Chemical constituents from underground part ofAstragaluscamptodontoides
2016-12-22HANBingYangZHANGYuTIANXinYanXIAOChaoJiangDONGXiangJIANGBei
HAN Bing-Yang, ZHANG Yu, TIAN Xin-Yan, XIAO Chao-Jiang,DONG Xiang, JIANG Bei
( Institute of Materia Medica, Dali University, Dali 671000, Yunnan, China )
Abstract: For understanding the chemical constituents of Astragalus camptodontoides, nineteen compounds were isolated from the ethyl acetate fraction of the methanol extract of underground part. By physical-chemical properties and spectroscopic date, their structures were identified as isobavachin (1), 4′-hydroxyisolonchocarpin (2), 5-deoxyeuchrenone (3), shinflavanone (4), khonklonginols H (5), 4′-O-methylpreglabridin (6), 3′-hydroxy-4′-O-methylglabridin (7), 4′-O-methylglabridin (8), 8-prenyl-phaseollinisoflavan (9), xambioona (10), glabrol (11), glyasperin H (12), methylnissolin (13), phthalic acid isodibutyl ester (14), butul isobutyl phthalate (15), β-sitosterol (16), daucosterol (17), oleanic acid (18), and (2S,3S,4R,9E)-1,3,4-trihydroxy-2- [(2′R)-2′-hydroxytetracosanoylamino]-9-octadecene (19). All compounds were isolated from this plant for the first time, including compounds 1-7 obtained from Astragalus genus for the first time.
Chemical constituents from underground part ofAstragaluscamptodontoides
HAN Bing-Yang, ZHANG Yu, TIAN Xin-Yan, XIAO Chao-Jiang,DONG Xiang, JIANG Bei*
(InstituteofMateriaMedica,DaliUniversity, Dali 671000, Yunnan, China )
Abstract: For understanding the chemical constituents ofAstragaluscamptodontoides, nineteen compounds were isolated from the ethyl acetate fraction of the methanol extract of underground part. By physical-chemical properties and spectroscopic date, their structures were identified as isobavachin (1), 4′-hydroxyisolonchocarpin (2), 5-deoxyeuchrenone (3), shinflavanone (4), khonklonginols H (5), 4′-O-methylpreglabridin (6), 3′-hydroxy-4′-O-methylglabridin (7), 4′-O-methylglabridin (8), 8-prenyl-phaseollinisoflavan (9), xambioona (10), glabrol (11), glyasperin H (12), methylnissolin (13), phthalic acid isodibutyl ester (14), butul isobutyl phthalate (15),β-sitosterol (16), daucosterol (17), oleanic acid (18), and (2S,3S,4R,9E)-1,3,4-trihydroxy-2- [(2′R)-2′-hydroxytetracosanoylamino]-9-octadecene (19). All compounds were isolated from this plant for the first time, including compounds 1-7 obtained fromAstragalusgenus for the first time.
Astragaluscamptodontoides, underground part, chemical constituents, isolation and identification
Astragaluscamptodontoides, a species ofAstragalusgenus, grows on grassland with altitude over 3 100 m and is mainly distributed in South Tibet, Southwest Sichuan, and Northwest Yunnan in China (China Flora Editorial Board, 1993; Kunming Institute for Botany, 2006). This plant is often used as substitute of Chinese medicine “Huang Qi” by local folks, and therefore, it is supposed to have the major constituents similar to Huangqi. However, research concerning its chemical composition has not been reported yet. In order to investigate the chemical patterns of its major constituents, a detailed chemical study on the underground part ofA.camptodontoideswas carried out recently. As a result, nineteen compounds were isolated from the EtOAc fraction of its MeOH extract. Their structures were identified as isobavachin (1), 4′-hydroxyisolonchocarpin (2), 5-deoxyeuchrenone (3), shinflavanone (4), khonklonginols H (5), 4′-O-methylpreglabridin (6), 3′-hydroxy-4′-O-methylglabridin (7), 4′-O-methylglabridin (8), 8-prenyl-phaseollinisoflavan (9), xambioona (10), glabrol (11), glyasperin H (12), methylnissolin (13), phthalic acid isodibutyl ester (14), butul isobutyl phthalate (15),β-sitosterol (16), daucosterol (17), oleanic acid (18), and (2S,3S,4R,9E)-1,3,4-trihydroxy-2- [(2′R)-2′-hydroxytetracosanoylamino]-9-octadecene (19) (Fig. 1). All of these compounds were isolated from this plant for the first time, and compounds 1-7 were isolated fromAstragalusgenus for the first time.
1 Materials and Methods
1.1 Plant Materials
Astragaluscamptodontoideswas collected from Diqing (Yunnan, China) in September 2012 and identified by Dr. ZHANG De-Quan, Laboratory of Pharmacognosy of Dali University. A voucher specimen (20120918-2-A) was deposited in Institute of Materia Medica at Dali University.
1.2 Experimental Instruments
EI-MS spectra were obtained on VG Auto Spec-3000 and API QSTAR Pulsari Spectrometer.1H-NMR and13C-NMR spectra were recorded on a Bruker-400 MHz Spectrometer using TMS as an internal standard. TLC was performed on silica gel G and GF254plates (Qingdao Marine Chemical Factory). Column chromatography was carried out on silica gel (200-300 mesh; Qingdao Marine Chemical Factory), Sephadex LH-20 (Amersham Biosciences), and RP-18 gel (40-75 μm; J. T. Baker). TLC spots were visualized by 10% H2SO4with heating or by UV light.
