肉骨粉部分替代鱼粉对大菱鲆幼鱼氨基酸应答信号通路的影响*
2016-11-17许丹丹周慧慧麦康森
宋 飞, 许丹丹, 周慧慧, 麦康森, 徐 玮, 何 艮
(中国海洋大学水产动物营养与饲料农业部重点实验室,海水养殖教育部重点实验室,山东 青岛 266003)
肉骨粉部分替代鱼粉对大菱鲆幼鱼氨基酸应答信号通路的影响*
宋 飞, 许丹丹, 周慧慧, 麦康森, 徐 玮, 何 艮**
(中国海洋大学水产动物营养与饲料农业部重点实验室,海水养殖教育部重点实验室,山东 青岛 266003)
为研究肉骨粉部分替代鱼粉(添加或不添加晶体氨基酸)对大菱鲆(ScophthalmusmaximusL.)幼鱼的氨基酸应答(AAR)信号通路中关键调控因子基因表达量的影响。设计了3种等氮等能的饲料:鱼粉对照组(FM,60%鱼粉),肉骨粉替代组(MBM,肉骨粉替代45%鱼粉蛋白),肉骨粉替代添加晶体必需氨基酸组(MBM+AA,肉骨粉替代45%鱼粉蛋白,添加晶体必需氨基酸至鱼粉组必需氨基酸水平)。实验选取初始体质量(9.01±0.01)g的大菱鲆,分别饱食投喂3种不同的饲料30天,检测肌肉和肠道中AAR信号通路中关键调控因子的基因表达量。研究表明:与FM组相比,MBM组显著提高了大菱鲆幼鱼肌肉中谷氨酰胺合成酶(Asparagine synthesis,ASNS)、转录激活因子3(Activating transcription factor3, ATF3)、转录激活因子4(Activating transcription factor4, ATF4)、CCAAT增强子结合蛋白(CCAAT-enhance binding protein homology protein, CHOP)、发育和DNA损伤应答调节因子1(Regulated in development and DNA damage responses 1, REDD1)和真核翻译起始因子4E结合蛋白1 (Eukaryotic initiation factor 4E binding protein 1,4E-BP1)表达量的峰值。在摄食后2h,MBM+AA组的ASNS、ATF3、ATF4、CHOP、REDD1和4E-BP1表达量峰值与FM组的表达量峰值无显著性差异,却显著低于MBM组的表达量峰值。3个处理组肌肉中酵母转录激活因子2(General control nonderepressible 2, GCN2)的基因表达量无显著性差异。同时,与FM组相比,MBM组显著上调肠道中GCN2、ASNS、ATF4、CHOP和4E-BP1表达量的峰值,MBM+AA组对于降低这些基因的表达量无显著性效果。研究结果表明:肉骨粉替代45%鱼粉蛋白上调了肌肉和肠道中AAR信号通路中关键因子的表达,添加晶体氨基酸可以在一定程度上改善肉骨粉替代鱼粉对于肌肉中AAR信号通路中关键调控因子表达量的上调作用。
大菱鲆;肉骨粉;鱼粉;氨基酸应答信号通路;关键调控因子;基因表达
