基于偶氮联吡啶配体的银和钴配位聚合物的构筑与性质
2016-12-15蹇源彭述明刘宁李明凤何成
蹇源 彭述明 刘宁 李明凤 何成*,
基于偶氮联吡啶配体的银和钴配位聚合物的构筑与性质
蹇源1,2彭述明1,2刘宁1李明凤3何成*,3
(1中国工程物理研究院核物理与化学研究所,绵阳621900)
(2四川大学辐射物理及技术教育部重点实验室,原子核科学技术研究所,成都610064)
(3大连理工大学精细化工国家重点实验室,大连116024)
通过将2个4,4′-联吡啶基团用偶氮基团连接,我们合成了新的配体顺式-和反式-1,2-二((4,4′-联吡啶)-3-氮烯)(cis-L和trans-L),并利用trans-L与银离子和钴离子构筑了配位聚合物{[Ag2(trans-L)(ClO4)2]·4CH3CN}n(1)和{[Co(trans-L)2(H2O)2](ClO4)2}n(2)。其中1为一维梯形链,链与链之间通过π-π以及Ag…Ag相互作用堆积;2为三维无限dendrimer结构,其Co中心具有合适的氧化还原电位,在以荧光素为光敏剂的条件下,可作为光催化剂实现光解水放氢。
偶氮联吡啶;配位聚合物;银离子;钴离子;光催化放氢
0 Introduction
Metal-organic coordination polymers have received intense interests,especially in the fast developed field on metal-organic frameworks(MOFs) with well defined pore architectures,due to their tunable structural features and high surface areas[1-3]. The research for these materials with functional properties had led to intense studies because they possess advantageous cumulative attributes emerging from both metal and ligand components[4-5].The structural and functional diversity of thesecoordination polymers has brought these materials wide attention in recent years.They had the tenability,versatility and original flexibility,of which place themselves at the frontier between zeolites and enzymes[6-8].
Compounds containing azo groups are well known for their excellent photoisomerizable properties[9-12]. The MOFs constructed with azo-based ligand also have shown interesting application on such as the photoswitchable CO2adsorption capacity[13-18].Among the azo-based ligands,azo-4,4′-bipyridine,in which two pyridine rings linked by the azo moiety,has been used to construct several MOFs which exhibited a wide variety of architectures and attractive electric/ magnetic properties due to its controllability on the coordination configuration of the metal centers[19-20]. Herein,we extend the construction strategy of azopyridine by linking two 4,4′-bipyridine groups by an azo linker.The syntheses of two silver and cobalt complexes were reported respectively,and the application of Co complex as a catalyst on the light driven hydrogen evolution is shown.
1 Experimental
1.1 Reagents and measurements
All chemicals were of reagent grade quality obtained from commercial sources and used without further purification.The elemental analyses of C,H and N were performed on a Vario ELⅢelemental analyzer.1H NMR spectra were measured on a Varian INOVA 400M spectrometer.Solution fluorescent spectra were measured on JASCO FP-6500.Both excitation and emission slit widths were 2 nm.
Electrochemical measurements were carried under nitrogen at room temperature,performed on ZAHNER ENNIUM Electro-chemical Workstation with a conventional three-electrode system with a homemade Ag/AgClelectrode as a reference electrode, a platinum silk with 0.5 mm diameter as a counter electrode,and glassy carbon electrode as a working electrode.The solution concentrations in cyclic voltammograms were ca.1.0 mmol·L-1for the cobaltbased compounds and 0.1 mol·L-1for the supporting electrolyte,(n-Bu4N)PF6.The addition of NEt3HCl (0.1 mmol·L-1in DMF)was carried out with syringe.
Photoinduced hydrogen evolution was performed in a 40 mL flask.The flask was sealed with a septum, pre-degassed by bubbling nitrogen for 15 min under atmospheric pressure at room temperature.In the experiment,the catalyst was solved in little amount of DMF,and the EtOH/H2O solution(1∶1,V/V)containing fluorescein(FL)and triethylamine were added with the total volume of 5.0 mL.The pH value of the solution was adjusted to a suitable pH value by adding HCl or NaOH and was measured by the pH value meter. Then the samples were irradiated by a 500 W Xenon lamp,and the reaction temperature was maintained at 293 K by using a water filter to absorb heat.The generated photoproduct of H2was characterized by GC 7890T instrument analysis using a 5A molecular sieve column(0.6 m×3 mm),thermal conductivity detector, and nitrogen used as carrier gas.The amount of H2generated was determined by the external standard method.Hydrogen in the resulting solution was not measured and the slight effect of the hydrogen gas generated on the pressure of the flask was neglected for calculation ofthe volume ofhydrogen gas.