2 Extraction and Isolation
The dried and powdered roots ofA.camptodontoides(1.05 kg) were extracted with MeOH for six times, 6 h each time. The extracts were combined and concentratedinvacuumto give a crude extract. The crude extract was suspended in water and partitioned with EtOAc and butanol, successively. Removal of the solvent from each phase gave the EtOAc fraction, butanol fraction, and water-soluble extract, respectively. The EtOAc fraction (123.5 g) was subjected to a silica gel column and eluted with CHCl3-Me2CO (1∶0-0∶1) to provide Fr. 1-7. Fr. 1 (4 g) was subjected repeatedly to column chromatography on silica gel and eluted with petroleum ether-Me2CO to yield compounds 2 (5.4 mg), 3 (12.0 mg), 5 (15.3 mg), 6 (3.2 mg), 9 (5.5 mg), 14 (5.7 mg), 17 (5.6 mg) and 18 (8.7 mg). Fr. 2 (7.5 g) was subjected repeatedly to column chromatography padded with silica gel and eluted with petroleum ether-Me2CO to yield compounds 1 (5.2 mg), 4 (5.4 mg), 7 (8.3 mg), 8 (5.6 mg), 10 (15.3 mg), 12 (5.4 mg), 15 (3.8 mg), and 16 (20.2 mg). Fr. 3 (3 g) was purified repeatedly on silica gel column and eluted with petroleum ether-Me2CO to yield Compound 11 (8.2 mg). Fr. 6 (8 g) was subjected to a RP-18 chromatographic column and eluted with MeOH-H2O and followed by Sephadex LH-20 (MeOH) purification to yield compounds 13 (8.1 mg) and 19 (5.7 mg).
3 Results and Analysis
Isobavachin (1) Yellow powder; C25H26O4;1H-NMR (CDCl3, 400 MHz)δ: 7.76 (1H, d,J= 8.7 Hz, H-5), 6.96 (2H, d,J= 2.2 Hz, H-2′, 6′), 6.69(2H, d,J= 8.3 Hz, H-3′, 5′), 6.36 (1H, d,J= 9.8 Hz, H-6 ), 5.59 (1H, t,J= 9.8 Hz, H-2″), 5.36 (1H, dd,J= 13.3, 2.8 Hz, H-2), 3.15 (2H, overlap, H-1″), 3.01 (1H, dd,J= 16.8, 13.3 Hz, H-3b), 2.81 (1H, dd,J= 16.8, 2.9 Hz, H-3a), 1.49 (3H, s, H-4″), 1.46 (3H, s, H-5″);13C-NMR (CDCl3, 100 MHz)δ: 185.5 (s, C-4), 159.6 (s, C-7), 157.7 (s, C-9), 156.8 (s, C-4′), 131.7 (s, C-3″), 131.1 (s, C-1′), 128.8 (d, C-5), 127.9 (d, C-2′, 6′), 121.9 (d, C-2″), 116.0 (d, C-3′, 5′), 115.6 (s, C-10), 113.1 (s, C-8), 111.1 (d, C-6), 79.5 (d, C-2), 44.2 (t, C-3), 22.7 (q, C-4″), 22.7 (t, C-1″), 14.1 (q, C-5″). These data are consistent with the literature values (Ali et al, 2011), and hence was identified as isobavachin.