近年来,随着海水鱼饲料业的快速发展,鱼粉作为优质蛋白源,其价格和产量已显著的限制了海水鱼饲料业和养殖业的发展[1-2]。因此,研究者越来越多的关注于寻找优质的植物、动物蛋白源替代鱼粉的研究[3-4]。肉骨粉作为一种优质动物蛋白源,广泛的应用于海水鱼饲料中以部分替代鱼粉[5-6]。但是与鱼粉相比肉骨粉有氨基酸组成不平衡、适口性差和消化率低等缺点,这些因素严重限制了肉骨粉在水产饲料业中的使用[7]。海水鱼类上关于肉骨粉替代鱼粉的研究主要关注其替代后对鱼体生长、鱼体组成以及生化相关指标的影响。研究发现,过高比例的肉骨粉替代鱼粉能够显著的降低鱼类生长、鱼体营养物质积累和扰乱机体代谢水平。然而,对于上述现象的背后的机制研究尚未见报道。
氨基酸应答(Amino acid response,AAR)信号通路是生物体内一条高度保守的感知体内营养缺乏的信号通路[8-9]。当营养物质缺乏时,机体会激活体内的AAR信号通路,从而抑制体内整体的蛋白质翻译过程,并激活某些特定的蛋白的表达,例如转录因子、氨基酸合成和氨基酸转运基因的表达[10-11]。机体内的AAR信号通路的关键基因主要包括酵母转录激活因子(General control nonderepressible 2,GCN2)、转录激活因子4(Activating transcription factor4,ATF4)、天冬酰胺合成酶(Asparagine synthesis,ASNS)、转录激活因3(Activating transcription factor3,ATF3)、CCAAT增强子结合蛋白(CCAAT-enhance binding proteinhomology protein,CHOP)和发育和DNA损伤应答调节因子1(Regulated in development and DNA damage responses 1,REDD1)[12-14]。机体缺乏必需氨基酸情况下会产生大量非负载tRNA,其能够与GCN2蛋白结合从而使GCN2激酶去磷酸化被激活,进而使eIF2α磷酸化使体内大部分蛋白质合成减少,但是会通过转录水平促进其下游的转录因子ATF4的表达,ATF4又与CHOP结合,激活大量下游基因(ATF3,REDD1,ASNS)的转录,从而保证机体在饥饿条件下自身稳态[15-16]。在许多哺乳动物上的研究发现,AAR信号通路显著的受到食物中的营养素组成的影响[17-18],同时AAR信号通路参与了体内的多种营养素的代谢调控过程[19-21]。目前,鱼类上关于营养物质对AAR信号通路的影响相对较少。Xu等[22]报道,豆粕替代鱼粉能够显著激活AAR信号通路。Wang等[23]研究发现,军曹鱼(Rachycentroncanadum)摄食蛋氨酸缺乏饲料能够显著上调AAR通路相关基因的表达。但在鱼类上,饲料中肉骨粉替代鱼粉对于氨基酸应答信号相关基因表达的调控研究还未见报道。
