单分散g-C3N4量子点修饰一维棒状BiPO4微晶的合成及其对光催化活性增强机理
2016-12-05王丹军申会东岳林林
王丹军 申会东 郭 莉,2 岳林林 付 峰
(1延安大学化学与化工学院,陕西省化学反应工程重点实验室,延安716000) (2陕西师范大学材料科学与工程技术学院,西安716000)
单分散g-C3N4量子点修饰一维棒状BiPO4微晶的合成及其对光催化活性增强机理
王丹军*,1申会东1郭莉1,2岳林林1付峰*,1
(1延安大学化学与化工学院,陕西省化学反应工程重点实验室,延安716000) (2陕西师范大学材料科学与工程技术学院,西安716000)
利用水热法合成了一维棒状BiPO4微晶,在此基础上采用浸渍-焙烧法进行g-C3N4量子点表面修饰获得新颖的g-C3N4/ BiPO4异质结。借助X射线衍射(XRD)、场发射扫描电镜(FE-SEM)、透射电镜(HRTEM)、能谱(EDS)、紫外-可见漫反射(UV-Vis-DRS)等测试手段对所得样品的相组成、形貌和谱学特征进行了表征。选择罗丹明B(RhB)和苯酚作为模型污染物研究了所得在可见光下的催化活性。结果表明,样品16%(w/w)g-C3N4/BiPO4对RhB降解的速率常数分别是纯g-C3N4和BiPO4的4.6倍和15倍。g-C3N4量子点与BiPO4之间形成异质结,抑制了光生电子-空穴对的复合,从而提高了催化剂的活性。自由基捕获实验进一步表明,超氧负离子自由基(·O2-)是催化降解RhB和苯酚的主要活性物种。
一维棒状BiPO4微晶;g-C3N4量子点;表面修饰;活性增强机理
0 Introduction
During the past few decades,semiconductorbased photocatalysis has been widely investigated for its potential application in environmental remediation and solar energy transformation.Up to date,the most strategy to construct efficient visible-responsive photocatalyst is to extend the light absorption range,and prolong the life of photogenerated charge carriers by element doping and surfacemodification[1-3].Unfortunately,element doping is difficult to be controlled,and low thermal stability,which limits its application[4-5]. Therefore,the surface modification has become an important strategy is to develop more efficient photocatalyst with visible light responsiveness and low recombination rate of photogenerated electron and holes[6-8].
As one of an important Bi-based photocatalyst materials,BiPO4has receivedmuch attention owing to itpotential applications as a oxy-acid saltphotocatalyst with wide band-gap and high separation efficiency of e-/h+pairs[9].Moreover,PO43-helps the e-/h+separation, which plays an important role in its excellent photocatlaytic activity.However,the potential application of BiPO4is limited by inherent constraints such as inefficient use of the visible portion and low lifetime of photogenerated carriers.So,the photocatalytic efficiency of BiPO4needs further enhancement prior to practical applications[10-13].Graphitic carbon nitride(g-C3N4)is a novelmetal free organic photocatalystwith a narrow band gap of 2.7 eV[14],which make it can utilize visible light directly.In addition, g-C3N4is extremely stable owing to its tristriazine-ring structure and high degree of condensation[15].So,ithas been widely used as a narrow band gap semiconductor to construct hetero-photocatalyst by coupling over wider band gap semiconductor photocatalyst.Recently, Zhu′s groups have prepared the core/hell structured g-C3N4/BiPO4photocatalyst via ultrasonic dispersion method[16].Although core/hell structured g-C3N4/BiPO4exhibits high photocatalytic activities,the preparation process is high energy consumption.So,it′s vital to explore the facile and practicable method for fabrication g-C3N4/BiPO4photocatalyst.Very recently,Lietal. reported a spherical g-C3N4/BiPO4composite via a associated sonochemical and heat-treating process and the experimental results revealed that g-C3N4/BiPO4exhibited high photocatalytic activity for methyl orange[17].The above work inspired us to construct g-C3N4/BiPO4composite photocatalyst and investigate it activity enhancementmechanism.
Very recently,we have successfully fabricated AgBr/BiPO4heterojunction by loading AgBr QDs on the surface of BiPO4microcrystals[18].In thiswork, one-dimensional(1D)rod-like BiPO4was designed and fabricated by thehydrothermalmethod according to ourprevious report[18-19].Then,g-C3N4QDswasdecorated on the surface of rod-like BiPO4to construct the novel heterojunctions via the followed impregnation-calcinations process.Furthermore,the mechanism of enhanced catalytic activity for g-C3N4/BiPO4heterojunctionswas also discussed.
