SCUBE2过表达通过Wnt/β-catenin抑制结直肠癌细胞上皮-间质转化
2017-01-04张龙江席作武
王 丽, 张 宁, 张龙江, 席作武
(河南省中医院肛肠科,河南 郑州 450002)
SCUBE2过表达通过Wnt/β-catenin抑制结直肠癌细胞上皮-间质转化
王 丽, 张 宁△, 张龙江, 席作武
(河南省中医院肛肠科,河南 郑州 450002)
目的: 研究信号肽-CUB-EGF结构域蛋白2(SCUBE2)对结直肠癌细胞上皮-间质转化(EMT)的影响及可能的分子机制。方法:首先,用real-time PCR与Western blot法检测人结直肠癌HCT116细胞和正常人结直肠上皮FHC细胞SCUBE2的表达;HCT116细胞转染GV144-SCUBE2质粒过表达SCUBE2后,MTT、Transwell和流式细胞术分别检测其对细胞活力、迁移和凋亡的影响。转染GV144-SCUBE2质粒6 h后加入10 μg/L重组转化生长因子(TGF)-β1蛋白继续培养48 h,real-time PCR或者Western blot法检测EMT标志物[E-钙黏蛋白(E-cadhe-rin)、波形蛋白(vimentin)和Snail]、β-catenin、c-Myc和细胞周期蛋白(cyclin)D1的表达。随后,在HCT116细胞中使用Wnt/β-catenin通路激活剂氯化锂(LiCl)或抑制剂XAV93920并加入TGF-β1且过表达SCUBE2,检测Wnt/β-catenin通路激活或阻断后对HCT116细胞EMT的影响。结果:HCT116细胞中SCUBE2的表达明显低于FHC细胞;过表达SCUBE2能够抑制HCT116细胞的活力与迁移,但是对其凋亡影响不大;SCUBE2增加了E-cadherin的表达,降低了TGF-β1诱导的vimentin、Snail、β-catenin、c-Myc和cyclin D1的表达,XAV93920增强了SCUBE2对EMT的影响,而LiCl阻碍SCUBE2对EMT的影响。结论:过表达SCUBE2能够抑制结直肠癌细胞的生长与迁移,抑制其EMT的发生,这一过程可能是通过抑制Wnt/β-catenin信号通路发挥作用的。
SCUBE2; 结直肠癌; 上皮-间质转化; Wnt/β-catenin信号通路
结直肠癌是全球最常见的恶性肿瘤之一,在男性中居于第2位,在女性中居于第3位[1]。在结直肠癌中,上皮-间质转化(epithelial-to-mesenchymal transition, EMT)会导致基底膜缺失,且与不良预后有关[2]。信号肽-CUB-EGF结构域蛋白2 [signal peptide-CUB (complement C1r/C1s, Uegf and Bmp1)-EGF (epidermal growth factor) domain-containing protein 2, SCUBE2]是SCUBE家族中一个分泌型且与膜相关的多结构域糖蛋白[3]。近年来的研究显示,SCUBE2在乳腺癌中是一个新的肿瘤抑制剂,它能够通过逆转EMT来抑制乳腺癌细胞的迁移和侵袭[4],且它也是乳腺癌的一个重要预后标志物[5]。最新的研究显示SCUBE2在结直肠癌组织中表达降低,且与临床表现密切相关[6],但是SCUBE2对结直肠癌细胞EMT的作用及机制还不清楚。本研究通过在结直肠癌细胞中过表达SCUBE2,探究其对肿瘤细胞生长、迁移和凋亡的影响,并分析SCUBE2对转化生长因子(transforming growth factor, TGF)-β1诱导的EMT的影响及其作用机制。