大菱鲆(ScophthalmusmaximusL.)属于鲽亚目(Pleuronectoidei)菱鲆科(Scophthalmidae)瘤棘鲆属(Scophthalmus),为中国北方重要的养殖肉食性海水鱼[24]。近年关于鱼粉替代的研究已经在大菱鲆上进行了广泛的研究[25-26]。然而,关于肉骨粉替代鱼粉对于大菱鲆AAR信号通路中关键基因表达的调控机制尚未见报道。因此,本研究拟通过配制肉骨粉替代鱼粉的饲料投喂大菱鲆,以探讨饲料肉骨粉替代鱼粉对于大菱鲆肌肉和肠道氨基酸应答相关基因表达的影响,为海水鱼新蛋白源开发和利用提供理论依据。
1 材料方法
1.1 饲料原料和饲料配方
实验饲料以鱼粉、肉骨粉、谷朊粉和小麦粉为主要蛋白源,棕榈油和大豆卵磷脂为主要的脂肪源,配制3种粗蛋白质约50%,总能量约为20 kJ/g的等氮等能的实验饲料,分别是含有60%鱼粉蛋白的全鱼粉组(FM),含有33%鱼粉蛋白和34.2%肉骨粉蛋白的肉骨粉替代组(MBM)以及含有33%鱼粉蛋白和34.2%肉骨粉蛋白,添加晶体氨基酸至鱼粉组必需氨基酸水平的肉骨粉替代添加必需氨基酸组(MBM+AA)。实验饲料配方及常规营养成分分析见表1,3种实验饲料氨基酸组成见表2。
实验原料彻底粉碎后过80目筛,将过筛后的原料按照饲料配方中的比例充分混合均匀后再加入鱼油搓匀,再加入适量比例的水分搓匀后制粒。制成的颗粒在45 ℃的烘箱中恒温烘干12 h,烘干后的饲料颗粒放置在-20 ℃冰箱中备用。
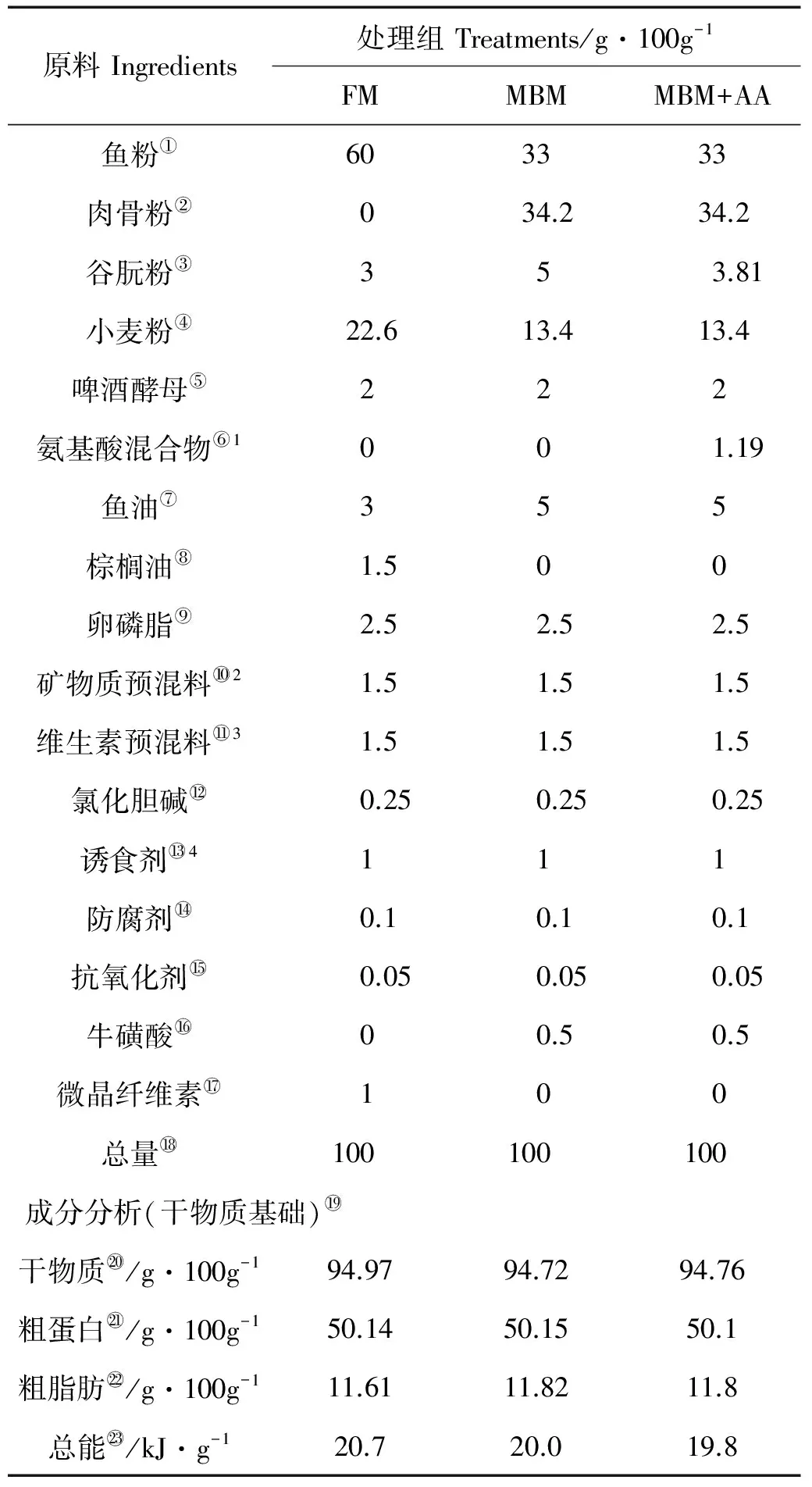
表1 实验饲料配方和主要营养成分(干物质)
注:FM:全鱼粉对照组;MBM:肉骨粉替代45%鱼粉蛋白组;MBM+AA:肉骨粉替代45%鱼粉蛋白添加晶体氨基酸至鱼粉组必需氨基酸水平组;1氨基酸混合物(g/kg饲料):L-蛋氨酸(包膜氨基酸,90%),2.4;L-赖氨酸(包膜氨基酸,60%),6.1;L-亮氨酸(晶体氨基酸,99.4%),1.6;L-组氨酸(晶体氨基酸,99.1%),0.5;L-苏氨酸(晶体氨基酸,99.9%)1.3;2矿物质预混料(mg/kg饲料):CoCl2·6H2O(1%),50;CuSO4·5H2O(25%),10;FeSO4·H2O(30%),80;ZnSO4·H2O(34.50%),50;MnSO4·H2O(31.80%),45;MgSO4·7H2O(15%),1200;Na2SeO3(1%),20;碘酸钙(1%),60;沸石粉,11470;3维生素预混料(mg/kg饲料):维生素B1(98%),25;维生素B2(80%)45;维生素B6(99%)20;维生素B12(1%)10;维生素K3(51%)10;肌醇(98%)800;泛酸钙(98%)60;烟酸(99%)200;叶酸(98%)20;生物素(2%)60;维生素A(500000 IU/g)32;维生素D(500000 IU/g)5;维生素E(50%)240;维生素C(35%)2000;抗氧化剂(100%)3;稻壳粉(100%)11470;4诱食剂:甜菜碱∶二甲基-β-丙酸噻亭∶甘氨酸∶丙氨酸∶5-磷酸肌苷=4∶2∶2∶1∶1。
