红桦幼苗根系对水-氮耦合效应的生理响应
2016-12-28孙誉育尹春英贺合亮
孙誉育,尹春英,贺合亮,唐 波,刘 庆
1 中国科学院成都生物研究所, 中国科学院山地生态恢复与生物资源利用重点实验室, 生态恢复与生物多样性保育四川省重点实验室, 成都 610041 2 中国科学院大学, 北京 100049 3 成都理工大学材料与化学化工学院, 成都 610059
红桦幼苗根系对水-氮耦合效应的生理响应
孙誉育1,2,尹春英1,*,贺合亮1,3,唐 波1,2,刘 庆1
1 中国科学院成都生物研究所, 中国科学院山地生态恢复与生物资源利用重点实验室, 生态恢复与生物多样性保育四川省重点实验室, 成都 610041 2 中国科学院大学, 北京 100049 3 成都理工大学材料与化学化工学院, 成都 610059
采用两因素随机区组设计,设置了5个水分梯度,即40%(W1)、50%(W2)、60%(W3)、80%(W4)、100%(W5)的土壤田间持水量(FC)和3个施氮梯度,即模拟氮沉降施加0(对照,N0)、20(N1)、40(N2)gN m-2a-1的硝酸铵,研究了水-氮耦合效应对川西亚高山主要阔叶树种红桦(Betulaalbosinensis)幼苗根系生理活性的影响及根系在土壤水、氮胁迫下的生理调控机制。结果表明:1)随土壤含水量降低,根系活力和根系呼吸速率显著降低,膜脂过氧化产物(丙二醛)、渗透调节物质(脯氨酸、可溶性蛋白质和可溶性糖)含量及抗氧化物酶(超氧化物歧化酶、过氧化物酶和过氧化氢酶)活性显著升高。2)水-氮耦合效应对红桦幼苗根系生理特征影响显著:施氮(N1和N2)在土壤水分良好(W4和W5)时使根系活力和根系呼吸速率显著升高,而在土壤水分不足(W1和W2)时显著降低了根系活力和根系呼吸速率;且在水分不足时,施氮浓度越大根系活力、MDA含量、脯氨酸及可溶性蛋白质含量变化越显著;在W3条件下,只有N1对根系生理功能促进作用显著。3)根系活力和根系呼吸速率与丙二醛含量呈显著负相关性。因此,一定范围内的氮沉降在土壤水分状况良好时对植物根系生理特征具有显著正效应,而在土壤水分不足时则使根系细胞膜系统受损,抑制根系生理活性,但根系可通过增加渗透调节物质含量和增强抗氧化物酶活性来抵御一定范围的环境胁迫。
水-氮耦合;根系活性;膜脂过氧化;渗透调节;抗氧化物酶
根系是植物重要的功能器官,它不仅担负着吸收水分和养分的重要生理功能,而且通过呼吸和周转消耗光合产物并向土壤输入有机质[1],同时也是首先感知土壤环境变化的植物器官[2- 4]。根系在受到土壤环境胁迫时,细胞膜脂过氧化程度加剧[5],根系活性降低,吸收水分和养分的能力受到抑制[6],但植物细胞可通过主动积累溶质的方式,降低渗透势和水势,维持膨压,进行渗透调节;此外,环境胁迫使根系在代谢过程中通过多种途径产生的活性氧及其清除系统的平衡遭到破坏,但轻中度的胁迫能使清除酶(抗氧化物酶)的活性增强,根系抗氧化能力提高,缓解活性氧造成的伤害[7]。由此可见,渗透调节物质的积累[8]和抗氧化能力的提高[9]是根系抵御环境胁迫的两种重要生理响应机制。