1 Experimental section
1.1Sample preparation
The g-C3N4/BiPO4were obtained by a simple impregnation-calcinations processmethod.In a typical procedure,a certain amountofmelamine was dissolved in methanol.Then,1.0 g as-prepared BiPO4was dispersed in the above solution and vigorously stirred for 60 min to obtain a uniform suspension and then dried to get dry powder.Finally,the powder was heated to 550℃with speed of 2℃·min-1in muffle furnace,then kept for 4 h,and the resulted powders were ground for further use.According to above method,the contents of g-C3N4in g-C3N4/BiPO4heterojunction range from 2.0%to 20.0%(w/w,the same below)were prepared.
1.2Characterizations
X-ray diffraction(XRD)patterns were measured with a Shimadzu XRD-7000 X-ray diffractometer using Cu Kαradiation.Scanning electron microscopy(SEM) images and energy dispersive X-ray spectroscopy(EDS) maps were obtained with a Hitachi a JEOL JSM-6610LV field emission scanning electron microscope. Transmission electron microscopy(TEM)observations were performed on a JEOL JEM-2100 electron microscope with an accelerating voltage of 200 kV. Diffuse reflectance spectra(UV-Vis-DRS)of the samples were recorded on a Shimadzu UV-2550 UVVisible spectrometer using BaSO4as the reference.
1.3Photocatalytic activity test
The photocatalytic activities of samples were evaluated by degradation rhodamine B(RhB)and phenol under visible light irradiation of a 400 W metal halide lamp with a cutoff filter to cut off the lightbelow 420 nm.The experiment detailwas similar to our previous report[18].Chemical oxygen demand (COD)was determined at a COD rapid monitor(5B-3B,LanHua Co.,LTD,China).To investigate the active species generated in the photocatalytic system, different scavengers,including tertiary butanol(TBA, 10 mmol·L-1),benzoquinone(BQ),ethylenediamine tetraacetic acid disodium salt(EDTA-2Na,10mmol· L-1)[20-22],were introduced into the photocatalysis solution to examine·OH,O2-·and h+,respectively. Theexperimentalprocedureswere conducted as follows: 200 mg of photocatalyst and 200 mL fresh aqueous solution of RhB was continuously magnetically stirred in dark for 1.0 h to establish an adsorption/desorption equilibrium of solution.Then,scavenger was added into the solution to obtained a concentration of 10 mmol·L-1.At given irradiation time intervals,then,5 mL of the suspension were sampled,centrifuged to remove the catalyst particles,and measured the concentration of RhB.
2 Result and discussion
2.1XRD,SEM and EDS of g-C3N4/BiPO4
Fig.1exhibited the XRD patterns of as-prepared samples.It is clearly seen that the diffraction peaks of samples could be assigned to orthorhombic g-C3N4(JCPDS No.08-0209)and monoclinic BiPO4(JCPDS No.89-0287)[13,16].Because some characteristic perks of g-C3N4were near those of BiPO4,the intensity changes in the BiPO4peaks were not obvious.In comparison, the intensitiesof the peaks at29.08 was clearly raised in the g-C3N4/BiPO4composites with an increasing amount of g-C3N4.Meanwhile,no impurities were detected,indicating the high purity of the obtained samples.
<1),且各件产品是否为不合格品相互独立.
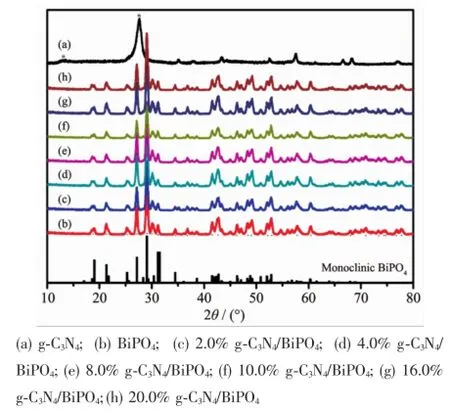
Fig.1 XRD patterns of g-C3N4/BiPO4samples
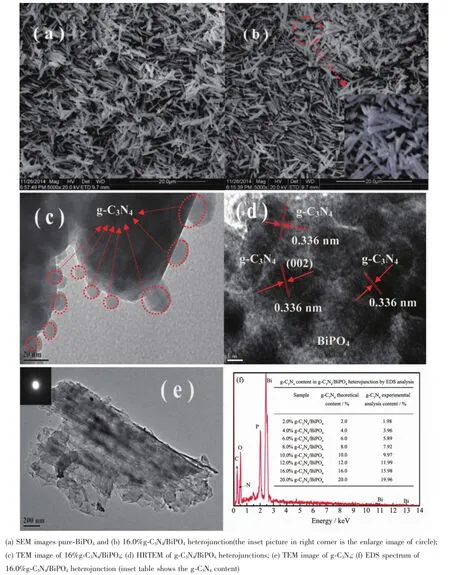
Fig.2 SEM and TEM images of samples
The morphology of the as-synthesized pure-BiPO4,g-C3N4,and g-C3N4/BiPO4composites were observed by SEM.As shown in Fig.2a~b,BiPO4exhibited an 1D rod-like shape with smooth surface. In order to form g-C3N4/BiPO4heterojunctions,g-C3N4QDs were loaded onto the surface of the rod-like BiPO4microcrystals(Fig.2b).The morphology of g-C3N4/BiPO4was not changed obviously because the g-C3N4contentwas very low,but g-C3N4/BiPO4microrod become wider than pure BiPO4.Further information g-C3N4/BiPO4heterojunctions was obtained for TEM images(Fig.2c~d).The locationsofg-C3N4nanoparticles on the surface of rod-like BiPO4are indicated byarrows in the TEM images(Fig.2c).It reveals that high dispersed spherical heteroparticles with size of about 20 nm loaded the surface of rod-like BiPO4. Fig.2d show the lattice fringeof0.336nm,corresponding to the(002)plane of g-C3N4,is clearly observed in the g-C3N4/BiPO4composite and that the interfaces between g-C3N4and BiPO4are smooth,which further verifies the formation of a g-C3N4/BiPO4heterojunction.In addition,it is observation from Fig.2e that pure g-C3N4displays plate-like shape morphology. SEM and TEM information clearly exhibited that g-C3N4QDs were highly dispersed on the surface of BiPO4and form the novel heterojunction structure. Fig.2f indicated that the content of g-C3N4in g-C3N4/ BiPO4in the sampleswere also close to the theoretical calculated value of g-C3N4/BiPO4(inset picture in Fig. 2f).