Scheme 1 Synthesis of trans-and cis-1,2-di((4,4′-bipyridine)-3-yl)diazene
1.2 Syntheses and characterizations
1.2.1 Synthesis of trans-1,2-di((4,4′-bipyridine)-3-yl)diazene acetate(trans-L acetate)
3-amino-4,4′-bipyridine(0.8 g,5 mmol)[21]was added to 40 mL water at 0℃,then NaOCl(80 mL) was added,and the reaction mixture was stirred for 24 h(Scheme 1).After filtering the reaction mixture, the filtrate was then reduced in volume in vacuo to afford the orange solid.The solid then recrystallization in glacial acetic acid to give the trans-L acetate. Yield:0.65 g(46%).Anal.Calcd.for C28H30N6O8(%):C 58.13,H 5.23,N 14.53;Found(%):C 58.33,H 5.19, N 14.32.1H NMR(400 MHz,CDCl3):δ2.23(s,6H) 7.45(d,2H),7.51(d,1H),7.71(s,1H),7.76(d,2H), 8.81(d,1H).
1.2.2 Synthesis of cis-1,2-di((4,4′-bipyridine)-3-yl) diazene(cis-L)
When the aforementioned orange solid recrystallized in CH2Cl2,the pure cis-L was obtained. Yield:0.34 g(40%).Anal.Calcd.for C20H14N6(%):C 70.99,H 4.17,N 24.84;Found(%):C 58.33,H 5.19, N 14.32.1H NMR(400 MHz,CDCl3):δ7.35(d,1H), 7.38(d,2H),7.60(s,1H),8.57(d,2H),8.71(d,1H).
1.2.3 Synthesis of{[Ag2(trans-L)(ClO4)2]·4CH3CN}n(1)
Complex 1 was obtained from the mixture of trans-L acetate(43 mg,0.075 mmol)in dichloromethane and AgClO4(31 mg,0.15 mmol)in acetonitrile as orange crystals.Yield:41 mg(60%). Anal.Calcd.for C28H26Ag2Cl2N10O8(%):C 36.67,H 2.86,N 15.27;Found(%):C 36.22,H 2.76,N 14.84. >
1.2.4 Synthesis of{[Co(trans-L)2(H2O)2](ClO4)2}n(2)
Complex 2 was obtained from the mixture of trans-L acetate(86 mg,0.15 mmol)in dichloromethane and Co(ClO4)2·6H2O(27 mg,0.075 mmol)in methanol after two days as orange red crystals.Yield:29 mg(40%).Anal.Calcd.for C40H32Cl2Co N12O10(%):C 49.50,H 3.32,N 17.32; Found(%):C 49.92,H 3.52,N 17.80.
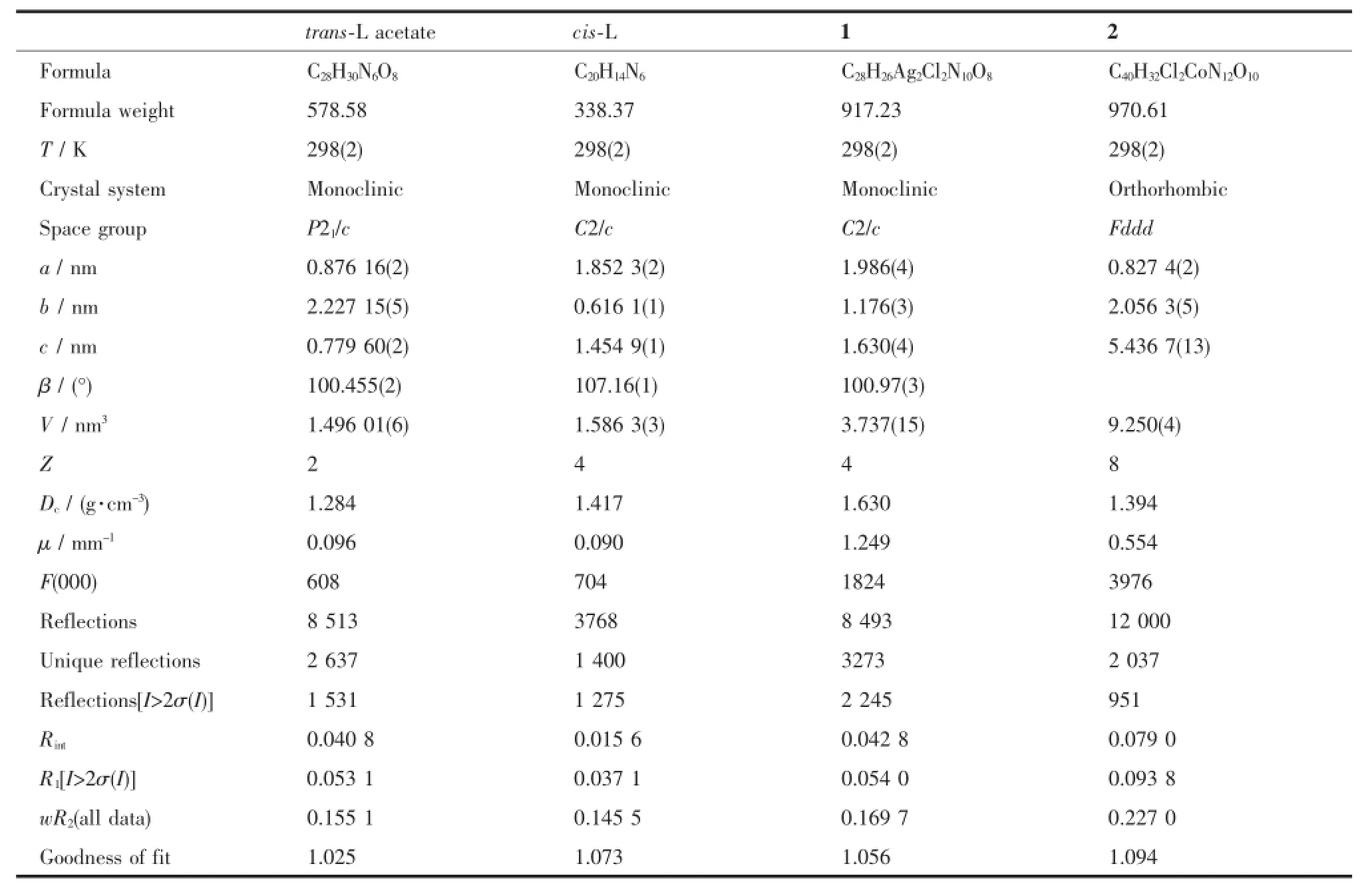
Table 1 Crystal data of the complexes
1.3 Crystallography
The intensities of the complexes were collected on a Bruker SMART APEX CCD diffractometer with graphite-monochromated Mo Kα(λ=0.071 073 nm) using the SMART and SAINT programs[22].The structure was solved by direct methods and refined on F2by full-matrix least-squares methods withSHELXTL program[23].In all of the structural refinements,the non-hydrogen atoms were refined anisotropically.Hydrogen atoms were fixed geometrically at calculated distances and allowed to ride on the parent non-hydrogen atoms.For the refinement of 2,one of the perchlorate ions was restrained with idealized distance on the Cl-O bond. Crystaldata ofthe complexes were listed in Table 1.