近年来,大气氮沉降持续稳定增长,严重影响着陆地及水生生态系统的生产力和稳定性,大范围较高浓度的氮沉降导致了不同程度的土壤酸化和森林退化[10-11]。同时,大气氮沉降效应也因全球降水分布不均而异[10]。我国西南地区地处青藏高原东部,地形独特复杂,生态环境脆弱,对气候变化反应十分敏感,最明显的特点是干湿季明显、冬季低温、土壤养分有效性较低[12]。该区是IPCC预测的气候变暖和氮沉降的主要区域[13],也是研究全球变化对森林生态系统影响的关键地区和重要森林类型[14],红桦(Betualalbosinensis)是该区主要阔叶树种,其根系生理特性与土壤水、氮有效性密切相关。因此,本试验着重研究不同土壤水、氮条件对该区红桦幼苗根系活性的影响,探讨幼苗根系在水、氮胁迫环境下的生理响应机制,有助于了解该区主要植被地下生理特性及加深根系生态学的理论研究。
1 材料和方法
1.1 试验地基本概况
本试验选址于四川省阿坝州茂县的中国科学院成都生物研究所茂县生态定位研究站(103.90°E, 31.70°N,海拔1826 m),地处四川西部边缘向青藏高原腹地过渡的高山峡谷区,是长江的重要支流——岷江上游的重要地区,该地区年平均降雨量、年平均蒸发量和年平均气温分别为825.2 mm、968.7 mm、9.3℃,属暖温带气候。植被属针阔叶落叶常绿混交林,红桦是该区主要的阔叶树种。
1.2 试验材料和试验设计
本试验采用两因素(水分和氮素)随机区组设计,根据实测的当地土壤旱季极端含水量,设置5个水分梯度(结合前期红桦对土壤水分敏感性预实验结果及着重研究其在干旱环境下响应的试验目的,将土壤低含水量梯度设置得相对密集),即 40%(W1)、50%(W2)、60%(W3)、80%(W4)和 100%(W5)的土壤田间持水量(即实际土壤含水量分别为11.9%、14.9%、17.9%、23.9%、29.9%);基于本区域相关研究[15],设置施氮梯度为 0(N0)、20(N1)和 40(N2)gN m-2a-1,共15个处理,每处理重复3次,5株为1个重复。

1.3 试验方法
于2014年8月中旬(生长季中期)进行幼苗根系取样,每处理随机选取3株幼苗,用根钻将根系最大限度带土取出,以得到其完整根系,用清水洗净并置于4℃冰箱保存,用于根系部分生理指标的测定。其中,根系活力采用TTC还原法[17]测定;丙二醛(MDA)含量参照Hodges等[18]的方法测定;脯氨酸含量的测定参照Bates等[19]的方法;可溶性蛋白质含量采用考马斯亮蓝G- 250法[20]测定;可溶性糖含量参照Shi等[21]的方法测定;超氧化物歧化酶(SOD)活性的测定参照Dhindsa等[22]的方法;过氧化物酶(POD)活性和过氧化氢酶(CAT)活性分别参照Chance和Maehly[23]的方法测定。
此外,氧气流速(表示根系呼吸速率)参照Sun等[24]的方法,利用非损伤微测系统(BIO-IM-XY,Younger,USA)测定。实验使用的金属微电极来自旭月(北京)科技有限公司。电极在使用前进行校正,校正液O2浓度设置为0和21%,其他成分与测试液(0.2 mmol/L CaCl2,0.1 mmol/L KCl,0.5 g/L MES,0.1 mmol/L NH4NO3)相同,校正斜率在-9000—-2000范围内的电极用于检测。测试前将根系置于装有10—20 mL测试液的测试盒中平衡30 min后更换新的测试液开始测试,测试区域为距离根尖20 mm左右根毛分布密集的成熟区。每处理测试5个样品,每个样品稳定测定10 min。