Note:FM: fishmeal diet;MBM: meat and bone meal replaced 45% fishmeal protein diet;MBM+AA: meat and bone meal replaced 45% fishmeal protein and supplemented essential amino acids to match the essential amino acids profile of the FM diet;1Amino acid mixture (g/kgdiet): L-Methionine(Coated amino acid obtained, 90%), 2.4; L-Lysine (Coated amino acid, 60%), 6.1; L-Leucine (Crystalline amino acid, 99.4), 1.6; L- Histidine (Crystalline amino acid, 99.1%), 0.5; L-Threonine (Crystalline amino acid, 99.9%), 1.3;2Mineral premix (mg/kg diet): CoCl2·6H2O (1%), 50; CuSO4·5H2O (25%), 10; FeSO4·H2O (30%), 80; ZnSO4·H2O (34.50%), 50; MnSO4·H2O (31.80%), 45; MgSO4·7H2O (15%), 1200; Sodium selenite (1%), 20; Calcium iodine (1%) 60; Zeolite, 11470;3Vitamin premix (mg/kg diet): thiamin (98%), 25; riboflavin (80%), 45; pyridoxine-HCl (99%), 20; vitamin B12 (1%), 10; vitamin K3 (51%), 10; inositol (98%), 800; pantothenic acid (98%), 60; niacin acid (99%), 200; folic acid (98%), 20; biotin (2%), 60; retinol acetate (500000 IU/g), 32; cholecalciferol (500000 IU/g), 5; alpha-tocopherol (50%), 240; ascorbic acid (35%), 2000; anti-oxidants (oxygen ling grams, 100%), 3; rice husk powder (100%), 11470;4Attractant: Betaine∶ Dimethyl-propiothetin∶ Glycine∶ Alanine∶ inosine5'-phosphate=4∶2∶2∶1∶1.