材 料 和 方 法
1 材料
人结肠癌细胞HCT116及正常人结直肠上皮FHC细胞来源于ATCC;DMEM培养基、胎牛血清、青霉素/链霉素双抗溶液和胰酶均购自Gibco;NanoFectin转染试剂购自上海依科赛生物制品有限公司;氯化锂(lithium chloride,LiCl)购自Sigma;MTT及细胞毒性试剂盒购自碧云天生物技术公司;Annexin V-FITC/PI细胞凋亡试剂盒购自BD;TRIzol购自Invitrogen; SuperQuickRT cDNA第一链合成试剂盒与UltraSYBR Mixture均购自北京康为世纪公司;重组人TGF-β1蛋白、SCUBE2抗体、E-钙黏蛋白(E-cadherin)抗体、波形蛋白(vimentin)抗体、Snail抗体和β-catenin抗体均购自Abcam;细胞周期蛋白(cyclin)D1 和c-Myc 购自Cell Signaling Technology;XAV93920购自Selleck Chemicals;BCA法蛋白定量试剂盒购自上海捷瑞生物工程有限公司。
2 方法
2.1 细胞培养和转染 HCT116和FHC细胞均培养在含有10%胎牛血清、1×105U/L青霉素和100 mg/L链霉素的DMEM完全培养基中,置于37 ℃、5% CO2培养箱中常规培养。将对数期的HCT116细胞胰酶消化传代,接种到特定的细胞培养板中。待细胞融合度达到60%时,按照转染试剂说明书操作,分别转染GV144和GV144-SCUBE2质粒。转染6 h后加入10 μg/L TGF-β1继续培养48 h进行后续检测,或者加入TGF-β1培养24 h后加入20 mmol/L LiCl或10 μmol/L XAV93920再常规培养24 h。
2.2 细胞活力分析 采用MTT法检测过表达SCUBE2对HCT116细胞活力的影响。将转染24 h的HCT116细胞按照每孔1×103个的密度接种到96孔板中,继续培养24 h、48 h和72 h后,按照MTT试剂盒说明书操作检测各孔在570 nm处的吸光度值。
2.3 细胞迁移的检测 利用Transwell法检测过表达SCUBE2对HCT116细胞迁移的影响。将转染24 h的细胞在无血清的培养中培养12 h,然后将200 μL含有5×104个细胞的无血清培养基加入到Transwell上室,下室加入500 μL含有10%胎牛血清的DMEM培养基,继续培养48 h后,取出小室,4%多聚甲醛固定30 min,用棉签轻轻擦掉上层未迁移细胞,0.1%结晶紫染色20 min,PBS冲洗后,显微镜下随机选取5个视野计数穿膜细胞数,实验重复3次,取平均值。
2.4 细胞凋亡的检测 采用Annexin V-FITC/PI双染进行流式细胞术检测过表达SCUBE2对HCT116细胞凋亡的影响。用不含EDTA的胰酶消化并收集转染48 h的细胞,预冷的PBS洗涤细胞2次,按照Annexin V-FITC/PI细胞凋亡试剂盒说明书操作,用FACSCalibur流式细胞仪检测细胞的凋亡情况。实验重复3次,凋亡细胞包括凋亡早期和凋亡晚期细胞。