1.4 统计分析
数据的统计分析使用SPSS 17.0完成,利用两因素方差分析(Two- way ANOVA)检验水分和施氮效应,相同水分条件不同施氮梯度间的多重比较采用邓肯式新复极差法进行检验。
2 结果和分析
2.1 水-氮耦合效应对根系活力和根系呼吸速率的影响
红桦幼苗根系活力和根系呼吸速率受到土壤水分、氮素供应及其交互作用的极显著影响(图1)。二者均随土壤含水量增加而增大,W4和W5时达到最大。图1表明,当土壤水分不足(W1和W2)时,施氮(N1和N2)显著抑制了红桦幼苗根系活力和根系呼吸速率(W1条件下的N1除外),且施氮浓度越高,对根系活力抑制作用越显著;在土壤水分充足(W4和W5)时,N1显著促进了幼苗根系活力和根系呼吸速率;W3条件下,N1表现为显著促进作用,而N2表现为抑制效应。
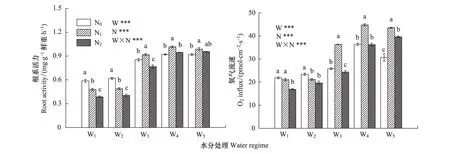
图1 不同水、氮供应对红桦幼苗根系活力和根系呼吸速率的影响(平均值±标准偏差)Fig.1 Effects of different soil water and nitrogen supply on root activity, root respiration rate in Betual albosinensis seedling (mean±SD)误差线上方不同的小写字母表示同一水分条件下不同施氮梯度差异显著(P<0.05);W: 水分效应;N: 氮肥效应;W×N: 水-氮交互作用;** P<0.05; *** P<0.005
2.2 根系MDA含量对水-氮耦合的响应及其与根系活力和呼吸速率的关系
随土壤含水量降低,幼苗根系MDA含量显著升高,且不同水、氮供应及其交互作用对MDA含量具有极显著影响(图2)。施氮只在土壤水分不足(W1和W2)时使MDA含量显著增大,且施氮浓度越高,作用越显著;而在W3和土壤水分状况良好(W4和W5)时,N1使MDA含量显著降低。此外,根系活力和呼吸速率均与根系MDA含量呈极显著负相关(图2)。
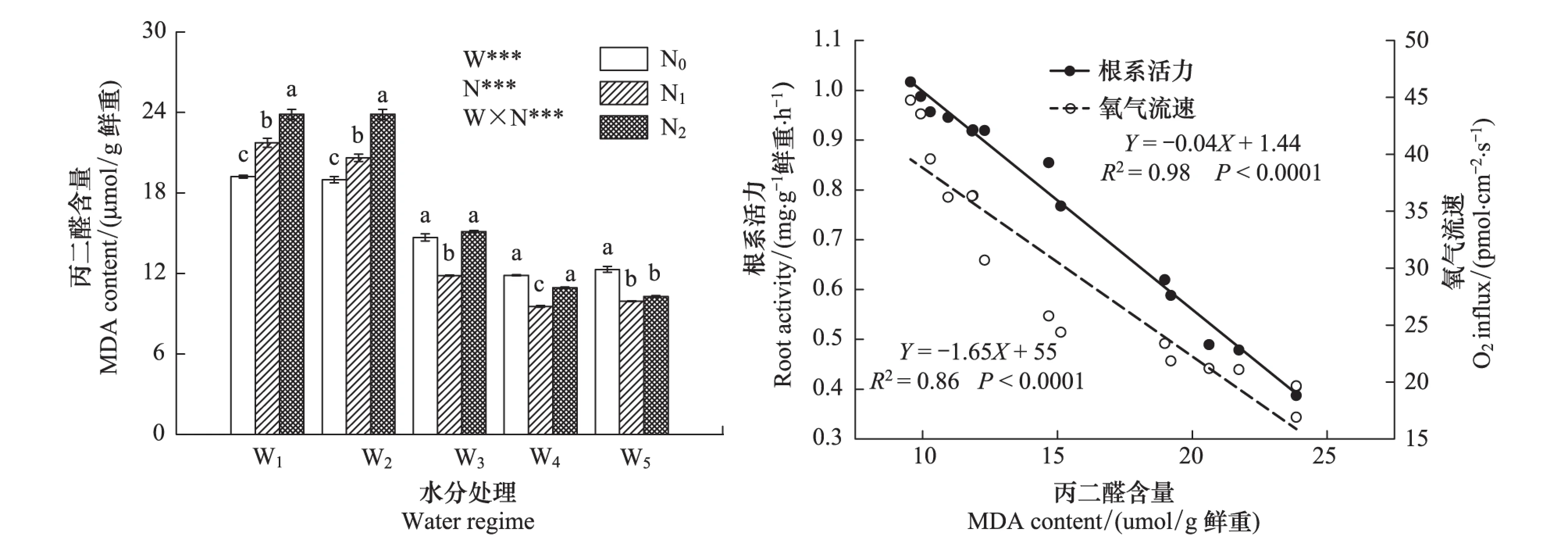
图2 红桦幼苗根系丙二醛(MDA)含量对水、氮供应的响应(平均值±标准偏差)及其与根系活力和呼吸速率的相关性Fig.2 Root MDA content response to different soil water and nitrogen supply (mean±SD) and its relationship with root activity and respiration rate in Betual albosinensis seedling
2.3 根系渗透调节物质对水-氮耦合效应的响应