2.2UV-Vis-DRS analysis
UV-Vis DRS spectra of the as-obtained samples are shown in Fig.3.According to Fig.3a,the absorption edge of pure-BiPO4and g-C3N4are occurred at about 320 nm and 465 nm,respectively.Moreover,the g-C3N4/BiPO4composites presented similar absorption characteristics to pure BiPO4due to the low contentg-C3N4of in g-C3N4/BiPO4heterojunction.The efficient visible light absorption abilities ensured that g-C3N4/ BiPO4generated sufficient electron-hole pairs under visible light irradiation.In addition,the band gap energies(Eg)of g-C3N4and BiPO4were calculated according to the formula:(αhγ)2=A(hν-Eg),whereα, h,ν,A and Egstand for the absorption coefficient, Planck′s constant,the light frequency,a constant and band gap energy,respectively[17,19].Therefore,the corresponding Egvalues of g-C3N4,BiPO4and 16.0%g-C3N4/BiPO4were determined from a plot of(αhν)2versus energy(hν)(Fig.3b)and estimated to be 2.6, 3.85 and 3.82 eV,respectively.
2.3Photocatalytic activity
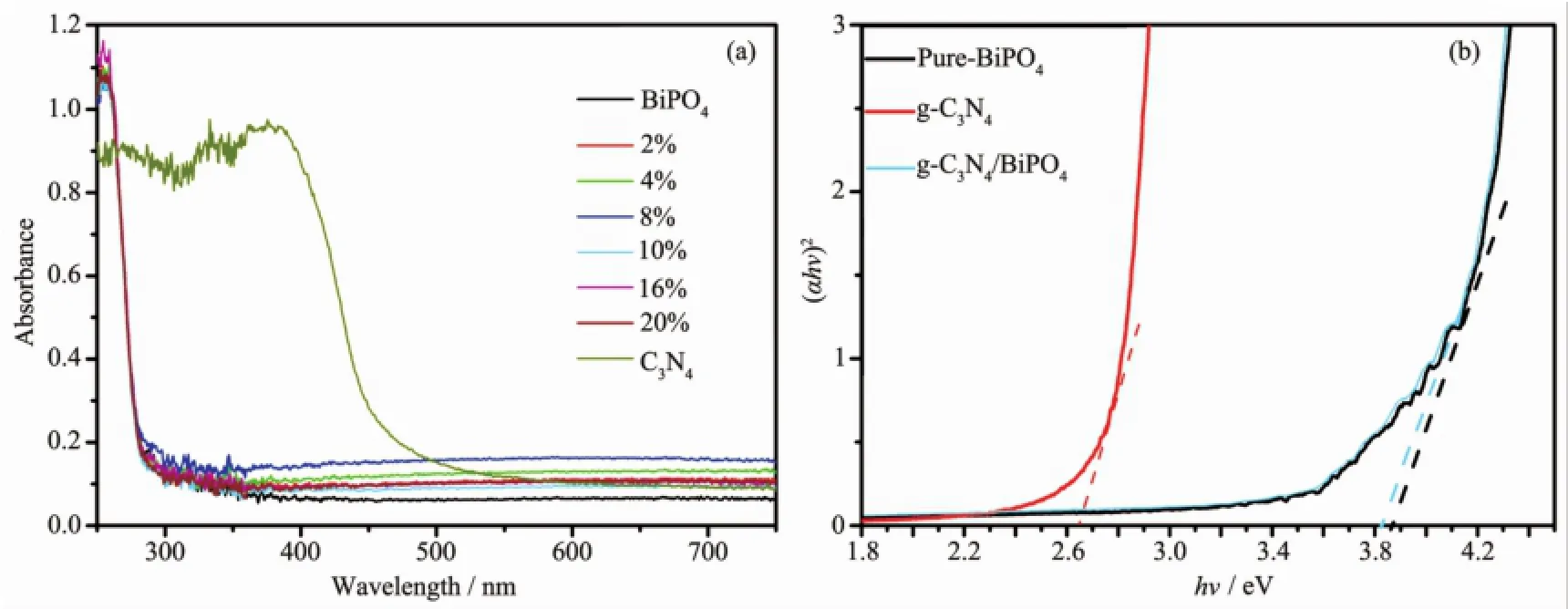
Fig.3 UV-Vis-DRS spectra of the as-obtained samples(a)and the band gap energies(Eg)of BiPO4,g-C3N4and 16.0%g-C3N4/BiPO4heterojunction(b)
The photocatalytic activities of the samples were evaluated by the degradation of RhB and phenol under visible light irradiation.The photocatalytic reactions follow pseudo-first-order kinetics law according to the Langmuir-Hinshelwood model for low concentration pollutant.The kinetics equation can be expressed as follows[23]:ln(C0/Ct)=kt+ln(C0/C1),where k is the pseudo-first-order rate constant,C0is the original concentration of RhB or phenol(10 mg·L-1),C1is the concentration after adsorption,and Ctrepresents the concentration at reaction time t.It can be seen from Fig.4a that the photocatalytic activity is enhanced gradually with the content of g-C3N4increasing from 4%to16%.However,further increasing the contentof g-C3N4in the heterojunctions leads to a decrease in the degradation rate.This resultmay be attributed to the agglomera-tion of g-C3N4QDs in the surface of BiPO4,which can weaken the heterojunction structure and decrease the catalytic