2.5 Real-time PCR实验 按照TRIzol说明书操作,提取细胞的总RNA,取2 μg总RNA进行逆转录,然后按照UltraSYBR Mixture说明书加样后进行Real-time PCR。以GAPDH为内参照,按照2-ΔΔCt的方法计算目的基因的相对表达量,引物序列见表1。
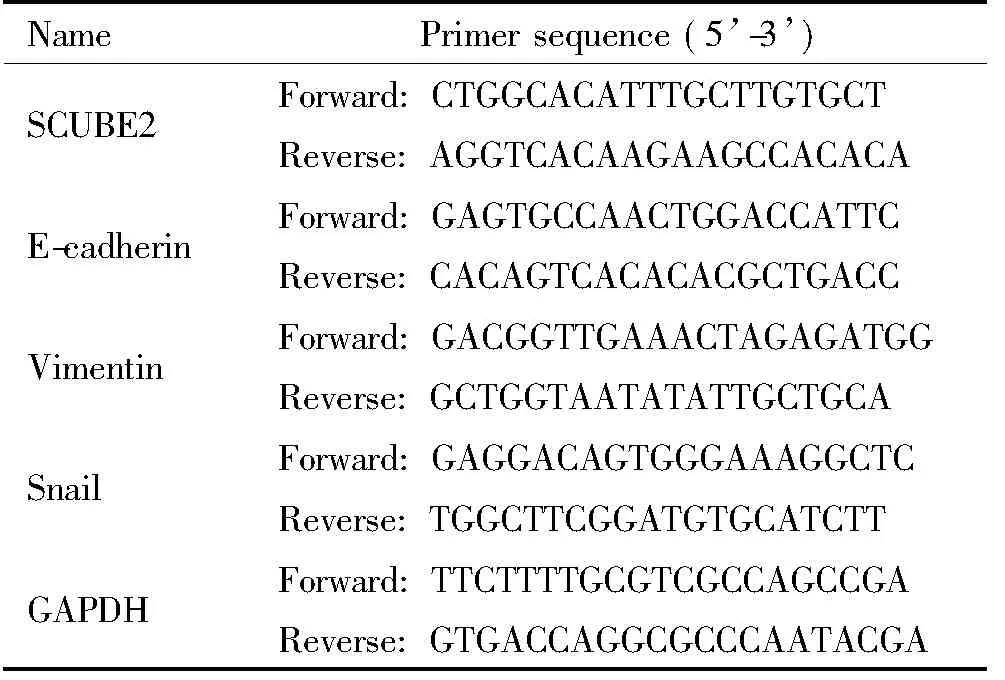
表1 引物序列
2.6 Western blot实验 RIPA裂解液将细胞充分裂解后,4 ℃、12 000×g离心15 min收集上清,即为提取细胞的总蛋白。BCA试剂盒测定蛋白质浓度后,将蛋白样品沸水浴煮沸5 min,每孔取相同质量的总蛋白上样进行SDS-PAGE分离蛋白,然后将蛋白转移至聚偏二氟乙烯(polyvinylidene difluoride,PVDF)膜上,5%脱脂奶粉室温封闭2 h,加入 I 抗 [SCUBE2(1∶500稀释)、E-cadherin(1∶800稀释)、vimentin(1∶1 000稀释)、Snail(1∶1 000稀释)、β-catenin(1∶8 000稀释)、c-Myc(1∶1 000稀释)和cyclin D1(1∶1 000稀释)] 4 ℃过夜孵育,TBST洗膜3次后加入HRP标记的 II 抗室温孵育45 min,再用TBST洗膜后加入新鲜配置的ECL发光液,将膜充分覆盖后拍照进行灰度值分析。
3 统计学处理
采用SPSS 13.0软件进行统计分析,计量资料用均数±标准差(mean±SD)表示,两组间比较采用t检验,多组间比较采用单因素方差分析。P<0.05表明差异具有统计学意义。
结 果
1 人结肠癌细胞中SCUBE2的表达降低
Real-time和Western blot实验检测人结肠癌细胞HCT116和正常人结直肠上皮FHC细胞中SCUBE2的表达,发现与FHC细胞相比,HCT116细胞中SCUBE2的表达在mRNA和蛋白水平均明显降低(P<0.05),见图1。
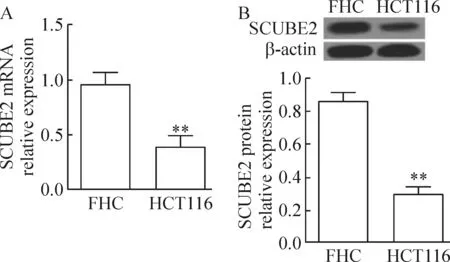
Figure 1.The expression of SCUBE2 at mRNA (A) and protein (B) levels in different colorectal cells were detected by real-time PCR and Western blot. Mean±SD.n=3.**P<0.01vsFHC group.
图1 不同结直肠细胞中SCUBE2的mRNA和蛋白表达
2 过表达SCUBE2抑制HCT116细胞的活力
HCT116细胞转染GV144 或GV144-SCUBE2质粒48 h后,Western blot法检测SCUBE2的表达结果显示,与GV144组相比,GV144-SCUBE2组中SCUBE2的表达明显升高(P<0.05)。转染24 h、48 h和72 h后分别用MTT法检测细胞的活力,与GV144组相比,GV144-SCUBE2组中HCT116细胞的活力明显降低(P<0.05),见图2。

Figure 2.The effect of SCUBE2 over-expression on the viability of HCT116 cells. A: Western blot analysis for SCUBE2 expression; B: MTT assay for cell viability. Mean±SD.n=3.**P<0.01vsGV144 group.