红桦幼苗根系渗透调节物质(脯氨酸、可溶性蛋白质和可溶性糖)含量也受土壤水分、氮素供应及其交互作用的极显著影响(图3)。与根系MDA含量的变化相似,根系渗透调节物质含量随土壤含水量降低而升高。当土壤水分不足(W1和W2)时,施氮使渗透调节物质含量显著升高,且施氮浓度越大,脯氨酸和可溶性蛋白质含量升高越显著;在W3和土壤水分状况良好(W4和W5)时,主要表现为N1显著降低了根系渗透调节物质含量。
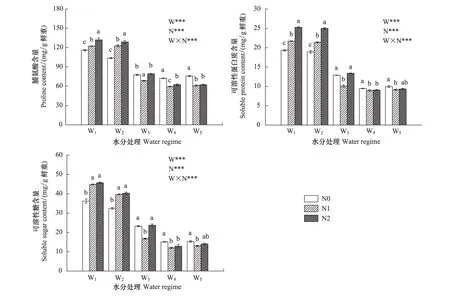
图3 红桦幼苗根系脯氨酸含量、可溶性蛋白质含量和可溶性糖含量(平均值±标准偏差)对不同水、氮供应的响应Fig.3 Responses of root proline content, soluble protein, soluble sugar in Betual albosinensis seedling (mean±SD) to different soil water and nitrogen supply
2.4 根系抗氧化物酶活性对水-氮耦合效应的响应
红桦幼苗根系抗氧化物酶(SOD、POD和CAT)活性也受水-氮耦合效应的极显著影响(图4),其随土壤水分、氮素供应的变化趋势与MDA含量及渗透调节物质含量一致。施氮在土壤水分胁迫(W1和W2)条件下,显著提高了SOD活性(图4),对POD和CAT活性无显著影响(图4)(W2条件下N2对POD的影响除外),在土壤水分充足(W4和W5)时,则显著降低了3种酶活性;在W3条件下,高、低浓度施氮效果不同。
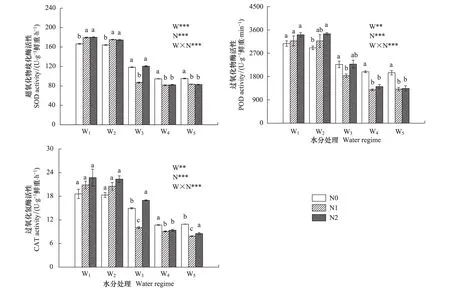
图4 红桦幼苗根系超氧化物歧化酶(SOD)、过氧化物歧化酶(POD)和过氧化氢酶(CAT)活性(平均值±标准偏差)对不同水、氮供应的响应Fig.4 Responses of root SOD, POD, CAT activities in Betual albosinensis seedling (mean±SD) to different soil water and nitrogen supply
3 讨论
根系活力和根系呼吸速率都是衡量根系生理活性的重要指标,在一定程度上反映了作物吸收水分和矿质养分的能力,直接影响着根系的生长发育状况,对植物的生长起着决定性作用[25]。通常,根系活力和呼吸速率越高,根系吸收水分和养分的能力就越强[24]。本研究中,根系活力和呼吸速率在土壤水分胁迫下显著降低,而氮素的施加更是加剧了其降低(图1),说明土壤水分胁迫不利于根系生理活性的发挥,且施氮加剧了胁迫程度,这可归因于低土壤含水量下,氮肥使土壤水分溶质势降低,导致植物吸收水分难度增大[26],从而降低了根系生理活性。在土壤水分充足的状况下,施氮使幼苗根系活力和呼吸速率显著增强,这表明充足的水分能使土壤氮素有效性增强,促进根系生理活性的发挥,提高根系吸收水分和养分的能力。本实验结果说明合适的水-氮配比才能使水、氮发挥其最大效应。但也有研究表明,当植物受到土壤环境胁迫时,根系活力有所增强[27],这可能是由于不同植物耐受环境胁迫能力及适应策略不同。