activity[24-25].Therefore,a suitable ratio and well dispersion of g-C3N4QDs in the composites are necessary.From Fig.4a,it also can be seen that pure-BiPO4can decompose 12.5%ofRhBafter 10 min illumination.Significantly,g-C3N4/BiPO4composites exhibited improved photocatalytic activities compared to pure BiPO4and pure g-C3N4.In particular, 16.0%g-C3N4/BiPO4showed the best photocatalytic activity than those of the others,corresponding to 97.85%of RhBwith 10min illumination.Fig.4b show the k value obtained from the fitted straight-line plots of ln(Ct/C0)versus time(t),which follow the order: pure-BiPO4<4.0%g-C3N4/BiPO4<8.0%g-C3N4/BiPO4<10.0%g-C3N4/BiPO4<20.0%g-C3N4/BiPO4<16.0%g-C3N4/BiPO4.The results shows that 16%g-C3N4/BiPO4photocatalysts possesses themaximal k value of 0.348 min-1,which is 15 and 4.6 times higher than that of pure BiPO4and g-C3N4,respectively.Moreover,phenol was chosen as another model environmental organic pollutant to further evaluate photocatalytic activity of g-C3N4QDs decorated rod-like BiPO4also investigated (Fig.5a~b).Similar to the RhB results,the g-C3N4QDs decoration on the surface results in an increase of phenol degradation.16%g-C3N4/BiPO4heterostructure also shows the best activity,with constants k=0.178 min-1.Fig.6shows that the COD removal ratio of 16% g-C3N4/BiPO4reaches a value of 87.8%after 60 min of irradiation,while that of pure-BiPO4and g-C3N4is 37.9%and 45.5%,respectively.The COD value reduction of 16%g-C3N4/BiPO4is slower than that of degradation of phenol.It is well-known that mineralization of organic compounds through two steps:ring cleavage and subsequently the oxidation of fragments. In our experiment,the COD removal rate of 16.0%g-C3N4/BiPO4exhibits different behavior before and after 20 min of irradiation.These results confirm that phenol is first ring cleaved and then converted to CO2and H2O.The loss of COD via mineralization can be lowered more than the removed amount of organic pollutants because these parent molecules are decomposed to smaller organic intermediates,and further degradation of these intermediates to CO2andH2Omay occur slowly[19-20].