图2 过表达SCUBE2对HCT116细胞活力的影响
3 过表达SCUBE2抑制HCT116细胞的迁移
Transwell实验的检测结果显示,与GV144组相比,GV144-SCUBE2组的迁移细胞数目明显降低,差异有统计学显著性(P<0.05),见图3。
4 过表达SCUBE2对HCT116细胞的凋亡无影响
流式细胞术检测细胞凋亡结果显示,与GV144组相比,GV144-SCUBE2组中细胞的凋亡率稍微增加,但是差异无统计学显著性,见图4。
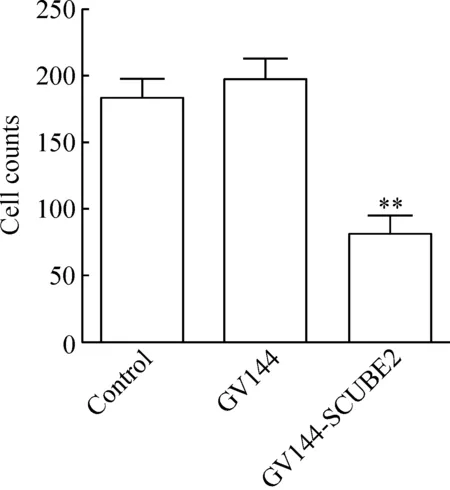
Figure 3.The effects of SCUBE2 over-expression on the number of migratory HCT116 cells. Mean±SD.n=3.**P<0.01vsGV144 group.
图3 过表达SCUBE2对HCT166细胞迁移数目的影响
5 过表达SCUBE2对HCT116细胞EMT的影响
Real-time PCR和Western blot实验检测E-cadherin、vimentin和Snail的表达。与对照组相比,TGF-β1组中E-cadherin的mRNA和蛋白表达均降低(P<0.05);vimentin和Snail的mRNA和蛋白表达均明显增加(P<0.05);与TGF-β1组相比,TGF-β1+SCUBE2组中E-cadherin的mRNA和蛋白表达均增加(P<0.05),vimentin和Snail的mRNA和蛋白表达均降低(P<0.05),见图5。
6 SCUBE2通过Wnt/β-catenin信号通路抑制EMT进程
Western blot实验检测HCT116细胞中β-catenin、c-Myc和cyclin D1的蛋白表达。与对照组相比,TGF-β1组中三者的表达均明显增加(P<0.05);与TGF-β1组相比,TGF-β1+SCUBE2组中三者的表达均明显降低(P<0.05),见图6A。

Figure 4.The effects of SCUBE2 over-expression on the apoptosis of HCT116 cells. Mean±SD.n=3.
图4 过表达SCUBE2对HCT116细胞凋亡的影响

Figure 5.The effects of SCUBE2 over-expression on the mRNA (A) and protein (B) expression of EMT markers. Mean±SD.n=3.**P<0.01vscontrol group;##P<0.01vsTGF-β1 group.
图5 过表达SCUBE2对HCT116细胞EMT标志物表达的影响
另外,HCT116细胞转染GV144-SCUBE2质粒6 h后加入TGF-β1蛋白(10 μg/L),培养24 h后加入LiCl(Wnt/β-catenin通路激活剂,20 mmol/L)或XAV93920 (Wnt/β-catenin通路抑制剂,10 μmol/L)继续培养24 h。Western blot法检测c-Myc、E-cadhe-rin、vimentin和Snail的表达。与TGF-β1+SCUBE2组相比,TGF-β1+SCUBE2+XAV93920组的E-cadherin表达明显增加,c-Myc、vimentin和Snail的表达明显降低(P<0.05);与TGF-β1+SCUBE2组相比,TGF-β1+SCUBE2+LiCl组中E-cadherin的表达明显降低,c-Myc、vimentin和Snail的表达明显增加(P<0.05),见图6B。

Figure 6.SCUBE2 inhibited EMT through Wnt/β-catenin signaling pathway. A: Western blot analysis for the expression of β-catenin, c-Myc, and cyclin D1; B: the expression of c-Myc and EMT markers in the HCT116 cells treated with 20 mmol/L LiCl or 10 μmol/L XAV93920 combined with SCUBE2 expression. Mean±SD.n=3.**P<0.01vscontrol;##P<0.01vsTGF-β1 group;△P<0.05,△△P<0.01vsTGF-β1+SCUBE2 group.