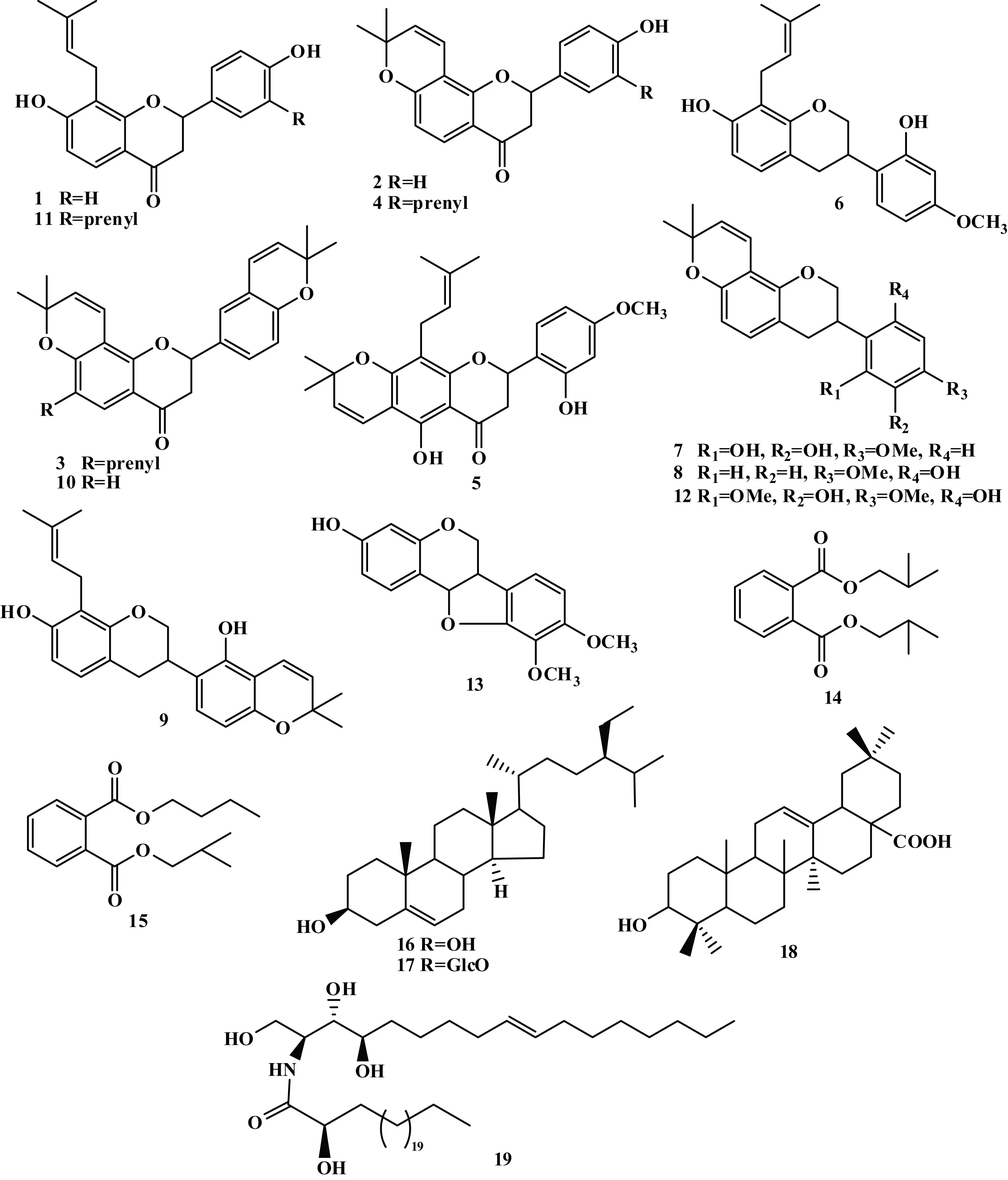
Fig. 1 Chemical structures of compounds 1-19
4′-Hydroxyisolonchocarpin (2) Yellow oil; C20H18O4;1H-NMR (CDCl3, 400 MHz)δ: 7.74 (1H, d,J= 8.7 Hz, H-5), 7.20 (2H, d,J= 8.6 Hz, H-2′, 6′), 6.81 (2H, d,J= 2.0 Hz, H-3′, 5′), 6.64 (1H, d,J= 10.5 Hz, H-1″), 6.49 (1H, d,J= 8.7 Hz, H-6), 5.55 (1H, d,J= 10.0 Hz, H-2″), 5.35 (1H, dd,J= 13.2, 2.8 Hz, H-2), 3.00 (1H, dd,J= 13.3, 3.6 Hz, H-3b), 2.79 (1H, dd,J= 16.8, 2.9 Hz, H-3a), 1.46 (6H, s, H-4″, 5″);13C-NMR (CDCl3, 100 MHz)δ: 190.5 (s, C-4), 159.6 (s, C-9), 157.8 (s, C-7), 155.4 (s, C-4′), 131.3 (s, C-1′), 128.8 (d, C-5), 127.9 (d, C-2″), 127.1 (d, C-2′, 6′), 124.3 (d, C-3′, 5′), 122.0 (d, C-1″), 116.5 (s, C-8), 116.0 (s, C-10), 111.1 (d, C-6), 79.6 (d, C-2), 77.3 (s, C-3″), 44.1 (t, C-3), 28.4 (q, C-4″), 28.1 (q, C-5″). Its1H-NMR and13C-NMR data were in accordance with those reported in the literature (Ryu et al, 2012). Therefore, Compound 2 was identified as 4′-hydroxyisolonchocarpin.
5-Deoxyeuchrenone (3) Yellow oil; C30H32O4;1H-NMR (CDCl3, 400 MHz)δ: 7.66 (1H, s, H-5), 7.13 (1H, dd,J= 2.2, 8.2 Hz, H-6′), 7.01 (1H, d,J= 2.2 Hz, H-2′), 6.74 (1H, m, H-5′), 6.57 (1H, d,J= 10.0 Hz, H-α), 6.27 (1H, d,J= 9.8 Hz, H-α′), 5.59 (1H, d,J= 9.8 Hz, H-β), 5.50 (1H, d,J= 10.0 Hz, H-β′), 5.30 (1H, dd,J= 2.8, 13.3 Hz, H-2), 5.27 (1H, bt, H-2″), 3.60 (1H, d,J= 4.5 Hz, H-1″), 2.94 (1H, m, H-3b), 2.72 (1H, dd,J= 2.9, 16.8 Hz, H-3a), 1.50 (6H, s, 2×CH3), 1.18 (12H, s, 4×CH3);13C-NMR (CDCl3, 100 MHz)δ: 192.7 (s, C-4), 166.5 (s, C-7), 156.7 (s, C-9), 152.3 (s, C-4′), 131.4 (s, C-1′), 130.3 (s, C-3″), 130.1 (d, C-2′), 129.9 (d, C-β′), 127.8 (d, C-β), 127.8 (d, C-6′), 126.9 (t, C-2″), 126.1 (d, C-α), 123.3 (s, C-5′), 120.9 (d, C-5), 115.4 (s, C-6), 115.0 (d, C-3′), 113.7 (d, C-α′), 110.1 (s, C-8), 108.3 (s, C-10), 78.6 (s, 2×-Me2C), 76.3 (d, C-2), 43.1 (t, C-3), 30.9 (t, C-1″), 29.9 (q, C-CH3), 28.7 (q, C-CH3), 28.3 (q, C-CH3), 27.4 (q, C-CH3), 26.7 (q, C-4″), 18.1 (q, C-5″). Its1H NMR and13C NMR data were identical with those reported in the literature (Mali et al, 1998). Thus, Compound 3 was identified as 5-deoxyeuchrenone.