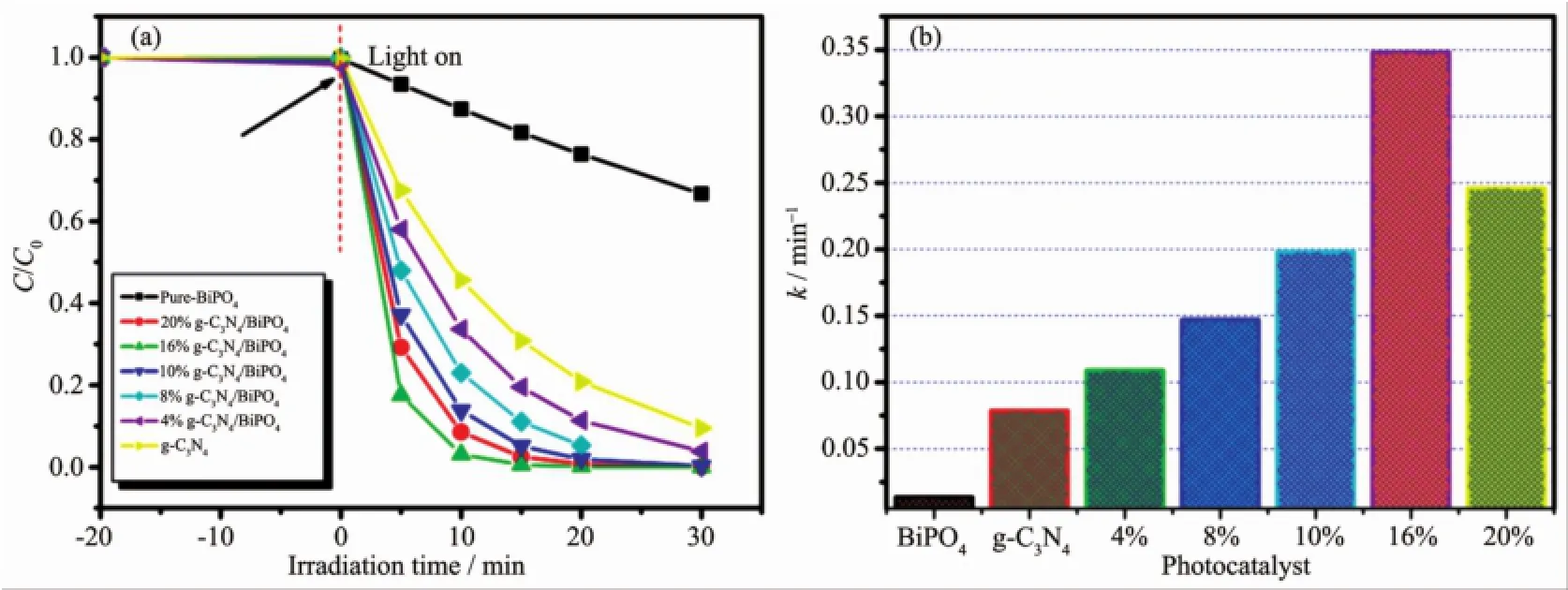
Fig.4 Photocatalytic activities of the prepared g-C3N4/BiPO4heterostructure for the RhB(a)and Corresponding k values of the different photocatalysts(b)under visible-light irradiation
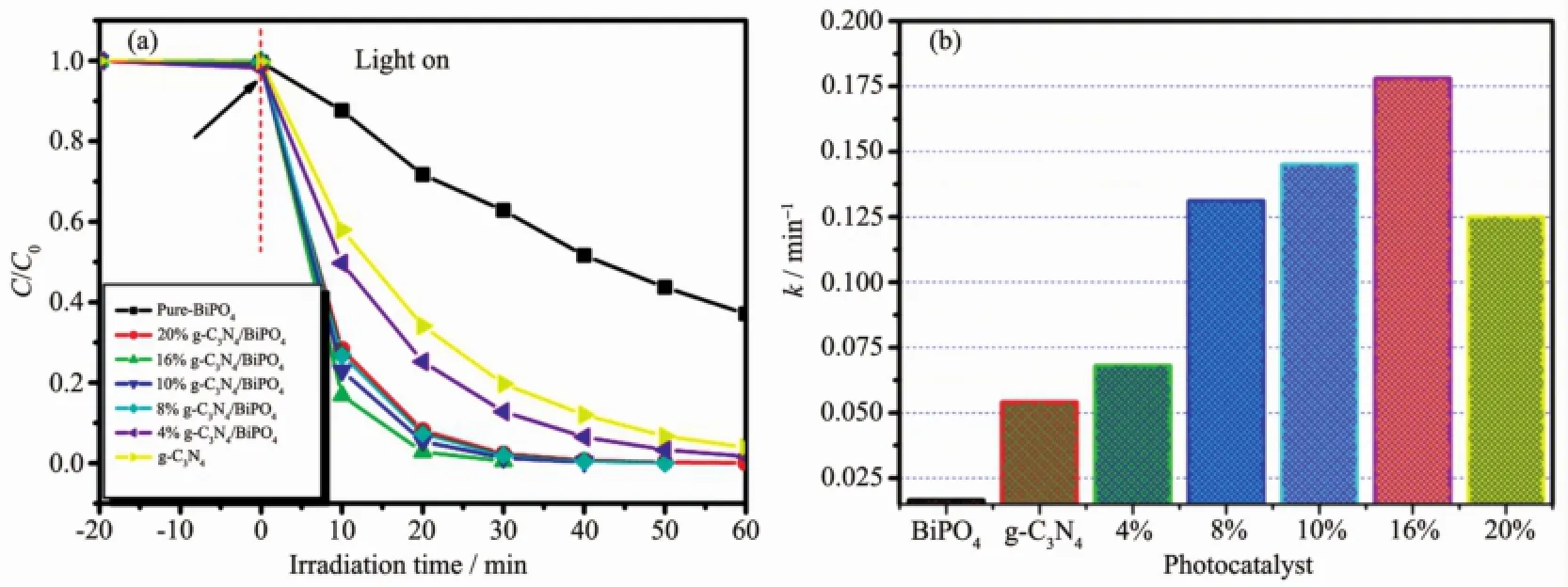
Fig.5 Photocatalytic activities of the g-C3N4/BiPO4composite photocatalysts for the phenol(a)and the corresponding k values of the different photocatalysts(b)under visible-light irradiation

Table1 SBETvalue and photocatalytic activities of g-C3N4/BiPO4heterojunctions
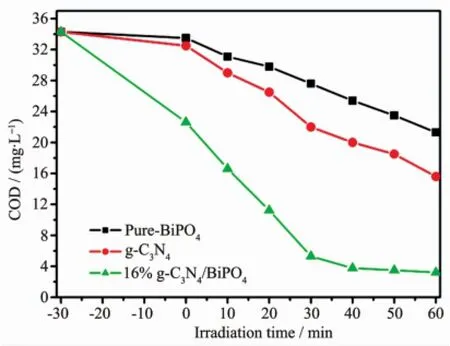
Fig.6 COD changes during the course of phenol photodegradation in the presence of pure-BiPO4,g-C3N4and 16%g-C3N4/BiPO4heterojunctions
To demonstrate the potential applicability of g-C3N4/BiPO4photocatalyst,the stability of the 16%g-C3N4/BiPO4photocatalyst was investigated(Fig.7). After five cycles for photo-degradation of RhB,the catalyst did not exhibit obvious loss of activity,as shown in Fig.7a,confirming thatg-C3N4QDs decorated 1D rod-like BiPO4have high stability and are easy to be recycled.Fig.7b shows phases composition 16.0%g -C3N4/BiPO4did not after five cycles.Therefore,g-C3N4/BiPO4heterojunctions can be used as stable visible-light-responsive photocatalyst.