2 Results and discussion
Ligand 1,2-di((4,4′-bipyridin)-3-yl)diazene was synthesized via the condensation of 3-amino-4,4′-bipyridine,and both trans-and cis-forms of the compound were present in the product.The cis-form was separated directly,and the trans-form was recrystallized from ice acetic acid as an acetate compound.The cis-configuration ligand crystallized in a space group of C2/c,with the two bipyridine groups sitting in the same side of N=N bond.While the transform ligand crystallized as an acetate salt in a space group of P21/c,with the two bipyridine groups sitting in the opposite side of N=N bond.The N=N distances of the azo group in both form are same(0.125 6(3) nm).
Both the trans-and cis-form ligand reacted with metal ion Ag and Co,however,only the trans-form gave the crystalline compound suitable for X-ray crystal analysis,perhaps due to the lower stretching force for the trans-configuration which is more suitable to form stable complex when coordinated with the metal ions.Complex 1 crystallized in C2/c space group.Single crystal analysis showed that there are one Ag ion,one half ligand,one perchlorate counter ion and two acetonitrile solvent molecules present in an asymmetric unit.It could be found that all the four N atom of the pyridine rings coordinated to the Ag ions,and each Ag ion was coordinated with two pyridine N atoms from two different ligands in a linear way with the Ag-N bond distance being 0.224 nm on average.The structure could be described as two infinite chain of alternating Ag(Ⅰ)separated by 1.176 nm linked by the azo group to form an infinite ladder chain.The closest introchain Ag…Ag distance is 0.672 nm.The pairs of ladder chains stack together byπ…πinteractions between the ligands and in which the ligands lie parallel to each other with a distance of 0.346 nm,and the weak interchain Ag…Ag interaction with a Ag…Ag distance of 0.365 nm between the differentladder chains,forming a stair up two-dimensional sheet.