植物生理生化指标的变化是植物对逆境条件的适应性反应,可反映植物生长状况及受伤害程度。环境胁迫可导致植物体内活性氧自由基积累并引发膜脂过氧化,MDA即是细胞膜脂过氧化产物[28],其含量可表征膜脂过氧化程度,反映细胞膜系统受伤害程度[29]。大量研究表明,在受到干旱、冷冻伤害以及高温胁迫时,植物细胞内产生和清除活性氧的平衡机制被破坏,从而使活性氧的产生和膜脂过氧化反应加剧,导致MDA大量积累[30-31]。本研究中,红桦幼苗根系MDA含量在土壤含水量不足时的变化(图2)说明土壤水分胁迫对根系细胞膜系统造成了伤害,施氮更是加剧了其受伤害程度。这应该是在较低土壤含水量的情况下,施加氮肥所导致的土壤渗透压增加,而在这种缓慢渗透胁迫下根系自由基积累引起膜脂过氧化作用[32]。相反,在土壤水分充足的条件下,施氮则减缓了根系细胞膜脂过氧化程度。该结果与Yin等人[33]的研究结果不完全一致,这可能与供试土壤水、氮条件以及研究物种对水、氮需求量不同有关。此外,本研究中根系MDA含量与根系活力和呼吸速率之间的显著负相关性(图2),说明幼苗根系膜脂过氧化程度的加剧是导致其生理活性降低的内在因素之一。
在一定范围内的环境胁迫下,根系往往可通过增强其渗透调节能力[8]和抗氧化能力[9]适应胁迫,进而维持根系及整个植株生理过程的正常进行[34]。本研究中,在土壤水分状况不足时,幼苗根系细胞膜系统受损(图2)、生理活性降低(图1),根系中渗透调节物质(脯氨酸、可溶性蛋白质和可溶性糖)含量相应升高(图3),这与Qayyum等[35]的研究结果相似,说明根系内的脯氨酸等可溶性物质能够通过细胞内渗透调节,去除活性氧,维持膜稳定性,从而缓解植株受到的胁迫[36-37]。但也有研究表明,干旱胁迫增加了脯氨酸含量却降低了可溶性蛋白质含量[27]和可溶性糖含量[38],这可能与研究物种的内在调节机制以及相关遗传因素[39]有关。此外,在土壤水分胁迫下,施氮则进一步提高了红桦幼苗根系渗透调节能力,尤其是脯氨酸和可溶性蛋白质含量随施氮浓度的增加显著增加(图3),说明红桦幼苗根系内脯氨酸和可溶性蛋白质对土壤环境胁迫的响应比可溶性糖更为敏感,因为脯氨酸作为水溶性最大的氨基酸,是植物渗透调节过程中的关键物质[34]。此外,根系可溶性蛋白质和可溶性糖含量的变化与植株体内氮含量和淀粉的积累量等也有关系[33]。
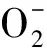
综上,土壤水分胁迫使根系细胞膜系统受损,根系生理活性受到抑制,施氮更是加剧了胁迫程度,但在一定范围内的胁迫下,根系可通过提高渗透调节能力和抗氧化调节能力来抵御环境胁迫;此外,在土壤水分状况良好时,施氮能够促进红桦幼苗根系生理活性增强,且对于本研究的供试土壤及试验设置的氮素梯度而言,低浓度施氮效果尤为明显。
[1] 王政权, 郭大立. 根系生态学. 植物生态学报, 2008, 32(6): 1213- 1216.
[2] Eshel A, Beeckman T. Plant Roots: the Hidden Half. New York: CRC Press, 2013.
[3] Lynch J P. Turner review no. 14. Roots of the second green revolution. Australian Journal of Botany, 2007, 55(5): 493- 512.