2.4Possible photocatalyticm echanism of g-C3N4/ BiPO4heterojunctions
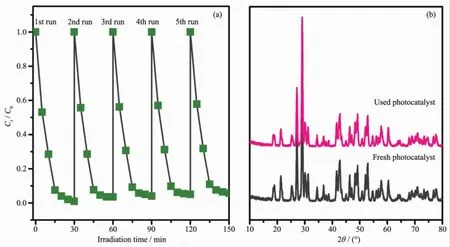
Fig.7 Repeated experiments of photocatalytic degradation of RhB on 16.0%g-C3N4/BiPO4photocatalystunder visible light irradiation(a)and XRD patterns of 16.0%g-C3N4/BiPO4photocatalyst before and after used for five cycles(b)
To further investigate the reactive species in the degradation of RhB,TBA,BQ,and EDTA-2Na were introduced as the scavenger of·OH,O2-·and h+, respectively.Fig.8shows the effects of different scavengers on the photocatalytic degradation of RhBover 16%g-C3N4/BiPO4.It can be seen that photocatalytic degradation of RhB was obviously suppressed by BQ and TBA,indicating that O2-·and·OH are the main reactive species.As shown in Fig.8,there is also a slight change for RhB photocatalytic degradation when h+scavenger EDTA-2Na was added.This indicates that h+is also one of the reactive species involved in the RhB photocatalytic oxidation process.

