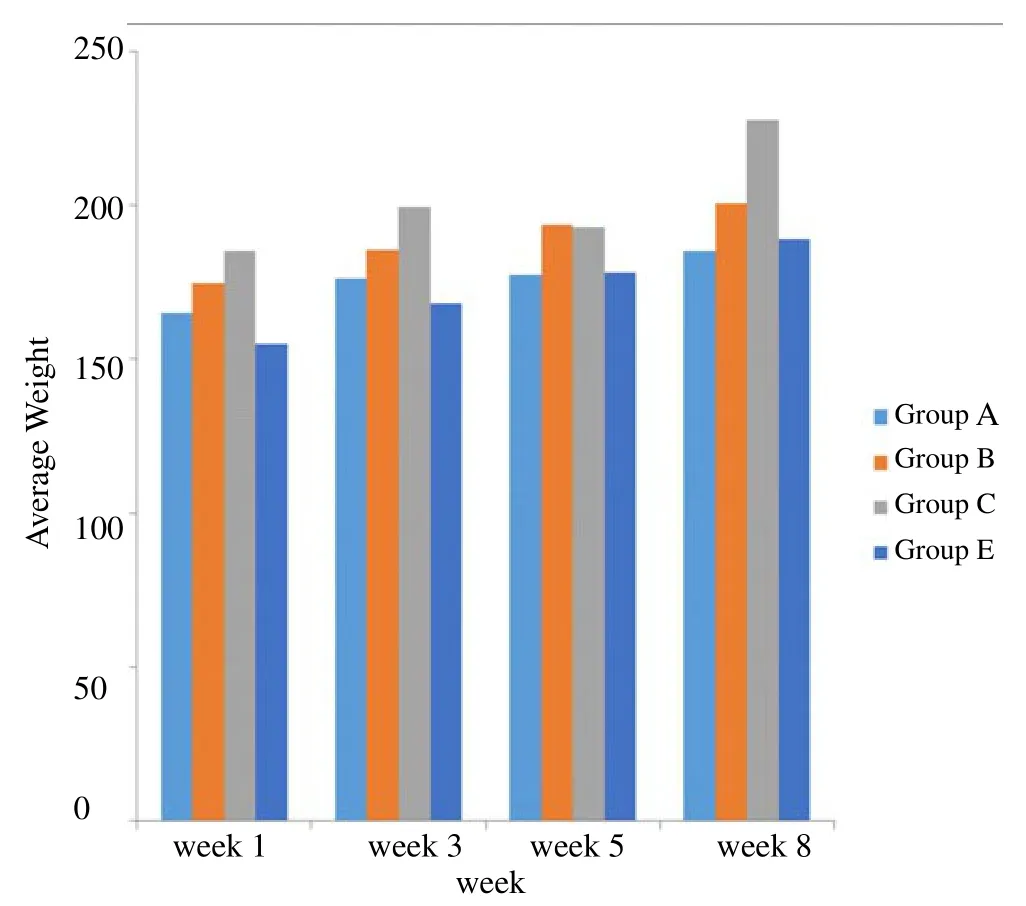
Figure 1. Average weight trend of male albino rats measured four times during the 8 weeks.
The number of deaths observed during the course of the experimentis shown in Figure 2. No death was recorded in the first 2 weeks including the first week of induction. Death occurrence was recorded in the 5th week when there was no induction. Highest death occurred in group C which was fed with 4mL of local gin (highest volume) with the loss of three animals in the total 8 weeks.
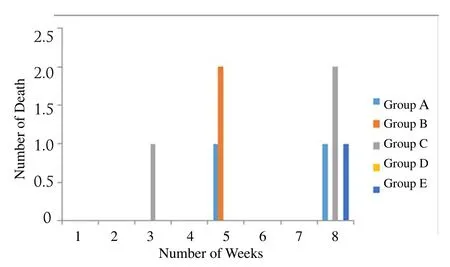
Figure 2.Number of deaths recorded for the total 8 weeks.