Fig.1 Structure of cis-form(left)and trans-form(right)of liagnd 1,2-di((4,4′-bipyridin)-3-yl)diazene
Complex 2 crystallized in Fddd space group. Single crystal analysis showed that the complex exists as a three-dimensional polymer in which each Co(Ⅱ)is coordinated in an octahedral geometry to four ligands and two water molecules occupied on the axialplaces. In this complex,there are only two opposite N atoms on the different pyridine rings(N1 and N1A as shown in Fig.1)of the ligand participate in the coordination to metal centers,thus the ligand act as aμ2-bridge ligand,the four ligand coordinated in one Co centerextend in a stair-up way,and the whole 3D structure compose of several infinite chains.It could not be found a cycle rings in the structure,thus the structure of 2 could be described as a dendrimer.Nevertheless, viewed from a axle,the structure seems to have a one dimension channel(Fig.3).
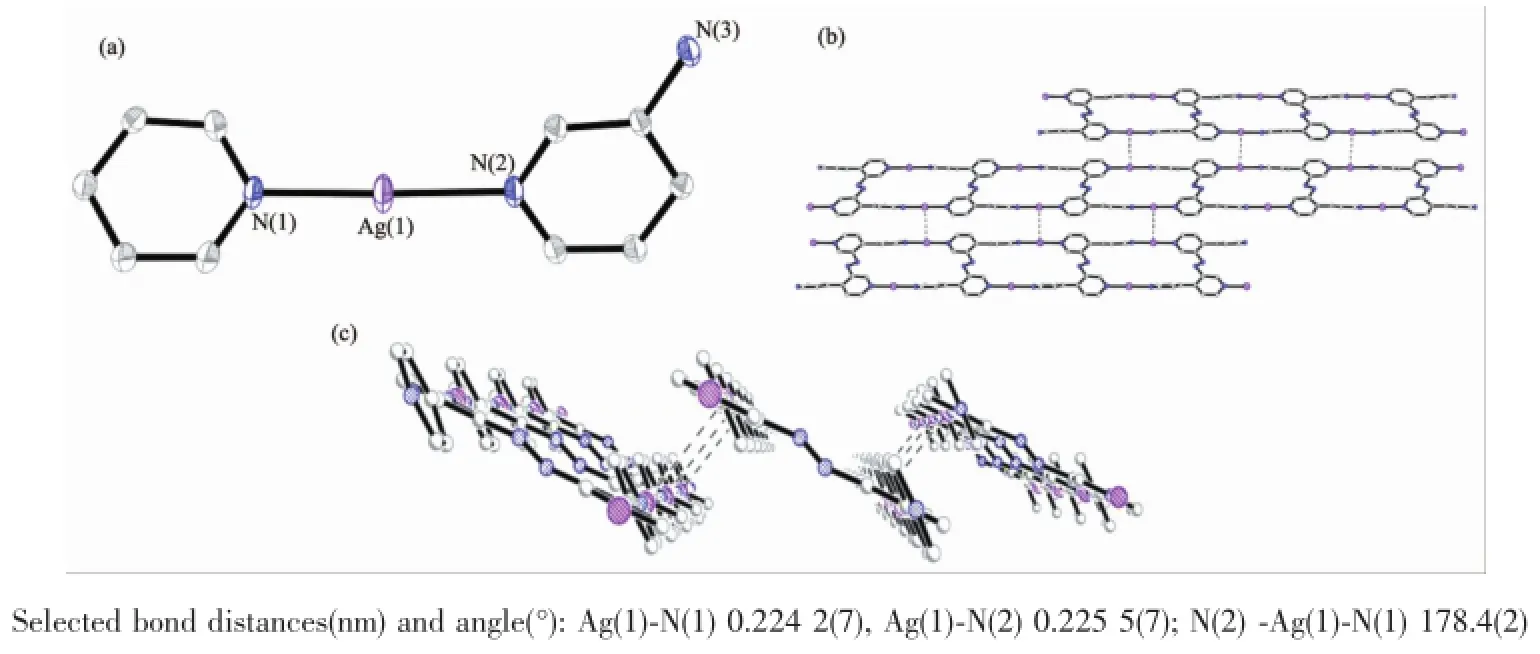
Fig.2(a)Coordination environment of Ag center in 1 with 30%probability displacement ellipsoids;Top(b) and side(c)view of complex 1
Solar energy conversion of water into the environmentally clean fuel hydrogen offers one of the best long-term solutions for meeting future energy demands.Current solar fuel research involves developing these molecular based systems containing a photosensitizer for light absorption,a catalyst for H2generation,an electron source for proton reduction and a means of electron transfer to the catalyst.The coordination atoms around the Co ions in this structure were quite similar with that of the normal used[Co(dmgBF2)2(OH2)2]photocatalyst[24],and 2 is soluble in DMF solution,providing the possibility of using it in the homogeneous light driven hydrogen production system.