Fig.8 Photocatalytic degradation of RhB over 16%g-C3N4/BiPO4with the addition of scavengers
It is known that the generation of O2-·could be via two different processes.On the one hand,RhB can be excited by visible light to form the excited state (RhB*).RhB*then injects electrons into the CB of g-C3N4/BiPO4to form eCB-,which is scavenged by the O2on the surface of the catalyst to form O2-·.So,it is reasonable that RhB may display a weak photosensitization effect on g-C3N4/BiPO4under visible light. On the other hand,when g-C3N4/BiPO4was irradiated under visible light,only g-C3N4could be activated. The electrons and hole were photogenerated in CB and VB of g-C3N4,then move to the empty bottom of the CB of BiPO4.Finally,the electron in the CB of BiPO4could reactwith O2to form O2-·(Fig.9).At the same time,·OH may produced via followed reaction. Based on our experimental results and the discussions above,themechanism of photocatalytic degradation of RhB on the g-C3N4/BiPO4heterojunctions may be proposed,as described in the Eq.(1)~(12):
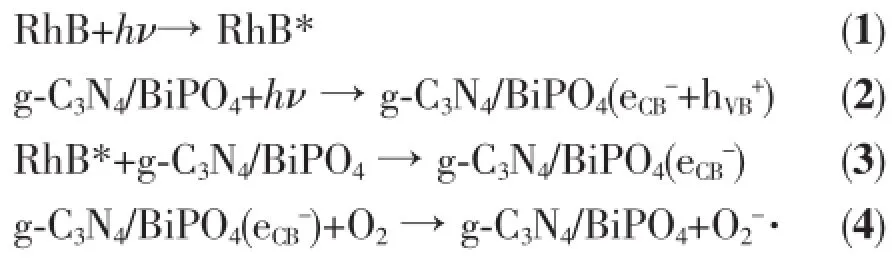
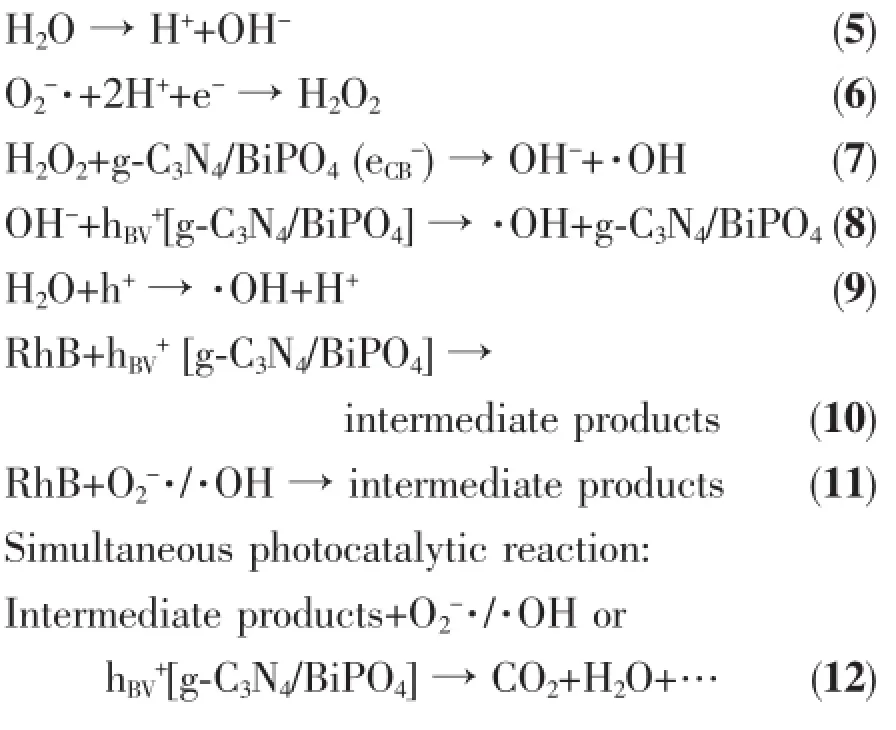

Fig.9 Potential of valence and conduction band for g-C3N4and BiPO4to illustrate the photocatalytic enhancementmechanism of g-C3N4/BiPO4heterojunction
3 Conclusions
1D rod-like BiPO4micro crystals was synthesized via a hydrothermal.Then,g-C3N4QDs with the size of about20 nm were deposited on the surface of rod-like BiPO4by employing a followed impregnation-calcinations method to construct the novel g-C3N4/BiPO4heterojunctions.The g-C3N4QDs decorated BiPO4exhibits enhanced photocatalytic activity in decomposition of RhB and phenol,which is much higher than thatof pure-BiPO4and g-C3N4,and the content of g-C3N4impacts the catalytic activity of g-C3N4/BiPO4heterojunction.The enhanced activity of as-fabricated g-C3N4/BiPO4heterojunctions is attributed to the efficient separation of electron-hole pairs in g-C3N4/ BiPO4due to the formation of heterojunction between the surface of two semiconductors.Both O2-·and·OH are main reactive species which responsible for thedecomposition of RhB and phenol.Furthermore,g-C3N4/BiPO4has high stability,suggesting that QDs decoration could be a promising strategy for designing new efficient photocatalyst.