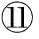
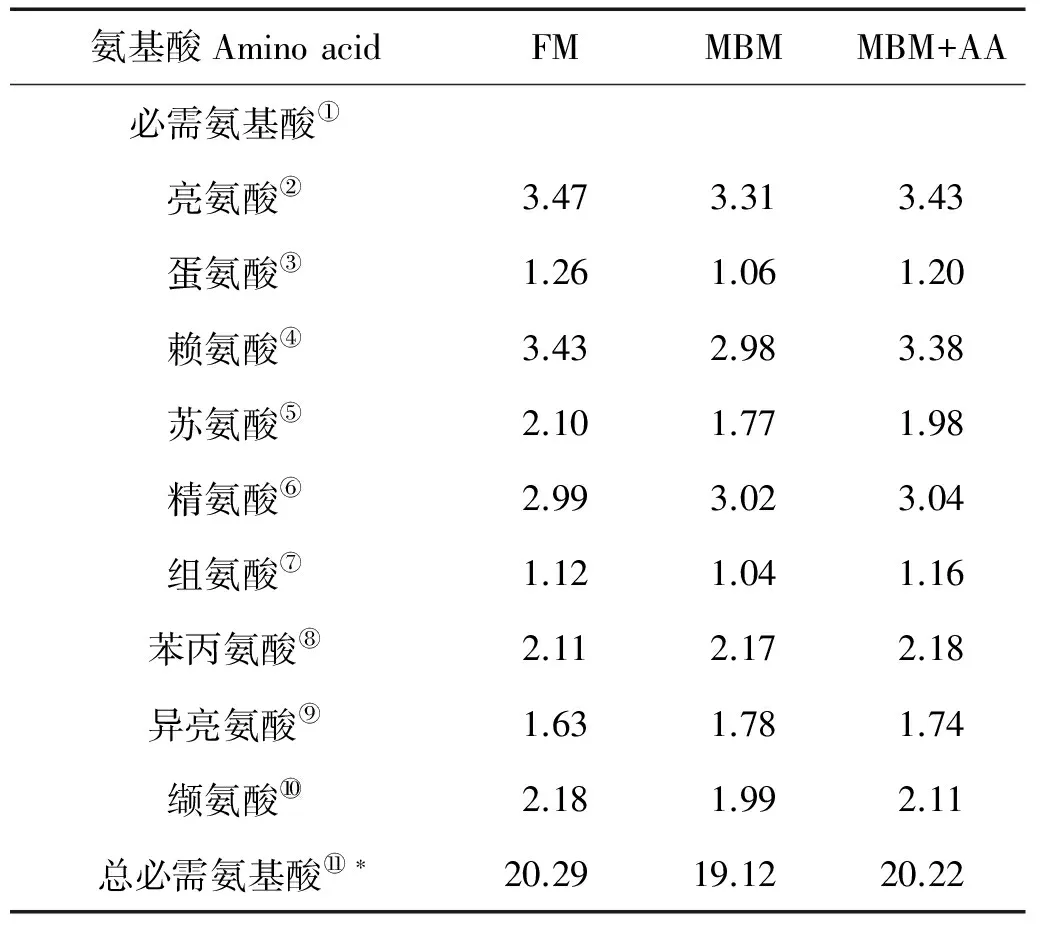
表2 实验饲料氨基酸组成(干物质)
续表2
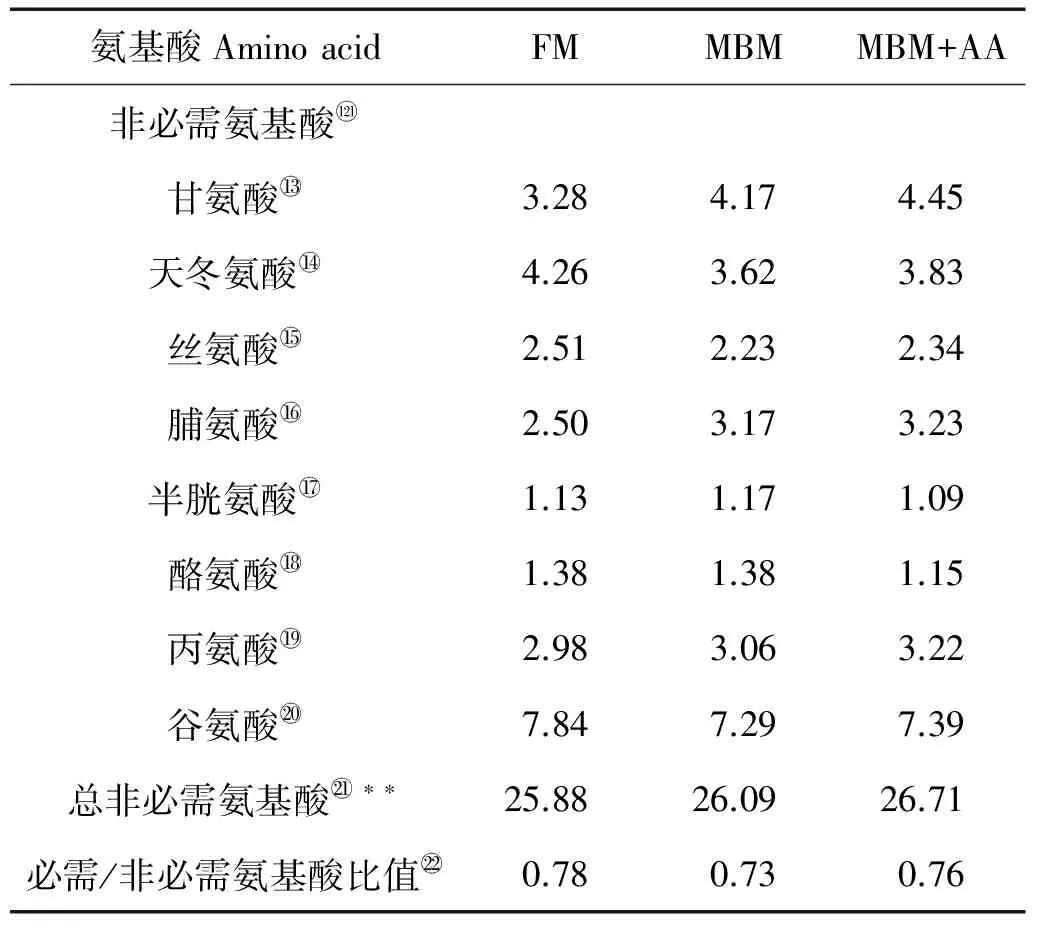
氨基酸AminoacidFMMBMMBM+AA非必需氨基酸甘氨酸3.284.174.45天冬氨酸4.263.623.83丝氨酸2.512.232.34脯氨酸2.503.173.23半胱氨酸1.131.171.09酪氨酸1.381.381.15丙氨酸2.983.063.22谷氨酸7.847.297.39总非必需氨基酸∗∗25.8826.0926.71必需/非必需氨基酸比值0.780.730.76
注:FM:全鱼粉对照组;MBM:肉骨粉替代45%鱼粉蛋白组; MBM+AA:肉骨粉替代45%鱼粉蛋白添加晶体氨基酸至鱼粉组必需氨基酸水平组。*:所有必需氨基酸量的总和;**:所有非必需氨基酸量的总和。
Note:FM: fishmeal diet;MBM: meat and bone meal replaced 45% fishmeal protein diet;MBM+AA: meat and bone meal replaced 45% fishmeal protein and supplemented essential amino acids to match the essential amino acids profile of the FM diet. *:Represents total EAA;**:Represents total NEAA.
1.2 实验用鱼及实验过程
实验用鱼大菱鲆幼鱼购买于山东省海阳市黄海水产有限公司。养殖实验在山东省海阳市黄海水产公司养殖基地开展,开始实验前,实验所用大菱鲆幼鱼在养殖系统中暂养2周以适应养殖环境,期间投喂等量混合的3种实验饲料。暂养后选取规格均一的大菱鲆幼鱼随机分组,其平均体重为(9.19±0.01)g。实验每个处理随机分配3个养殖桶(玻璃纤维,500 L),每桶放置40尾鱼。Cai等[27]研究饲料中脂肪水平对大黄鱼(Pseudosciaenacrocea)的脂质代谢基因的影响和Yuan等[28]探究饲料中脂肪水平对半滑舌鳎(Cynoglossussemilaevis)幼鱼脂质代谢相关基因的影响等实验的养殖周期为30天。依据Xu等[22]探究豆粕替代鱼粉对大菱鲆幼鱼营养感知信号通路的影响的实验周期设定了本实验的养殖周期为30天,每天分别在07:00和19:00饱食投喂2次,投喂1 h后记录剩余的残饵数量,并进行换水保证养殖水质。养殖期间,水温为19~22 ℃,盐度为29~33,PH为7.5~8.0,水溶氧含量为6.0~7.0 mg/L。
1.3 样品采集