[4] Zhang X Y, Chen S Y, Sun H Y, Wang Y M, Shao L W. Root size, distribution and soil water depletion as affected by cultivars and environmental factors. Field Crops Research, 2009, 114(1): 75- 83.
[5] Zhou R L, Zhao H L. Seasonal pattern of antioxidant enzyme system in the roots of perennial forage grasses grown in alpine habitat, related to freezing tolerance. Physiologia Plantarum, 2004, 121(3): 399- 408.
[6] Liu L J, Chen T T, Wang Z Q, Zhang H, Yang J C, Zhang J H. Combination of site-specific nitrogen management and alternate wetting and drying irrigation increases grain yield and nitrogen and water use efficiency in super rice. Field Crops Research, 2013, 154: 226- 235.
[7] Nakamura T, Yokota S, Muramoto Y, Tsutsui K, Oguri Y, Fukui K, Takabe T. Expression of a betaine aldehyde dehydrogenase gene in rice, a glycinebetaine nonaccumulator, and possible localization of its protein in peroxisomes. The Plant Journal, 1997, 11(5): 1115- 1120.
[8] Sanchez F J, de Andres E F, Tenorio J L, Ayerbe L. Growth of epicotyls, turgor maintenance and osmotic adjustment in pea plants (PisumsativumL.) subjected to water stress. Field Crops Research, 2004, 86(1): 81- 90.
[9] Wang X, Peng Y H, Singer J W, Fessehaie A, Krebs S L, Arora R. Seasonal changes in photosynthesis, antioxidant systems andELIPexpression in a thermonastic and non-thermonatic Rhododendron species: a comparison of photoprotective strategies in overwintering plants. Plant Science, 2009, 177(6): 607- 617.
[10] Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman J W, Fenn M, Gilliam F, Nordin A, Pardo L, De Vries W. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecological Applications, 2010, 20(1): 30- 59.
[11] Sparrius L B, Sevink J, Kooijman A M. Effects of nitrogen deposition on soil and vegetation in primary succession stages in inland drift sands. Plant Soil, 2012, 353(1/2): 261- 272.
[12] 王开运. 川西亚高山森林群落生态系统过程. 成都: 四川科学技术出版社, 2004.
[13] IPCC. Climate change 2007: mitigation of Climate change // Metz B, Davidson O R, Bosch P R, Dave R, Meyer L A, eds. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press, 2007.
[14] Xu Z F, Wan C, Xiong P, Tang Z, Hu R, Cao G, Liu Q. Initial responses of soil CO2efflux and C, N pools to experimental warming in two contrasting forest ecosystems, Eastern Tibetan Plateau, China. Plant and Soil, 2010, 336(1/2): 183- 195.
[15] Zhao C Z, Liang J, He J, Liu Q. Effects of elevated temperature and nitrogen fertilization on nitrogen metabolism and nutrient status of two coniferous species. Soil Science and Plant Nutrition, 2012, 58(6): 772- 782.
[16] Liu X J, Zhang Y, Han W X, Tang A H, Shen J L, Cui Z L, Vitousek P, Erisman J W, Goulding K W T, Christie P, Fangmeier A, Zhang F S. Enhanced nitrogen deposition over China. Nature, 2013, 494(7438): 459- 462.
[17] 常蓬勃, 李志云, 杨建唐, 霍晓婷, 王文亮. 氮钾锌配施对烟草超氧化物歧化酶和硝酸还原酶活性及根系活力的影响. 中国农学通报, 2008, 24(1): 266- 270.
[18] Hodges D M, DeLong J M, Forney C F, Prange R K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 1999, 207(4): 604- 611.
[19] Bates L S, Waldren R P, Teare I D. Rapid determination of free proline for water-stress studies. Plant and Soil, 1973, 39(1): 205- 207.
[20] Castillo F J, Penel C, Greppin H. Peroxidase release induced by ozone in Sedum album leaves Involvement of Ca2+. Plant Physiology, 1984, 74(4): 846- 851.
[21] Shi P, Körner C, Hoch G. End of season carbon supply status of woody species near the treeline in western China. Basic and Applied Ecology, 2006, 7(4): 370- 377.