References:
[1]Tong H,Ouyang SX,Bi Y P,et al.Adv.Mater.,2012,24(1): 229-251
[2]Kubacka A,Fernández-García M,Colón G.Chem.Rev., 2012,112(3):1555-1614
[3]Chen X B,Shen S,Guo L,et al.Chem.Rev.,2010,110(11): 6503-6570
[4]Liu J,Yang Q,Yang W T,et al.J.Mater.Chem.A,2013,1 (26):7760-7766
[5]Wang W H,Himeda Y,Muckerman JT,et al.Chem.Rev., 2015,115(23):12936-12973
[6]Paola A D,García-López E,MarcìG,et al.J.Hazard.Mater., 2012,211-212:3-29
[7]Kudo A,Miseki Y.Chem.Soc.Rev.,2009,38(1):253-278
[8]Cheng H.F,Huang B B,Wang P,et al.Chem.Commun., 2011,47(25):7054-7056
[9]Wang Y J,Guan X F,Li L P,et al.CrystEngComm,2012,14 (23):7907-7914
[10]Wang D J,Zhang J,Guo L.J.Inorg.Mater.,2015,30(7):683 -693
[11]Lin X P,Xing J C,Wang W D,et al.J.Phys.Chem.C, 2007,111(49):18288-18293
[12]Geng J,Hou W H,LüY N,et al.Inorg.Chem.,2005,44 (23):8503-8509
[13]Xu H,Xu Y G,H.Li M,et al.Dalton Trans.,2012,41(12): 3387-3394
[14]Wang X C,Meada K,Thomas A,et al.Nat.Mater.,2009,8 (1):76-80
[15]Wang Y,X.Wang C,Antonietti M.Angew.Chem.Int.Ed., 2012,51(1):68-89
[16]Pan C S,Xu J,Wang Y J,et al.Adv.Funct.Mater.,2012, 22(7):1518-1524
[17]Li Z S,Li B L,Peng S H,et al.RSC Adv.,2014,4(66): 35144-35148
[18]Wang D J,Guo L,Zhen Y Z,et al.J.Mater.Chem.A, 2014,2(10):11716-11727
[19]WANG Dan-Jun(王丹军),YUE Lin-Lin(岳林林),ZHANG Jie(张洁),et al.J.Synth.Cryst.(人工晶体学报),2014,43 (1):2977-2984
[20]Zhang L S,Wong K H,Chen Z G,et al.App l.Catal.A: Gen.,2009,363(1/2):221-229
[21]Wang Y J,Lin J,Zong R L,et al.J.Mol.Catal.A:Chem., 2011,349(1/2):13-19
[22]Lin H L,Cao J,Luo B D,et al.Chin.Sci.Bull.,2012,57 (22):2901-2907
[23]Li FT,Zhao Y,Hao Y J,etal.J.Hazard.Mater.,2012,239 -240:118-127
Synthesis of Monodispersed g-C3N4Quantum Dots(QDs)Decorated on the Surface of 1D Rod-like BiPO4w ith Enhanced Photocatalytic Activities
WANG Dan-Jun*,1SHEN Hui-Dong1GUO Li1,2YUE Lin-Lin1FU Feng*,1
(1College of Chemistry&Chemical Engineering,Yan′an University,Shaanxi Key Laboratory of Chemical Reaction Engineering,Yan′an,Shaanxi716000,China)
(2School of Materials Science and Engineering,ShaanxiNormal University,Xi′an 710119,China)
1D rod-like BiPO4have been successfully synthesized via a hydrothermal process,and g-C3N4quantum dots(QDs)was decorated on the surface of BiPO4to form a novel g-C3N4/BiPO4heterojunction via a followed impregnation-calcinationsmethod.XRD,FE-SEM,HR-TEM,EDSand UV-Vis-DRS techniques were employed to characterize the phase composition,morphology and spectrum properties of as-synthesized samples.The photocatalytic activities of samples were evaluated by degradation of RhB and phenol under visible light irradiation.The results also shows that 16%(w/w)g-C3N4/BiPO4photocatalysts possesses the maximal k value of 0.348 min-1,which is 15 and 4.6 times higher than that of pure BiPO4and g-C3N4,respectively.The catalytic efficiency enhancement of g-C3N4/BiPO4heterojunctions relative to pure-BiPO4can be attributed to the formation of heterojunctions between g-C3N4QDs and BiPO4,which suppresses the recombination of photogenerated electron-holes.The radical scavengers test further confirmed that·O2-was the main reactive species during thephotocatalytic process.Therefore,thiswork provides a facile process for the design of novel and efficient BiPO4-based photocatalystwithmulti-components.
rod-like BiPO4microcrystal;g-C3N4quantum dots(QDs);decoration;photocatlaytic activity enhancementmechanism
All reagents were analytical purity and without further purification.Rod-like BiPO4microcrystalwas prepared according to our previous report[18-19]. In a typical process,5 mmol Bi(NO3)3·5H2O was dissolved in 5 mL HNO3(4.0 mol·L-1),then NH4H2PO4solution were slowly added to above Bi(NO3)3solution drop-wise under vigorously stirring.Afterward,the suspension was transported into 50 mL Teflon-lined autoclave and heated at 190℃for 24 h.After hydrothermal reaction,the autoclave was naturally cooled to room temperature.Then,the resultedprecipitates were collected,washed with deionized water and absolute ethanol for several times,and dried in a vacuum oven at80℃for 4 h.
O647.32
A
1001-4861(2016)07-1246-09
10.11862/CJIC.2016.170
2016-01-02。收修改稿日期:2016-05-23。
国家自然科学基金(No.21373159)、陕西省科技项目(No.2013K11-08,2013SZS20-P01,2015YG174)、陕西省教育厅科研基金项目(No.15JS119)、延安大学基金(No.2013YDZ-07,YDBK2013-11)和延安大学研究生科研创新项目(No.YCX201602)资助项目。
*通信联系人。E-mail:wangdj761118@163.com,FengFu@126.com