Figure 3 is a rat with liver tumor. The tumors were seen in all the groups fed with different volumes (1, 2, 3 and 4mL) of local gin although of varying degrees but was absent in the control. These tumors could either be malignant or benign.
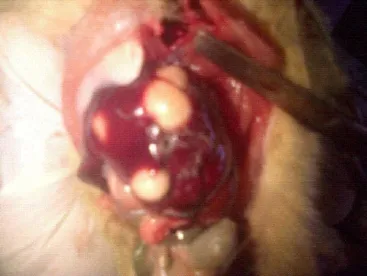
Figure 3. Liver tumors in a rat.
The amplicons were electrophoretically separated using 2% agarose gel at 60 ℃. At this concentration of agarose and annealing temperature, no band was seen. The temperature was too high for the denatured template single stranded DNA to bind to the primers (Data not shown).
Figure 4 is the electrophoretic pattern of the amplicon using 1.5% agarose gel and at 54 ℃. Lane 1 contains the DNA ladder to monitor the movement and sizes of the bands. There was null amplification in the negative (Lane 2 because it does not contain the analyzed sample) while the other groups gave different bands though faint in some as can be seen in lane 4 and 5. The sizes of the bands were 300, 1 000 and 1 400 bp. There was a close similarity in the thickness of band found on lanes 13 and 14. Lane 13 is the amplicon of rat given 1mL of local gin while lane 14 is the control group.
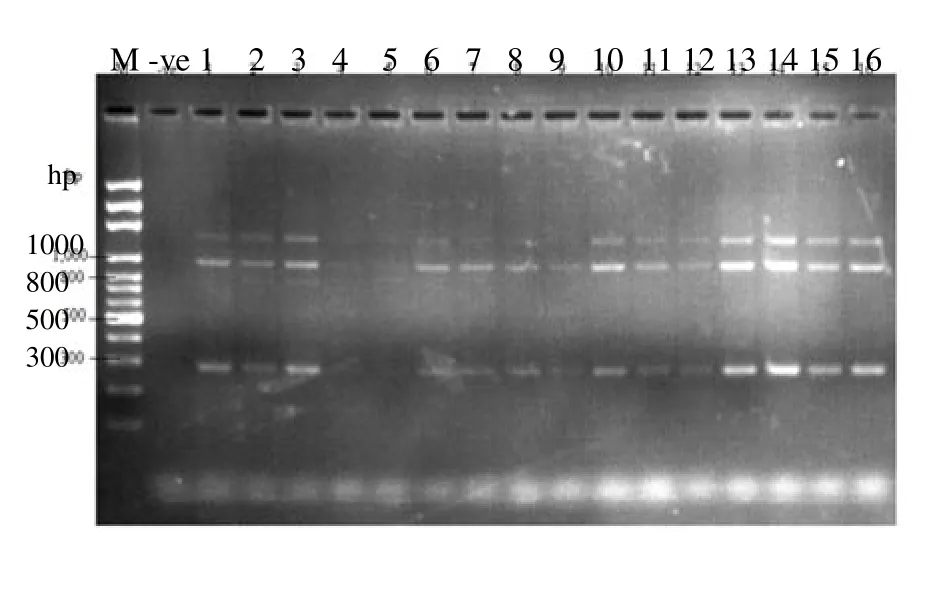
Figure 4. Bands on 1.5% agarose gel and annealing temperature of 54 C.
The PCR products were separated after digesting with BseR I restriction endonuclease. BseRI recognizes a six base pair nonpalindromic sequence 5’-GAGGAG-3’ and cleaves double stranded DNA ten nucleotides beyond this sequence on the top strand and eight nucleotides beyond the sequence on the complementary strand to produce a two nucleotide 3’ extension. There was no visible digestion of the amplicon which may be as a result of the insufficient time used for the incubation of the PCR product with the restriction enzyme (Data not shown).