30天养殖实验结束后,实验鱼饥饿48 h使鱼体代谢达到稳定水平[29],从每个处理中随机选取6尾鱼,即为0 h样品。所剩的实验鱼再重新投喂各自的饲料至饱食状态,分别在投喂后2、8和24 h分别随机取样,所选取样的鱼都确保充分摄食饲料,消化道中充满食物。肠道是鱼类吸收食物中包括氨基酸/蛋白质在内的营养成分的主要器官[30],饲料氨基酸首先被肠道感知;肌肉是鱼体蛋白质周转代谢的主要器官[31],氨基酸是组成蛋白质的底物,氨基酸应答信号通路是负调控机体蛋白质合成的主要途径,而肝脏是机体中间代谢的主要器官[32],因此本实验随机选取实验鱼放置于冰盘上解剖采取全肠和背肌,迅速将所取得样品置于液氮中,-80 ℃保存备用。
1.4 肠道、肌肉RNA的提取及反转录
采用Invitrogen公司的Trizol提取肠道和肌肉组织中总RNA,具体的提取过程参考李明珠[33]实验方法。使用1.2%的琼脂糖凝胶电泳检测RNA的完整性,并使用NanoDrop 2000检测提取RNA的质量和浓度。取1μg RNA使用PrimeScript RT reagent Kitwith gDNA Eraser(TAKALA, Japan)进行反转录,具体步骤按照试剂盒说明书。
1.5 实时荧光定量PCR(qRT-PCR)
以大菱鲆RNA聚合酶II亚基D(RNA polymerase II subunit D, RPSD, GeneBank: DQ848899.1)作为内参基因。通过克隆得到CHOP核心序列,根据已获得的CHOP部分核心序列设计特异性定量引物(见表3)。另外根据已有文献报道大菱鲆GCN2、ASNS、ATF3、ATF4、REDD1、4E-BP1定量引物(见表3)进行荧光定量PCR实验[34]。反应体系为:12.5 μL 2×SYBR Premix Ex TaqTMII (TAKALA, Japan)、2μL模板cDNA、1 μL特异性引物(上游和下游引物,10 μmol/L)和9.5 μL无菌水,总反应体系25μL。反应条件:95 ℃ 2 min;95 ℃ 10 s,58 ℃ 10 s,72 ℃ 20 s,40个循环。在每个循环后,通过溶解度曲线检测PCR扩增的特异性。制作梯度稀释的浓度标准曲线检测目的基因与内参基因的扩增效率是否一致且接近于1,扩增效率计算公式E=10(-1/Slop)-1[35]。本实验中GCN2、ASNS、ATF3、ATF4、CHOP、REDD1、4E-BP1的扩增效率均于内参基因RPSD的扩增效率一致,目的基因的相对表达量采用2-(△△Ct)方法进行测定[36]。
1.6 数据处理与分析
本实验所有的实验数据使用SPSS 17.0软件进行双因素方差分析(two-way ANOVA),实验数据用平均值±标准误(means±SE)表示,MBM处理组和MBM+AA处理组目的基因表达量以FM组0 h的数值作为参照求比值,将FM组0 h的数值设为1,得到其他各种不同时间点的各数值。先将数据进行方差齐性检验,若数据方差不齐,则将数据进行平方根(SQRT)转换[22]。然后各处理组间做Tukey’s多重比较,P<0.05表示处理组间差异显著。