Fig.3(a)Coordination environment of Co center in 2;View of compound 2 from a(a),b(b)and c(c)axle
Cyclic voltammogram of 2 in a DMF solution exhibited an irreversible Co2+/Co+redox process at -0.67 V(vs Ag/AgCl).Photolysis of a solution of FL (photosensitizer,3.0 mmol·L-1)and 2(20μmol·L-1)in a solvent mixture containing triethylamine(NEt3, electron donor,10%,V/V)in H2O/EtOH(1∶1,V/V)at25℃results the direct H2generation.The volume of H2was quantified at the end of the photolysis by GC analysis of the headspace gases[25].The amount of H2generation in 12 hours photolysis maximizes at pH 10.5(Fig.4a).The increase of pH values really decreased the efficiency,due to the lower proton concentration in solution and the fact that the H2generation became more thermodynamically unfavourable with increasing pH value.Whereas at lower pH value,the potential protonation of NEt3diminishes its ability to function as an electron donor, and the NEt3decomposition becomes less facile[26].The light induced H2evolution depends on the concentration of sacrificial reagent NEt3,the optimal concentration is 10%with a decrease in activity at both lower and higher concentration.The turnover number(TON)calculated was about 340 moles H2per mole of catalyst.Control experiment of this system without either the complex 2,the FL or the NEt3were carried out,the absence of any of them yielded unobservable amount of H2,demonstrating that they are essential for H2generation.Moreover,these artificial photosynthetic systems could not work well in the absence of the light.With the fixed concentration of FL(2 mmol·L-1),the TON reach the platform value when the concentration of 2 varied to 20μmol·L-1,further addition of 2 causes a very little hydrogen evolution enhancement corresponding to the catalyst(Fig.4b).These results suggest FL were decomposed during the photolysis,similar to those related systems containing photosensitizer FL and NEt3as sacrificialelectron donor[26].
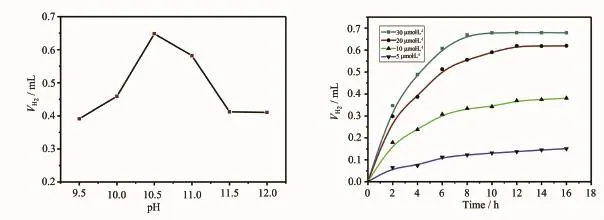
Fig.4(a)Hydrogen production of the systems containing 2(20μmol·L-1),FL(3.0 mmol·L-1)and NEt3(10%,V/V)in EtOH/H2O (1∶1,V/V)with the pH value varied;(b)Hydrogen production of the systems containing FL(3.0 mmol·L-1),NEt3(10%,V/V) in EtOH/H2O(1∶1,V/V)at pH=11.0 in 5 mL solution with different concentrations of the redox catalyst 2
3 Conclusions
In a summary,we prepared two metal organic coordination polymer assembled by Ag ion or Co ion with the azo based ligand trans-L.The complex 1 forms a one dimension infinite ladder chain,while the structure of complex 2 is a 3D infinite chain model. The application of the Co complex(2)as a catalyst is showed on the light driven hydrogen evolution.