图6 SCUBE2通过Wnt/β-catenin信号通路抑制EMT进程
讨 论
SCUBE2蛋白包含1个N端信号肽序列、9个EGF样重复序列、1个沉默区、3个富含半胱氨酸的重复序列和1个C端CUB结构域[3]。它主要表达于血管内皮细胞和一些非内皮类型的细胞中,如成纤维细胞、肾间质细胞和乳腺导管上皮细胞[5, 7]。SCUBE2在乳腺癌中发挥关键作用,它能结合并拮抗骨形态发生蛋白的活性,抑制β-catenin通路从而抑制乳腺癌细胞生长[3, 5];而且还能够逆转EMT过程从而抑制乳腺癌细胞的迁移和侵袭[4]。Parris等[8]的研究发现,SCUBE2的表达也与口腔鳞状细胞癌的临床病理特征相关,如癌周炎性浸润、颈部淋巴结转移和肿瘤大小等。最近的研究发现,SCUBE2与结直肠癌的疾病进程和预后相关[6],但是目前其相关机制还没有报道。我们结果中发现与正常结直肠上皮FHC细胞相比,HCT116结肠癌细胞中SCUBE2的表达明显降低。
EMT是上皮细胞在特定的生理或病理条件下向间充质细胞转化的现象,是肿瘤细胞获得迁移和侵袭能力的分子基础[9]。本研究中过表达SCUBE2能够抑制HCT116细胞的活力与迁移,这表明SCUBE2可能与其EMT进程相关。EMT过程伴随着上皮表型黏附分子E-cadherin的减少或缺失,间质表型vimentin 和Snail表达增加[10]。在EMT相关的信号通路中,TGF-β信号通路被认为在EMT过程中发挥关键作用[11],TGF-β能激活上皮细胞向间充质转化[12]。本研究中TGF-β1刺激后,E-cadherin的表达降低,vimentin 和Snail表达增加,表明TGF-β1成功诱导HCT116细胞EMT过程。随后本研究检测SCUBE2过表达对TGF-β1诱导的EMT标志物的影响发现,SCUBE2能够增加E-cadherin的表达,降低Vimentin 和Snail的表达,这表明过表达SCUBE2基因可以抑制TGF-β1诱导的EMT进程。
Wnt/β-catenin通路在人类肿瘤中常被激活,它与肿瘤细胞EMT进程密切相关[13-14],c-Myc和cyclin D1是该通路的2个下游蛋白[15]。本研究进一步检测过表达SCUBE2与HCT116细胞的EMT机制发现,增高SCUBE2的表达能够降低β-catenin、c-Myc和cyclin D1的表达,这表明SCUBE2抑制了Wnt/β-catenin通路的激活。糖原合成酶激酶3β(glycogen synthase kinase-3β,GSK3β)是一个丝氨酸/苏氨酸蛋白激酶,是Wnt信号通路中一个重要的负调控因子,能够磷酸化通路中的蛋白使其降解[16]。LiCl 是GSK3β的抑制剂,能够激活Wnt/β-catenin通路[17]。本研究使用LiCl和Wnt/β-catenin通路抑制剂XAV93920,进一步探究SCUBE2是否是通过Wnt/β-catenin通路抑制HCT116细胞的EMT进程。LiCl能够拮抗过表达SCUBE2引起的E-cadherin上调以及vimentin和Snail下调;XAV93920能够增强过表达SCUBE2对TGF-β1介导的HCT116细胞EMT的影响。这些结果表明过表达SCUBE2是通过Wnt/β-catenin通路抑制结直肠癌细胞的EMT进程。
[1] Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65(2):87-108.
[2] Spaderna S, Schmalhofer O, Hlubek F, et al. A tran-sient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer[J]. Gastroenterology, 2006, 131(3):830-840.
[3] Lin YC, Chen CC, Cheng CJ, et al. Domain and functional analysis of a novel breast tumor suppressor protein, SCUBE2[J]. J Biol Chem, 2011, 286(30): 27039-27047.
[4] Lin YC, Lee YC, Li LH, et al. Tumor suppressor SCUBE2 inhibits breast-cancer cell migration and invasion through the reversal of epithelial-mesenchymal transition[J]. J Cell Sci, 2014, 127(Pt 1):85-100.
[5] Cheng CJ, Lin YC, Tsai MT, et al. SCUBE2 suppresses breast tumor cell proliferation and confers a favorable prognosis in invasive breast cancer[J]. Cancer Res, 2009, 69(8):3634-3641.
[6] Song Q, Li C, Feng X, et al. Decreased expression of SCUBE2 is associated with progression and prognosis in colorectal cancer[J]. Oncol Rep, 2015, 33(4): 1956-1964.
[7] Yang RB, Ng CK, Wasserman SM, et al. Identification of a novel family of cell-surface proteins expressed in human vascular endothelium[J]. J Biol Chem, 2002, 277(48):46364-46373.
[8] Parris TZ, Aziz L, Kovacs A, et al. Clinical relevance of breast cancer-related genes as potential biomarkers for oral squamous cell carcinoma[J]. BMC Cancer, 2014, 14:324.