2 实验结果
2.1 肌肉中AAR信号通路中关键调控因子基因表达变化
如图1所示,FM、MBM和MBM+AA三个处理组禁食后再投喂都显著提高了肌肉中GCN2、ASNS、ATF3和ATF4 mRNA表达量(P<0.05),并且在进食后2~8 h表达量达到峰值,但是进食却显著降低了CHOP、REDD1和4E-BP1 mRNA的表达量(P<0.05)。与FM组相比,MBM组显著上调了肌肉中ASNS、ATF3、AFT4、CHOP、REDD1和4E-BP1基因峰值的表达量(P<0.05)。MBM+AA组的ASNS、ATF3、ATF4、CHOP、REDD1和4E-BP1的表达量的峰值与FM组相比没有显著性差异(P>0.05),但是却显著低于MBM组的表达量(P<0.05)。肌肉中GCN2的峰值表达量在3个处理组间没有显著差异(P>0.05)。

图1 摄食不同饲料对大菱鲆幼鱼肌肉中氨基酸应答信号通路中关键调节因子基因表达的影响
Fig.1 The key regulators involved in AAR signaling pathway gene expression level in muscle after juvenile turbot fed different diets
2.2 肠道中AAR信号通路中关键因子基因表达变化
如图2所示,与禁食相比,大菱鲆摄食3种实验饲料均显著促进了肠道中GCN2、ASNS、ATF3、ATF4和CHOP基因的表达量(P<0.05),且进食2~8 h时基因的表达量达到峰值,然而肠道中REDD1和4E-BP1的mRNA表达量却随着投喂时间的增加呈现显著下降的趋势(P<0.05)。与FM组相比,MBM组显著提高GCN2、ASNS、ATF3、ATF4、CHOP和4E-BP1的基因表达量的峰值(P<0.05)。MBM+AA组调控AAR信号通路中相关基因的表达量与MBM组的变化趋势相似,MBM+AA组也显著上调GCN2、ASNS、ATF3、ATF4、CHOP和4E-BP1的基因表达量的峰值(P<0.05)。


图2 摄食不同饲料对大菱鲆幼鱼肠道中氨基酸应答信号通路中关键调节因子基因表达的影响
Fig.2 The key regulators involved in AAR signaling pathway gene expression level in intestine after juvenile turbot fed different diets
3 讨论