Shinflavanone (4) Yellow powder; C25H26O4;1H-NMR (CDCl3, 400 MHz)δ: 7.74 (1H, d,J= 8.7 Hz, H-5), 7.22 (1H, d,J= 5.7 Hz, H-2′), 7.20 (1H, s, H-6′), 6.86 (1H, d,J= 8.2 Hz, H-1‴), 6.50 (1H, d,J=8.6 Hz, H-6), 6.63 (1H, d,J= 8.3 Hz, H-5′), 5.56 (1H, d,J= 9.8 Hz, H-2‴), 5.38 (1H, t,J= 2.4 Hz, H-2″ ) , 5.35 (1H, dd,J= 3.2, 12.8 Hz, H-2), 3.39 (2H, d,J= 6.7 Hz, H-1″), 3.02 (1H, dd,J= 16.8, 13.3 Hz, H-3b), 2.80 (1H, dd,J= 2.9, 16.8 Hz, H-3a), 1.78 (6H, s, H-4″, 5″), 1.47 (3H, s, H-4‴), 1.44 (3H, s, H-5‴);13C-NMR (CDCl3, 100 MHz)δ: 191.3 (s, C-4), 159.7 (s, C-9), 157.9 (s, C-7), 154.8 (s, C-4′), 134.9 (s,C-3″), 130.8 (s, C-1′), 128.8 (d, C-5), 128.0 (d, C-2′), 127.9 (s, C-3′), 127.4 (d, C-2‴), 125.4 (d, C-6′), 121.4 (d, C-2″), 116.0 (d, C-1‴), 115.8 (d, C-5′), 114.7 (s, C-8), 111.1 (s, C-10), 109.4 (d, C-6), 79.7 (s, C-3‴), 77.2 (d, C-2), 44.1 (t, C-3), 29.6 (t, C-1″), 28.4 (q, C-4‴), 28.1 (q, C-5‴), 25.8 (q, C-4″), 17.9 (q, C-5″). Compound 4 was identified as shinflavanone since its1H-NMR and13C-NMR data agreed with those reported literatures (Suh et al, 1999).
Khonklonginols H (5) Yellow oil; C26H28O6;1H-NMR (CDCl3, 400 MHz)δ: 7.18 (1H, d,J= 8.4 Hz, H-6′), 6.87 (2H, d,J= 6.6 Hz, H-1‴), 6.64 (1H, dd,J= 8.5, 2.2 Hz, H-5′), 6.32 (1H, d,J= 2.2 Hz, H-3′), 5.82 (1H, dd,J= 12.6, 2.0 Hz, H-2), 5.65 (1H, d,J= 7.0 Hz, H-2‴), 5.56 (1H, t,J= 9.7 Hz, H-2″), 3.89 (3H, s, -OCH3), 3.20 (2H, t,J= 7.0 Hz, H-1″), 3.01 (1H, dd,J= 17.6, 14.6 Hz, H-3a), 2.91 (1H, dd,J= 17.6, 3.1 Hz, H-3b), 1.76 (6H, s, H-4″, 5″), 1.46 (3H, s, H-4‴), 1.44 (3H, s, H-5‴);13C-NMR (CDCl3, 100 MHz)δ: 191.8 (s, C-4), 161.8 (s, C-4′), 159.8 (s, C-7), 158.0 (s, C-9), 157.9 (s, C-5), 154.9 (s, C-2′), 131.2 (s, C-3″), 130.5 (d, C-6′), 128.8 (d, C-2‴), 121.6 (d, C-2″), 116.0 (s, C-1′), 115.7 (t, C-1″), 114.6 (s, C-8), 113.1 (d, C-5′), 111.2 (s, C-6), 109.4 (d, C-3′), 108.1 (s, C-10), 79.8 (s, C-3‴), 77.6 (d, C-2), 56.2 (q, -OCH3), 44.0 (t, C-3), 28.4 (q, C-4‴), 28.1(q, C-5‴), 25.9 (q, C-4″), 22.4 (t, C-1″), 17.9 (q, C-5″). Its1H-NMR and13C-NMR data were in accordance with those reported in the literature (Sutthivaiyakit et al, 2009). Therefore, Compound 5 was identified as khonklonginols H.
4′-O-Methylpreglabridin (6) Yellow oil;C21H24O4;1H-NMR (CDCl3, 400 MHz)δ: 6.83 (1H, d,J= 8.2 Hz, H-5), 6.67 (1H, d,J= 8.3 Hz, H-6′), 6.60 (1H, d,J= 8.2 Hz, H-5′), 6.56 (1H, s, H-3′), 6.40 (1H, dd,J= 8.2, 8.3 Hz, H-6), 5.25 (1H, m, H-2″), 4.34 (1H, d,J= 9.1 Hz, H-2b), 3.90 (3H, s, -OCH3), 3.90 (1H, dd,J= 11.9, 6.0 Hz, H-2a), 3.41 (1H, m, H-3), 3.40 (2H, d,J= 4.8 Hz, H-1″), 2.93 (1H, ddd,J= 15.8, 10.2, 2.0 Hz, H-4), 1.81 (3H, s, H-5″), 1.74 (3H, s, H-4″);13C-NMR (CDCl3, 100 MHz)δ: 161.9 (s, C-7), 153.8 (s, C-9), 152.6 (s, C-4′), 151.7 (s, C-2′), 134.2 (s, C-3″), 129.1 (d, C-5), 127.6 (d, C-6′), 122.1 (d, C-2″), 120.8 (s, C-1′), 114.4 (s, C-8, 10), 108.1 (d, C-5′), 106.4 (d, C-3′), 97.7 (d, C-6), 69.3 (t, C-2), 56.2 (q, -OCH3), 31.6 (d, C-3), 31.0 (t, C-4), 25.8 (q, C-5″), 22.3 (t, C-1″), 17.9 (q, C-4″). Its1H-NMR and13C-NMR data were identical with those reported in the literature (Castro et al, 1986). Compound 6 was identified as 4′-O-methylpreglabridin.