[1]Long J R,Yaghi O M.Chem.Soc.Rev.,2009,38:1213-1214
[2]Xu Y,Luo F,Che Y X,et al.Inorg.Chem.Commun.,2009, 12:639-641
[3]Hasegawa S,Horike S,Matsuda R,et al.J.Am.Chem.Soc., 2007,129:2607-2614
[4]Han S S,Mendoza-Cortés J L,GoddardⅢW A.Chem.Soc. Rev.,2009,38:1460-1476
[5]Rosi N L,Kim J,Eddaoudi M,etal.J.Am.Chem.Soc.,2005, 127:1504-1518
[6]Yoon,M,Srirambalaji R,Kim K.Chem.Rev.,2012,112: 1196-1231
[7]Lee J Y,Farha O K,Roberts J,etal.Chem.Soc.Rev.,2009, 38:1450-1459
[8]Luo F,Che Y X,Zheng J M.Inorg.Chem.Commun.,2008, 11:142-144
[9]Wegner H A.Angew.Chem.Int.Ed.,2012,51:4787-4788
[10]Stoll R S,Hecht S.Angew.Chem.Int.Ed.,2010,49:5054-5075
[11]Yu H,Ikeda T.Adv.Mater.,2011,23:2149-2180
[12]Beharry A A,Woolley G A.Chem.Soc.Rev.,2011,40:4422-4437
[13]Lyndon R,Konstas K,Ladewig B P,et al.Angew.Chem. Int.Ed.,2013,52:3695-3698
[14]Buyukcakir O,Je S H,Park J,et al.Chem.Eur.J.,2015, 21:15320-15327
[15]Brown J W,Henderson B L,Kiesz M D,et al.Chem.Sci., 2013,4:2858-2864
[16]Modrow A,Zargarani D,Herges R,et al.Dalton Trans., 2011,40:4217-4222
[17]Modrow A,Zargarani D,Herges R,et al.Dalton Trans., 2012,41:8690-8696
[18]Gong L L,Feng X F,Luo F.Inorg.Chem.,2015,54:11587 -11589
[19]He C,Zhang B G,Duan C Y,et al.Eur.J.Inorg.Chem., 2000:2549-2554
[20]Halder G J,Kepert C J,Moubaraki B.Science,2002,298: 1762-1765
[21]Zhang L J,Jian Y,Wang J,et al.Dalton Trans.,2012,41: 10153-10155
[22]SMART and SAINT,Area Detector Control and Integration Software,Siemens Analytical X-ray Systems,Inc.,Madison, WI,1996.
[23]Sheldrick GM.SHELXTLVer5.1,Software Reference Manual, Bruker AXS,Inc.,Madison,WI,1997.
[24]Dong J F,Wang M,Zhang P.J.Phys.Chem.C,2011,115: 15089-15096
[25]Zhang P,Wang M,Na Y,et al.Dalton Trans.,2010,39: 1204-1206
[26]McNamara W R,Han Z,Alperin P J,et al.J.Am.Chem. Soc.,2011,133:15368-15371
Structures of Silver and Cobalt Coordination Polymers Constructed with Azo-Bipyridine Ligand
JIAN Yuan1,2PENG Shu-Ming1,2LIU Ning1LIMing-Feng3HE Cheng*,3
(1Institute of Nuclear Physics and Chemistry,China Academy of Engineering Physics,Mianyang,Sichuan 621900,China)
(2Key Laboratory of Radiation Physics and Technology(Sichuan University),Ministry of Education;Institute of Nuclear Science and Technology,Sichuan University,Chengdu 610064,China)
(3State Key Laboratory of Fine Chemicals,Dalian University of Technology,Dalian,Liaoning 116024,China)
By linking two 4,4′-pyridine groups through an azo linker,ligand trans-and cis-1,2-di((4,4′-bipyridine)-3-yl)diazene(trans-and cis-L)were obtained.And the syntheses oftwo silver and cobaltcomplexes{[Ag2(trans-L) (ClO4)2]·4CH3CN}n(1)and{[Co(trans-L)2(H2O)2](ClO4)2}n(2)with the trans-form ligand are reported respectively.The complexes exhibited diverse structure features.And the application of Co complex as a photocatalystwas showed on the lightdriven hydrogen evolution.CCDC:1497801,trans-L acetate;1497799,cis-L;1497798,1;1497800,2.
azo-bipyridine;coordination polymer;Ag ion;Co ion;light driven hydrogen production
O614.81+2;O614.122
A
1001-4861(2016)09-1676-07
10.11862/CJIC.2016.211
2016-04-18。收修改稿日期:2016-08-07。
国家自然科学基金(No.21531001)资助项目。
*通信联系人。E-mail:hecheng@dlut.edu.cn