4. Discussion
Alcohol abuse has been shown to result in the production of spermatozoa with less condensed chromatin, and this may be one possible cause of infertility following ethanol consumption[20]. From human studies, it is known that alcohol consumption produces significant morphological changes in the spermatozoa, which include breakage of the sperm head, distention of the midsection, and tail curling[21]. In addition, the seminiferous tubules in alcohol users mostly contain degenerated spermatids, with a consequent azoospermia. Sperm DNA damage is more common in infertile males and a significant number of patients diagnosed withunexplained (normozoospermic) infertility according to traditional diagnostic methods had remarkably high degrees of fragmented sperm DNA[22].
From this study, there was a significant increase in the body weights of the local gin treated rats. This trend was also observed in the control group. This suggests that alcohol contributed to the weights of the rats because the highest weight was recorded in group C that was administered 4 mL of local gin. Alcohol has been known to contribute to body weight by causing unhealthy eating habits. It also accounts for nearly 10% of the calorie intake amongst adults who drink because it has an energy value of 7 kcal/g, second only to fat which is the most energy dense macronutrient at 9 kcal/g[23]. Thus, the consumption of alcohol can contribute to obesity.
The toxic effect of local gin was evident in the incidence of mortality. The highest loss occurred in the group that received the highest volume of the local gin. Also, multiple tumors that were seen in the liver of all the local gin treated rats reveal the level of damage done to the liver. The liver is the organ for the metabolism of alcohol and these tumors can either be benign or malignant. Local gin (ogogoro) contains many impurities that are toxic and carcinogenic
[24].
The primer used was able to amplify the PRM1 gene which is essential to make the PRM1 protein needed for the compaction of sperm DNA. There were three distinct bands of different sizes. The control group had thick bands (14, 15 and 16) unlike the rest and there was variation in the pattern. As the volume of administered local gin increases, the thickness of the band decreases. Group C fed with 4mL of local gin had the thinnest band (Lanes 1 and 2). From this observation, it can be suggested that local gin probably may induce copy number variation in the chromosome. The three bands may suggest some form of heterozygosity. Some variants were observed by Ravel et al.[9] at some position by direct sequencing of DNA samples. 107G>C change was also observed in the heterozygote state in four men, each of whom had unexplained severe oligozoospermia. Aoki et al.[25] reported a rare silent polymorphic variant. He also reported that abnormal protein synthesis is associated with aberrant mRNA retention suggesting that defects in Protamine translational regulation may contribute to Protamine deficiency in infertile men. Iguchi et al.[26] also observed that an individual who carried a mutation in PRM1 gene was oligozoospermic and exhibited a high level of DNA fragmentation in his sperm measured using the TUNEL assay. This suggests that infertile men may have amino acid variants in the PRM1 gene that may affect spermatogenesis. Rare variants in this gene may potentially have a significant effect on male infertility by altering gene expression or modifying RNA transcripts during spermatogenesis.
From this study, it can be inferred that local gin can induce a considerable damage to the sperm DNA thus capable of reducing male reproductive capacity hence the consumption of alcohol should be minimized in man. Also, those who regularly consume alcohol or who have decreased sperm progressive motility should be subjected to sperm DNA integrity tests.
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] Odejide AO. Status of drug use/abuse in Africa: A review. Int J Mental Health Addiction 2006; 4(2): 87-102.
[2] Isidore SO. Drug and Alcohol Consumption by out of school Nigerian Adolescents. Afr JDrug Alcoh Stud 2001; 1(2): 99.
[3] World Health Organization. Global Status Report on Alcohol. World Health Organization, Department of Mental Health and Substance Abuse, Geneva; 2004.
[4] Arowojolu AO, Akinloye O, Shittu OB, Adejuwon CA. Correlations between seminal plasma hormones and sperm biophysical parameters ininfertile males in Ibadan. Tropical J Obstet Gynaecol 2003; 20(1): 7-11.
[5] Irvine DS. Epidemology and etiology of male infertility. Hum Reprod 1998; 13(1): 33-44.
[6] Agarwal A, Allamaneni SS. Sperm DNA damage assessment: A test whose time has come. Fertil Steril 2005; 84(4): 850-853.
[7] Zini A, Fischer MA, Sharir S, Shayegan B, Phang D, Jarvi K. Prevalence of abnormal sperm DNA denaturation in fertile and infertile men. Urology 2002; 60(6): 1069-1072.