3′-Hydroxy-4′-O-methylglabridin (7) White oil; C21H22O5;1H-NMR (Acetone-d6, 400 MHz)δ: 6.85 (1H, d,J= 8.2 Hz, H-5), 6.65 (1H, d,J= 2.3 Hz, H-1″), 6.63 (1H, d,J= 2.3 Hz, H-6′), 6.5 (1H, d,J= 8.6 Hz, H-5′), 6.31 (1H, d,J= 8.2 Hz, H-6), 5.64 (1H, d,J= 9.9 Hz, H-2″), 4.36 (ddd, 1H,J= 2.1, 3.4, 10.3 Hz, H-2a), 4.04 (t, 1H,J= 10.2 Hz, H-2b), 3.81 (3H, s, -OCH3), 3.51 (m, 1H, H-3), 3.04 (dd, 1H,J= 11.1, 15.6 Hz, H-4b), 2.83 (ddd, 1H,J= 1.8, 5.1, 15.7 Hz, H-4a), 1.38 (6H, s, H-4″, 5″);13C-NMR (Acetone-d6, 100 MHz)δ151.9 (s, C-7), 149.8 (s, C-9), 146.9 (s, C-4′), 143.5 (s, C-2′), 133.4 (s, C-3′), 129.3 (d, C-5), 128.7 (d, C-2″), 120.6 (s, C-1′), 116.9 (d, C-6′), 116.8 (d, C-1″), 114.6 (s, C-10), 109.6 (s, C-8), 108.4 (d, C-6), 102.9 (d, C-5′), 75.2 (s, C-3″), 69.9 (t, C-2), 55.4 (q, -OCH3), 32.0 (d, C-3), 30.2 (t, C-4), 27.1 (q, C-4″), 26.9 (q, C-5″). Compound 7 was identified as 3′-hydroxy-4′-O-methylglabridin by comparison of the1H-NMR and13C-NMR data with those reported in the literature (Kinoshita et al, 1996).
4′-O-Methylglabridin (8) White oil; C21H22O4;1H-NMR (CDCl3, 400 MHz)δ: 7.20 (1H, d,J= 8.7 Hz, H-5), 7.01 (1H, d,J= 8.7 Hz, H-6′), 6.85 (1H, d,J= 8.3 Hz, H-1″), 6.63 (1H, d,J= 12.5 Hz, H-5′), 6.39 (1H, d,J= 8.2 Hz, H-6), 6.34 (1H, d,J= 2.4 Hz, H-3′), 5.56 (1H, d,J= 9.8 Hz, H-2″), 4.37 (1H, ddd,J= 10.4, 3.3, 2.0 Hz, H-2a), 4.02 (1H, t,J= 10.4 Hz, H-2b), 3.89 (6H, s, -OCH3), 3.47 (1H, overlap, H-3), 3.00 (1H, dd,J= 10.9, 15.7 Hz, H-4b), 2.89 (1H, dd,J= 15.7, 5.3 Hz, H-4a), 1.45 (3H, s, H-4″), 1.44 (3H, s, H-5″);13C-NMR (CDCl3, 100 MHz)δ: 151.8 (s, C-4′), 149.8 (s, C-2′), 145.7 (s, C-7), 142.2 (s, C-9), 132.2 (d, C-6′), 129.2 (d, C-5), 128.9 (d, C-2″), 120.9 (s, C-1′), 117.7 (d, C-1″), 117.0 (s, C-10), 114.4 (s, C-8), 109.9 (d, C-6), 108.6 (d, C-5′), 102.6 (d, C-3′), 75.6 (s, C-3″), 69.9 (t, C-2), 56.1 (q, -OCH3), 32.0 (t, C-4), 30.4 (t, C-3), 27.8 (q, C-4″), 27.5 (q, C-5″). Its1H-NMR and13C-NMR data were in accordance with those reported in the literature (Kinoshita et al, 1996). Therefore, Compound 8 was identified as 4′-O-methylglabridin.
8-Prenyl-phaseollinisoflavan (9) Yellow oil; C25H28O4;1H-NMR (CDCl3, 400 MHz)δ: 6.90 (1H, d,J= 8.2 Hz, H-5), 6.81 (1H, d,J= 8.2 Hz, H-6′), 6.64 (1H, d,J= 10.0 Hz, H-1″), 6.49 (1H, d,J= 8.7 Hz, H-6), 6.34 (1H, d,J= 8.2 Hz, H-5′), 5.54 (1H, d,J= 9.8 Hz, H-2″), 5.28 (1H, m, H-2‴), 4.35 (1H, ddd,J= 9.8, 3.2, 2.1 Hz, H-2a), 4.08 (1H, dd,J= 9.8, 9.8 Hz, H-2b), 3.66 (1H, m, H-3), 3.34 (2H, d,J= 6.8 Hz, H-1‴), 2.94 (1H, ddd,J= 15.4, 5.5, 2.0 Hz, H-4a), 2.72 (1H, m, H-4b),1.81 (3H, s, H-5‴), 1.74 (3H, s, H-4‴), 1.46 (6H, s, H-4″, 5″);13C-NMR (CDCl3, 100 MHz)δ: 159.4 (s, C-7), 157.8 (s, C-9), 154.0 (s, C-2′), 153.2 (s, C-4′), 131.1 (s, C-3‴), 131.0 (d, C-2″), 128.9 (d, C-5), 127.9 (d, C-6′), 127.1 (d, C-2‴), 124.3 (s, C-1′), 122.0 (d, C-1″), 121.3 (s, C-10), 116.5 (s, C-8), 116.0 (s, C-3′), 111.1 (d, C-6), 109.4 (d, C-5′), 79.6 (d, C-3″), 71.8 (t, C-2), 44.2 (d, C-3), 32.0 (t, C-4), 29.7 (q, C-4″), 29.7 (q, C-5″), 27.7 (q, C-4‴), 22.7 (t, C-1‴), 19.2 (q, C-5‴). Compound 9 was identified as 8-prenyl-phaseollinisoflavan by comparison of the1H-NMR and13C-NMR data with those reported in the literature (Kinoshita et al, 1996).