[22] Dhindsa R S, PlumbD P, Thorpe T A. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 1981, 32(1): 93- 101.
[23] Chance B, Maehly A C. Assay of catalases and peroxidases. Methods in Enzymology, 1955, 2: 764- 775.
[24] Sun J, Chen S L, Dai S X, Wang R G, Li N Y, Shen X S, Zhou X Y, Lu C F, Zheng X J, Hu Z M. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiology, 2009, 149(2): 1141- 1153
[25] Richter A K, Frossard E, Brunner I. Polyphenols in the woody roots of Norway spruce and European beech reduce TTC. Tree Physiology, 2007, 27(1): 155- 160.
[26] 刘锁云, 陈磊庆, 胡廷会, 李立军, 刘景辉. 水氮耦合对燕麦光合特性的影响. 西北农业学报, 2013, 22(1): 60- 67.
[27] Yao X Q, Chu J Z, Wang G Y. Effects of drought stress and selenium supply on growth and physiological characteristics of wheat seedlings. Acta Physiologiae Plantarum, 2009, 31(5): 1031- 1036.
[28] De Vos C H R, Schat H, De Waal M A M, Vooijs R, Ernst W H O. Increased resistance to copper-induced damage of the root cell plasmalemma in copper tolerant Silene cucubalus. Physiologia Plantarum, 1991, 82(4): 523- 528.
[29] Xu Z Z, Zhou G S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta, 2006, 224(5): 1080- 1090.
[30] Bai L P, Sui F G, Ge T D, Sun Z H, Lu Y Y, Zhou G S. Effect of soil drought stress on leaf water status, membrane permeability and enzymatic antioxidant system of maize. Pedosphere, 2006, 16(3): 326- 332.
[31] Zhou R L, Wang H O. Correlation between resistance to dehydration and lipid peroxidation of desert plants under atmosphere dehydration and high temperature stresses. Journal of Desert Research, 1999, 19(S1): 49- 54.
[32] 张立军, 胡文玉, 戴俊英. 渗透胁迫下玉米幼苗离体叶片膜透性变化机理的研究. 沈阳农业大学学报, 1996, 27(3): 207- 210.
[33] Yin C Y, Pang X Y, Chen K, Gong R G, Xu G, Wang X. The water adaptability ofJatrophacurcasis modulated by soil nitrogen availability. Biomass and Bioenergy, 2012, 47: 71- 81.
[34] 王玉凤, 王庆祥, 商丽威. NaCl和 Na2SO4胁迫对玉米幼苗渗透调节物质含量的影响. 玉米科学, 2007, 15(5): 69- 71.
[35] Qayyum A, Razzaq A, Ahmad M, Ahmad M, Jenks M A. Water stress causes differential effects on germination indices, total soluble sugar and proline content in wheat (TriticumaestivumL.) genotypes. African Journal of Biotechnology, 2013, 10(64): 14038- 14045.
[36] Lee G, Carrow R N, Duncan R R, Eiteman M A, Rieger M W. Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environmental and Experimental Botany, 2008, 63(1/3): 19- 27.
[37] Yang C W, Shi D C, Wang D L. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyteSuaedaglauce(Bge.). Plant Growth Regulation, 2008, 56(2): 179- 190.
[38] Li J, Qu H, Zhao H L, Zhou R L, Yun J Y, Pan C C. Growth and physiological responses ofAgriophyllumsquarrosumto sand burial stress. Journal of Arid Land, 2015, 7(1): 94- 100.
[39] Rampino P, Pataleo S, Gerardi C, Mita G, Perrotta C. Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environment, 2006, 29(12): 2143- 2152.
[40] Willekens H, Langebartels C, Tiré C, Van Montagu M, Inzé D, Van Camp W. Differential expression of catalase genes inNicotianaplumbaginifolia(L.). Proceedings of the National Academy of Sciences of the United States of America, 1994, 91(22): 10450- 10454.
[41] Wang F Z, Wang Q B, Kwon S Y, Kwakb S S, Su W A. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. Journal of Plant Physiology, 2005, 162(4): 465- 472.