[9] 李航宇, 李 岩, 刘 丹, 等. 细胞外HSP70/HSP70-PCs对人肝癌HepG2细胞上皮-间充质转化的影响及机制研究[J]. 中国病理生理杂志, 2013, 29(9): 1631-1636.
[10]Lee GA, Hwang KA, Choi KC. Roles of dietary phytoestrogens on the regulation of epithelial-mesenchymal transition in diverse cancer metastasis[J]. Toxins (Basel), 2016, 8(6): E162.
[11]Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition[J]. Cell Res, 2009, 19(2):156-172.
[12]Koeck S, Amann A, Huber JM, et al. The impact of metformin and salinomycin on transforming growth factor beta-induced epithelial-to-mesenchymal transition in non-small cell lung cancer cell lines[J]. Oncol Lett, 2016, 11(4): 2946-2952.
[13]Huang J, Xiao D, Li G, et al. EphA2 promotes epithelial-mesenchymal transition through the Wnt/beta-catenin pathway in gastric cancer cells[J]. Oncogene, 2014, 33(21):2737-2747.
[14]Yang T, Zhang H, Qiu H, et al. EFEMP1 is repressed by estrogen and inhibits the epithelial-mesenchymal transition via Wnt/beta-catenin signaling in endometrial carcinoma[J]. Oncotarget, 2016, 7(18): 25712-25725.
[15]Song L, Liu D, He J, et al. SOX1 inhibits breast cancer cell growth and invasion through suppressing the Wnt/beta-catenin signaling pathway[J]. APMIS, 2016, 124(7):547-555.
[16]Yost C, Torres M, Miller JR, et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3[J]. Genes Dev, 1996, 10(12):1443-1454.
[17]Xu M, Wang S, Song YU, et al. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/beta-catenin signaling pathway[J]. Oncol Lett, 2016, 11(5):3075-3080.
(责任编辑: 林白霜, 罗 森)
Over-expression of SCUBE2 suppresses epithelial-mesenchymal transition through Wnt/β-catenin signaling pathway in colorectal cancer cells
WANG Li, ZHANG Ning, ZHANG Long-jiang, XI Zuo-wu
(AnorectalDepartment,HenanProvinceHospitalofTCM,Zhengzhou450002,China.E-mail:wenyinxian029@163.com)
AIM: To study the effect of SCUBE2 on epithelial-mesenchymal transition (EMT) in colorectal cancer cells and its mechanism. METHODS: The expression of SCUBE2 in human colorectal cancer cell line HCT116 and normal colonic cell line FHC was detected by real-time PCR and Western blot. HCT116 cells were transfected with GV144-SCUBE2 to over-express SCUBE2, and then the cell viability, migration, and apoptosis were determined by MTT assay, Transwell assay and flow cytometry, respectively. The expression of EMT markers (E-cadherin, vimentin, and Snail), β-catenin, c-Myc and cyclin D1 in the HCT116 cells was analyzed by real-time PCR or Western blot after transfection with GV144-SCUBE2 for 6 h, followed by the stimulation of 10 μg/L recombinant TGF-β1 protein for 48 h. Additionally, the EMT process of HCT116 cells, which were stimulated by TGF-β1, over-expressed SCUBE2, and treated with Wnt/β-catenin pathway activator lithium chloride (LiCl) or inhibitor XAV93920, was analyzed by Western blot. RESULTS: Compared with FHC cells, the expression of SCUBE2 in the HCT116 cells was significantly decreased. The viability and migration ability of the HCT116 cells were suppressed by SCUBE2 over-expression, but the apoptosis was not markedly changed. Elevated expression of SCUBE2 increased E-cadherin expression, and decreased the expression of vimentin, Snail, β-catenin, c-Myc and cyclin D1 induced by TGF-β1. Treatment with LiCl blocked but treatment with XAV93920 enhanced the effects of SCUBE2 on EMT. CONCLUSION: Over-expression of SCUBE2 may inhibit the cell growth and migration, and suppress EMT through Wnt/β-catenin signaling pathway.
SCUBE2; Colorectal cancer; Epithelial-mesenchymal transition; Wnt/β-catenin signaling pathway
1000- 4718(2016)12- 2245- 06
2016- 06- 06
2016- 09- 18
R735.3; R730.23
A
10.3969/j.issn.1000- 4718.2016.12.020
杂志网址: http://www.cjpp.net
△通讯作者 Tel: 0371-53312157; E-mail: wenyinxian029@163.com