Xambioona (10) Yellow powder; C25H24O4;1H-NMR (CDCl3, 400 MHz)δ: 7.74 (1H, d,J= 8.7 Hz, H-5), 7.20 (1H, dd,J= 2.1, 8.3 Hz, H-6′), 7.08 (1H, d,J= 2.1 Hz, H-2′), 6.81 (1H, m, H-5′), 6.64 (1H, d,J= 14.0 Hz, H-α), 6.49 (1H, d,J= 8.7 Hz, H-6), 6.34 (1H, d,J= 9.8 Hz, H-α′), 5.66 (1H, d,J= 9.8 Hz, H-β), 5.56 (1H, d,J= 10.0 Hz, H-β′), 5.36 (1H, dd,J= 2.7, 13.2 Hz, H-2), 3.01 (1H, m, H-3b), 2.79 (1H, dd,J= 2.9, 16.8 Hz, H-3a), 1.46 (12H, s, 4×CH3);13C-NMR (CDCl3, 100 MHz)δ: 191.0 (s, C-4), 159.6 (s, C-9), 157.8 (s, C-7), 153.3 (s, C-4′), 131.3 (d, C-5), 131.1 (s, C-1′), 128.8 (d, C-6′), 127.9 (d, C-β′), 127.1 (d, C-β′), 124.3 (d, C-2′), 122.0 (d, C-α), 121.3 (s, C-3′), 116.5 (d, C-5′), 116.0 (d, C-α′), 114.7 (s, C-8), 111.1 (d, C-6), 109.4 (s, C-10), 79.6 (d, C-2), 77.5 (s, 2×-Me2C), 44.1 (t, C-3), 28.4 (q, C-CH3), 28.2 (q, C-CH3), 28.1 (q, 2×C-CH3). These data are consistent with the literature values (Mizuno et al, 1989). Therefore, Compound 10 was identified as xambioona.
Glabrol (11) Yellow oil;C25H28O4;1H-NMR (CDCl3, 400 MHz)δ: 7.76 (1H, d,J= 8.7 Hz, H-5), 7.38 (1H, s, H-2′), 7.17 (1H, d,J= 2.5 Hz, H-6′), 6.67 (1H, d,J= 8.1 Hz, H-5′), 6.56 (1H, d,J= 8.6 Hz, H-6), 5.33 (2H, dd,J= 2.4, 13.2 Hz, H-2), 5.27 (2H, m, H-2″, 2‴), 3.75 (2H, m, H-1″, 1‴), 3.34 (1H, dd,J= 8.5, 10.6 Hz, H-3b), 2.82 (1H, dd,J= 16.8, 2.9 Hz, H-3a), 1.62 (6H, s, H-4″, 5″), 1.61 (6H, s, H-4‴, 5‴);13C-NMR (CDCl3, 100 MHz)δ: 191.5 (s, C-4), 161.4 (s, C-7), 160.7 (s, C-9), 144.5 (s, C-4′), 131.8 (s, C-1′), 131.1 (s, C-3″, 3‴), 126.5 (d, C-6′), 121.9 (d, C-5), 121.1 (d, C-2′), 121.1 (d, C-2″, 2‴), 115.5 (s, C-10), 114.9 (d, C-6), 114.5 (s, C-3′), 112.9 (d, C-5′), 110.5 (s, C-8), 79.4 (d, C-2), 44.0 (t, C-3), 29.2 (t, C-1‴), 25.8 (q, C-5″, 5‴), 22.3 (t, C-1″), 17.9 (q, C-4″, 4‴). The1H-NMR and13C-NMR data above were identical with those reported in the literature (Cho et al, 2012). Thus, Compound 11 was identified as glabrol.
Glyasperin H (12) Yellow oil; C22H24O5;1H-NMR (CDCl3, 400 MHz)δ: 6.83 (1H, d,J= 4.5 Hz, H-1″), 6.65 (1H, d,J= 8.6 Hz, H-6′), 6.63 (1H, d,J= 9.2 Hz, H-5′), 6.38 (1H, d,J= 7.0 Hz, H-6), 5.58 (1H, d,J= 11.8 Hz, H-2″), 4.35 (1H, d,J= 8.0 Hz, H-2a), 3.99 (1H, d,J= 9.2 Hz, H-2b), 3.89 (6H, s, 2×OCH3), 3.54 (1H, m, H-3), 2.92 (1H, d,J= 11.1 Hz, H-4b), 2.84 (1H, d,J= 15.8 Hz, H-4a), 1.43 (3H, s, H-5″), 1.42 (3H, s, H-4″), 7.74 (1H, d,J= 8.7 Hz, H-5);13C-NMR (CDCl3, 100 MHz)δ: 151.9 (d, C-7), 149.7 (s, C-9), 146.6 (s, C-4′), 145.3 (s, C-2′), 138.7 (s, C-3′), 129.2 (s, C-5), 129.0 (d, C-2″), 127.5 (s, C-1′), 117.0 (d, C-6′), 116.9 (d, C-1″), 114.4 (s, C-10), 109.9 (s, C-8), 108.7 (d, C-6), 106.5 (d, C-5′), 75.6 (s, C-3″), 70.6 (t, C-2), 61.1 (q, 2′-OCH3), 56.2 (q, 4′-OCH3), 31.6 (d, C-3), 31.6 (t, C-4), 27.8 (q, C-5″), 27.5 (q, C-4″). Compound 12 was identified as glyasperin H by comparison of the1H-NMR and13C-NMR data with those reported in the literature (Sairafianpour et al, 2002).