[42] Neill S J, Desikan R, Clarke A, Hurst R D, Hancock J T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. Journal of Experimental Botany, 2002, 53(372): 1237- 1247.
[43] 葛体达, 隋方功, 白莉萍, 吕银燕, 周广胜. 水分胁迫下夏玉米根叶保护酶活性变化及其对膜脂过氧化作用的影响. 中国农业科学, 2005, 38(5): 922- 928.
[44] 桂世昌, 杨峰, 张宝艺, 张新全, 黄琳凯, 马啸. 水分胁迫下扁穗牛鞭草根系保护酶活性变化. 草业学报, 2010, 19(5): 278- 282.
The physiological responses ofBetulaalbosinensisseedling roots to soil water-nitrogen coupling
SUN Yuyu1,2, YIN Chunying1,*, HE Heliang1,3, TANG Bo1,2, LIU Qing1
1KeyLaboratoryofMountainEcologicalRestorationandBioresourceUtilization&EcologicalRestorationBiodiversityConservationKeyLaboratoryofSichuanProvince,ChengduInstituteofBiology,ChineseAcademyofSciences,Chengdu610041,China2UniversityofChineseAcademyofSciences.Beijing100049,China3CollegeofMaterialandChemistry&ChemicalEngineering,ChengduUniversityofTechnology,Chengdu610059,China
The increasing deposition of nitrogen into the atmosphere has affected the stability and productivity of ecosystem. The effects of nitrogen deposition vary in regards to soil water content. There is uneven precipitation distribution and significant water-nitrogen coupling, which directly affects the growth and development of plants. In order to better understand the responses of plant roots to soil water-nitrogen coupling, we exposed seedlings ofBetulaalbosinensisto five different water regiments of field water holding capacity (FC) (40% [W1], 50% [W2], 60% [W3], 80% [W4], and 100% [W5]). As well as three different nitrogen (N) regiments (control, 0 [N0], 20 [N1], and 40 [N2] gN m-2a-1with NH4NO3). These experimental conditions allowed us to determine the root activity and physiology of the seedlings when exposed to various water and nitrogen supplies. The results indicated that a decrease of soil water content prompted the root vigor and root respiration rate to decrease while membrane lipid peroxide(MDA), osmotic adjustment substances (i.e. proline, soluble protein, and soluble sugar) content, and the activity of antioxidant enzymes (i.e. superoxide dismutase, peroxidase and catalase) all increased. Moreover, root physiological characteristics were significantly affected by the water-nitrogen coupling effect. When soil moisture was deficient (W1and W2), the addition of nitrogen markedly reduced root activity. However, when adequate soil water (W4and W5) was present, root activity was significantly enhanced. Additionally, the greater the concentration of nitrogen applied, the greater the effects were upon root activity and the contents of proline and soluble MDA. Only the lower concentration of nitrogen addition (N1) significantly improved root physiological functions under 60%FC. Finally, both root vigor and nitrate reductase activity had a significant negative correlation with membrane lipid peroxide level (MDA content). In conclusion, atmospheric nitrogen deposition could significantly promote root physiological characteristics ofB.albosinensisseedlings when soil moisture is plentiful. However, if soil water is deficient, the deposition of atmospheric nitrogen could cause serious damage to the cell membrane systems and consequently restrain the activity of the root system. Therefore, plant roots could defend themselves against these stresses by increasing the osmotic regulation substances content and antioxidant enzyme activity within a certain range.
water-nitrogen coupling; root activity; membrane lipid peroxidation; osmotic adjustment; antioxidant enzyme
国家自然科学基金项目(31370495, 31400424)
2015- 02- 15;
日期:2016- 03- 03
10.5846/stxb201502150360
*通讯作者Corresponding author.E-mail: yincy@cib.ac.cn
孙誉育,尹春英,贺合亮,唐波,刘庆.红桦幼苗根系对水-氮耦合效应的生理响应.生态学报,2016,36(21):6758- 6765.
Sun Y Y, Yin C Y, He H L, Tang B, Liu Q.The physiological responses ofBetulaalbosinensisseedling roots to soil water-nitrogen coupling.Acta Ecologica Sinica,2016,36(21):6758- 6765.