[8] Dare WN, Noronha CC, Kusemiju OT, Okanlawon OA. The effect of Ethanol on spermatogenesis and fertility in male Sprague-Dawley Rats pretreated with acetylsalicylic acid. Niger Postgrad Med J 2002; 9(4): 194-198.
[9] Kuku SF, Akinyanju PA, Ojeifo JO. Serum levels of gonadotropins, prolactin and testosterone in oligo/azoospermic Nigerian males. Int J Fertil 1988; 33(1): 40-44.
[10] Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril 2005; 84(4): 919-924.
[11] Suenaga E, Nakamura H. Evaluation of three methods for effective extraction of DNA from human hair. J Chromatogr B Analyt Technol Biomed Life Sci 2005; 820(1): 137-141.
[12] Wu SF, Zhang H, Cairns BR. Genes for embryo development are packaged in blocks of multivalent chromatin in zebrafish sperm. Genome Res 2011; 21(4): 578-589
[13] Sakkas D, Manicardi G, Bianchi PG, Bizzaro D, Bianchi U. Relationship between the presence of endogenous nicks and sperm chromatin packaging in maturing and fertilizing mouse spermatozoa. Biol Reprod 1995; 52(5): 1149-1155.
[14] Tesarik J, Greco E, Cohen-Bacrie P, Mendoza C. Germ cell apoptosis in men with complete and incomplete spermiogenesis failure. Mol Hum Reprod 1998; 4(8): 757-762.
[15] Riesco MF, Robles V. Quantification of DNA damage by q-PCR in cryopreserved zebrafish Primordial Germ Cells. J Appl Ichthyol 2012; 28(6): 925-929.
[16] Manicardi GC, Bianchi PG, Pantano S, Azzoni P, Bizzaro D, Bianchi U, et al. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod 1995; 52(4): 864-867.
[17] Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl 2000; 21(1): 33-44.
[18] World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. 59th WMA General Assembly, Seoul. World Medical Association Inc; 2008.
[19] Ravel C, Chantot-Bastaraud S, El Houate B, Berthaut I, Verstraete L, De Larouziere V, et al. Mutations in the protamine 1 gene associated with male infertility. Mol Hum Reprod 2007; 13(7): 461-464.
[20] Talebi AR, Sarcheshmeh AA, Khalili MA, Tabibnejad N. Effects of ethanol consumption on chromatin condensation and DNA integrity of epididymal spermatozoa in rat. Alcohol 2011; 45(4): 403-409.
[21] La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero AE. Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl 2013; 15(2): 221-225.
[22] Oleszczuk K, Augustinsson L, Bayat N, Giwercman A, Bungum M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013; 1(3): 357-360.
[23] Dennis EA, Flack KD, Davy BM. Beverage consumption and adult weight management: A review. Eating Behaviors 2009; 10(4): 237-246.
[24] Idonije OB, Festus OO, Asika EC, Ilegbusi MI, Okhiai O. Article ID sjmct-179. Sci J Med Clin Trials 2012; 2012: 4.
[25] Aoki VW, Liu L, Carrell DT. A novel mechanism of protamine expression deregulation highlighted by abnormal protamine transcript retention in infertile human males with sperm protamine deficiency. Mol Hum Reprod 2006; 12(1): 41-50.
[26] Iguchi N, Yang S, Lamb DJ, Hecht NB. An SNP in protamine 1: A possible genetic cause of male infertility. J Med Genet 2006; 43(4): 382 -384.
ment heading
10.1016/j.apjr.2016.10.013
*Corresponding author: Joseph Bamidele Minari (Ph.D), Department of Cell Biology and Genetics (Molecular Biology Research Group Laboratory), University of Lagos, Akoka, Lagos, Nigeria.
Mobile: (+234) 8032488513
E-mail: baminjoe@yahoo.co.uk
Protamine1
Local gin (ogogoro)
BseR I endonuclease
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
- Nicotine effect toward the oocyte level of rats(Rattus novergicus)
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
- Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
- The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