Methylnissolin (13) White oil; C17H16O5;1H-NMR (CD3OD, 400 MHz)δ: 7.47 (1H, d,J= 8.5 Hz, H-1), 7.01 (1H, d,J= 4.2 Hz, H-7), 6.66 (1H, d,J= 2.2 Hz, H-8), 6.55 (1H, dd,J= 8.2, 2.2 Hz, H-2), 6.48 (1H, d,J= 8.7 Hz, H-4), 5.57 (1H, d,J= 6.2 Hz, H-11a), 4.28 (1H, dd,J= 9.6, 3.4 Hz, H-6e), 3.84 (3H, s, 9-OCH3), 3.82 (3H, s, 10-OCH3), 3.81 (1H, m, H-6a), 3.33 (1H, m, H-6);13C-NMR (CD3OD, 100 MHz)δ: 158.6 (s, C-3), 156.5 (s, C-4a), 154.8 (s, C-9), 152.8 (s, C-11b), 131.8 (s, C-10), 129.8 (d, C-1), 122.0 (s, C-6b), 118.5 (d, C-7), 110.3 (s, C-1a), 104.8 (d, C-2), 104.2 (d, C-8), 102.8 (d, C-4), 78.9 (d, C-11a), 66.0 (t, C-6), 61.0 (q, -OCH3), 55.5 (q, -OCH3), 39.8 (d, C-6a). Compound 13 was identified as methylnissolin by comparison of the1H-NMR and13C-NMR data with the data reported in the literature (Lee et al, 2008).
Phthalic acid isodibutyl ester (14) Yellow powder; C16H22O4;1H-NMR (CDCl3, 400 MHz)δ: 7.74 (2H, m, H-3, 6), 7.53 (2H, m, H-4, 5), 4.10 (2H, d,J= 7.2 Hz, H-1′), 2.04 (1H, m, H-2′), 0.99 (6H, d,J= 7.2 Hz, H-1″, 3′);13C-NMR (CDCl3, 100 MHz)δ: 167.6 (s, C-α), 132.3 (s, C-2), 132.3 (s, C-1), 130.8 (d, C-6), 130.8 (d, C-3), 128.7 (d, C-5), 128.7 (d, C-4), 71.8 (t, C-1′), 29.7 (q, C-2′), 19.2 (q, C-3′), 19.2 (q, C-4′). Its1H-NMR and13C-NMR data were identical with those reported in the literature (Zhang et al, 2003). So, Compound 14 was identified as hthalic acid isodibutyl ester.
Butul isobutyl phthalate (15) Yellow oil; C16H22O4;1H-NMR (CDCl3, 400 MHz)δ: 7.74 (2H, m, H-3, 6), 7.53 (2H, m, H-4, 5), 4.10 (2H, d,J= 7.2 Hz, H-1′), 2.04 (1H, m, H-2′), 0.99 (6H, d,J= 7.2 Hz, H-1″, 3′);13C-NMR (CDCl3, 100 MHz)δ: 167.8 (s, C-α), 132.9 (s, C-1, 2), 130.9 (d, C-4, 5), 128.8 (d, C-3, 6), 71.8 (t, C-1′), 67.7 (t, C-1″), 29.7 (t, C-2′), 27.7 (d, C-2″), 19.2 (q, C-3″), 18.5 (t, C-3′), 14.1 (q, C-4′). These data are consistent with the literature values (Liu et al, 2011), and Compound 15 was therefore identified as butul isobutyl phthalate.
β-Sitosterol (16) White powder. The compound was developed withβ-sitosterol standard on co-TLC experiments eluted with different solvent systems, and they had same Rfvalues. Therefore, it was identified asβ-sitosterol.
Daucosterol (17) White powder. This compound was identified by co-TLC experiments and it showed the same Rfvalues with daucosterol standard in different develop systems. Therefore, it was determined as daucosterol.
Oleanic acid (18) White powder. By co-TLC experiments, it was identified as oleanic acid due to the same Rfvalues with oleanic acid standard in different elution systems.
(2S,3S,4R,9E)-1,3,4-Trihydroxy-2- [(2′R)-2′-hydroxytetracosanoylamino]-9-octadecene (19) White powder; C42H83O5N; EI-MS: 681 [M]+;1H-NMR (CD3OD, 400 MHz)δ: 8.56 (1H, d,J= 8.7 Hz, NH), 5.52 (2H, m, H-9 and H-10), 5.08 (1H, m, H-2), 4.60 (1H, dd,J= 7.5, 3.6 Hz, H-2′), 4.47 (1H, dd,J= 10.8, 4.8 Hz, H-1a), 4.41 (1H, dd,J= 10.8, 4.6 Hz, H-1b), 4.32 (1H, dd,J= 6.2, 5.1 Hz, H-3), 4.26 (1H, m, H-4), 2.15-2.18 (4H, m, H-5a, H-8a, H-9a and H-3′a), 1.94-2.05 (5H, m, H-5b, H-8b, H-9b, H-3′b and H-4′a), 1.71-1.77 (3H, m, H-6a, H-6b and H-4′b), 1.26-1.32 (methylene band), 0.87 (6H, brt,J= 7.0 Hz, H-18 and H-24′);13C-NMR (CD3OD, 100 MHz)δ: 175.2 (s, C-1′), 131.0 (d, C-9 or C-10), 130.8 (d, C-9 or C-10), 77.0 (d, C-3), 73.0 (d, C-4), 72.6 (d, C-2′), 62.1 (t, C-1), 53.1 (d, C-2), 35.8 (t, C-3′), 34.0 (t, C-5), 33.5 (t, C-8), 33.1 (t, C-11), 32.3 (t, C-16′ and C-22′), 29.6-30.4 (methylens), 26.9 (t, C-4′), 26.0 (t, C-6), 23.1 (t, C-17 and C-23′), 14.4 (q, C-18 and C-24′). Compound 19 was identified as (2S, 3S, 4R, 9E)-1, 3, 4-trihydroxy-2- [(2′R)-2′-hydroxytetracosanoylamino]-9-octadecene by comparison of the1H-NMR and13C-NMR data above with those reported in the literature (Su et al, 2002).
ALI MS, ALI MI, ONOCHA PA,et al, 2011. Bis-Sigmodiol: a new prenylflavanone dimer fromErythrinasigmoideaHua (Fabaceae) of Nigeria [J]. J Asian Nat Prod Res, 13:182-187.
CASTRO O, LOPEZ J, VERGARA A. 1986. Isoflavans and a stilbene from wood of the decay-resistant tropical treeDiphysarobinioides[J]. J Nat Prod, 49:680-683.
CHINA FLORA EDITORIAL BOARD, CHINESE ACADEMY OF SCIENCE, 1993. The Flora of China [M]. Beijing: Science Press:94.
CHO S, PARK JH, PAE AN, 2012. Hypnotic effects and GABAergic mechanism of licorice (Glycyrrhizaglabra) ethanol extract and its major flavonoid constituent glabrol [J]. Bioorg Med Chem, 20:3493-3501.
(Continueonpage1352)(Continuefrompage1388)
KINOSHITA T, KAJIYAMA K, HIRAGA Y, et al, 1996. Isoflavan derivatives fromglycyrrhizaglabra(licorice) [J]. Heterocycles, 43:581-588.
KUNMING INSTITUTE OF BOTANY,CHINESE ACADEMY OF SCIENCE, 2006. Flora of Yunnan [M]. Beijing: Science Press: 733.
LEE EJ, YEAN MH, JUNG HS, et al, 2008. Phytochemical studies onAstragalusroot (2)-flavonoids and a lignan [J]. Natl Prod Sci, 14:131-137.
LIU M, ZHANG W, QIU L, et al, 2011. Synthesis of butyl-isobutyl-phthalate and its interaction with α-glucosidaseinvitro[J]. J Biochem, 1:27-33.MALI RS, KULKARNI-JOSHI P, 1998. Synthesis of 6-prenylpyranoflavanones: Total synthesis of (+/-)-maxima flavanone A [J]. Ind J Chem, Section B: Org Chem Incl Med Chem, 38:596-599.MIZUNO M, TAMURA KI, TANAKA T, et al, 1989. Six flavanones from the roots ofEuchrestaformosana[J]. Phytochemistry, 10:2811-2812.
RYU HW, LEE JH, KANG JE, et al, 2012. Inhibition of xanthine oxidase by phenolic phytochemicals fromBroussonetiapapyrifera[J]. J Kor Soc Appl Biol Chem, 55:587-594.
SAIRAFIANPOUR M, KAYSER O, CHRISTENSEN J, et al, 2002. Leishmanicidal and antiplasmodial activity of constituents ofSmirnowiairanica[J]. J Nat Prod, 65:1754-1758.
SU BN, MISICO R, PARK EJ, et al, 2002. Isolation and characterization of bioactive principles of the leaves and stems ofPhyaslisphiladelphica[J]. Tetrahedron, 58:3453-3466.SUH H, LEE S,KIM N, et al, 1999. Syntheses of (+/-)-shinflavanone and its structural analogues as potent inhibitors of bone resorption pits formation [J]. Bioorg Med Chem Lett, 9:1433-1436.SUTTHIVAIYAKIT S, THONGNAK O, LHINHATRAKOOL T, et al, 2009. Cytotoxic and antimycobacterial prenylated flavonoids from the roots ofEriosemachinense[J]. J Nat Prod, 72:1092-1096.ZHANG W, LOU HX, LI GY, et al, 2003. A new triterpenoid fromEntodonokamuraebroth [J]. J Asian Nat Prod Res, 3:189-195.
2015-07-29
2015-12-20
国家自然科学基金(31170313) [Supported by the National Natural Science Foundation of China (31170313)]。
韩冰洋(1987-),男,湖北襄阳人,硕士,主要从事药用植物研究,(E-mail) hby31510@163.com。
类芒齿黄芪地下部分化学成分研究
韩冰洋, 张 宇, 田新雁, 肖朝江, 董 相, 姜 北*
( 大理大学 药物研究所, 云南 大理 671000 )
为了解类芒齿黄芪(Astragaluscamptodontoides)主要化学成分,从其地下部分甲醇提取物的乙酸乙酯部位分离出19个单体化合物,通过现代波谱分析及理化性质等手段分别鉴定为异补骨脂黄酮 (1),4′-hydroxyisolonchocarpin (2),5-去氧山豆根黄酮(3),shinflavanone (4),khonklonginols H (5),4′-O-methylpreglabridin (6),3′-hydroxy-4′-O-methylglabridin (7),4′-O-methylglabridin (8),8-prenyl-phaseollinisoflavan (9),xambioona (10),光甘草酚 (11),粗毛甘草素H (12),methylnissolin (13),邻苯二甲酸异丁酯 (14),邻苯二甲酸丁酯异丁酯 (15),β-谷甾醇(16),胡萝卜苷(17),齐墩果酸(18),(2S,3S,4R,9E)-1,3,4-trihydroxy-2- [(2′R)-2′- hydr-oxytetracosanoylamino]-9-octadecene (19)。化合物1~19均为首次从该植物中获得,化合物1~7为首次从黄芪属(Astragalus)植物中分离得到。
类芒齿黄芪, 地下部分, 化学成分, 分离与鉴定
10.11931/guihaia.gxzw201505031
*通讯作者: 姜北,博士,教授,主要从事天然药物、民族医药研究,(E-mail) dalinorthjiang@163.com。
Q946.8 Document code: A Article ID: 1000-3142(2016)11-1382-08
韩冰洋, 张宇, 田新雁, 等. 类芒齿黄芪地下部分化学成分研究 [J]. 广西植物,2016,36(11):1382-1388HAN BY, ZHANG Y, TIAN XY, et al. Chemical constituents from underground part ofAstragaluscamptodontoides[J]. Guihaia, 2016, 36(11):1382-1388