AAR信号通路是机体应对氨基酸等营养不平衡的极端环境时启动的一条主要信号通路[37]。生物体的生长发育过程中不断的处于外界营养和能量环境的变化中,生物体通过这条信号通路维持机体在变化的环境中自身稳定[20,38]。本研究投喂大菱鲆不同实验饲料后发现,肠道和肌肉AAR信号通路相关基因表达均随着投喂时间显著变化。肌肉中GCN2、ATF4、ATF3、CHOP和ASNS的基因表达均在大菱鲆摄食饲料后随时间呈现出先升高后下降的趋势,并于2~8 h达到表达量峰值。而肌肉中REDD1和4EBP-1的表达量则在禁食时显著高于摄食后的表达量。肠道中的AAR相关基因的表达量在饥饿再投喂后,随着进食时间的变化具有相似的变化趋势。机体内相关基因的表达受到摄食时间的影响。在虹鳟中的研究结果表明,血浆中游离氨基酸的含量显著受到进食时间的影响,这与本实验的研究结果一致[39]。本实验研究发现,大菱鲆摄食不同实验饲料后,氨基酸应答基因表达的出现的峰值时间均相同,在豆粕替代鱼粉的实验中发现,大菱鲆摄食后其氨基酸转运载体和糖类、脂类代谢基因表达出现峰值的时间均没有受到摄食饲料成分的影响[22]。这在一定程度上说明了食物的营养组成对于大菱鲆营养感知基因表达周期性变化没有显著的调控作用。
鱼类摄食食物并感知食物中的营养组成首先从肠道开始,食物在肠道中被消化酶消化后进入肠道细胞,后转运到机体其他组织细胞中参与多种营养物质的代谢过程。研究表明,肠道不仅是机体消化和吸收的主要场所,也是机体感知营养变化的主要器官[22,30],而肌肉则是鱼类营养物质的沉积和储存最重要的器官[31]。本研究发现,大菱鲆摄食不同实验饲料显著影响肠道和肌肉中AAR信号通路中关键因子的表达,大菱鲆摄食MBM组饲料后,其肌肉和肠道中AAR信号通路中相关基因(ATF4、ATF3、CHOP和ASNS)表达相较于摄食FM组均显著的上调,而相比于大菱鲆摄食MBM组饲料,摄食MBM+AA组饲料其肌肉中AAR信号通路中相关基因表达显著降低,但是肠道中相关基因的表达却没有显著降低的变化。这一实验结果与豆粕替代鱼粉对大菱鲆AAR信号通路的研究结果一致[22]。在军曹鱼的研究中发现,摄食缺乏蛋氨酸的饲料时其AAR信号通路被显著激活[23]。本研究分析MBM组实验饲料的氨基酸组成发现,肉骨替代鱼粉后蛋氨酸、赖氨酸和苏氨酸的含量低于FM组,添加晶体氨基酸处理组的饲料必需氨基酸组成与FM组没有显著性差异,可以下调肌肉中AAR信号通路中关键调控因子的表达使之与FM组没有显著差异,但是未能降低AAR信号通路中关键调控因子在肠道中的表达,这表明肉骨粉替代鱼粉后造成肌肉和肠道成氨基酸应答基因表达上调作用,在一定程度上是由其替代鱼粉后造成食物中缺乏必需氨基酸造成的。然而,添加晶体氨基酸并未能够完全抑制AAR信号通路相关基因表达,这可能是由于晶体氨基酸吸收不同步造成机体同样出现一定程度的氨基酸缺乏现象[40]。
TOR信号通路是机体内调控营养代谢的核心通路[41],最近的研究发现AAR信号通路能够感知机体氨基酸变化进一步调控TOR信号通路[13]。研究表明,REDD1在AAR信号通路调控TOR信号通路的过程中发挥着联接的作用,激活的AAR上调REDD1的表达,其能够使TOR上游的关键复合体TSC1与TSC2分开,从而抑制了mTOR的活性[42-43]。本研究发现,REDD1的基因表达量在肉骨粉替代组显著的上调。同时分析TOR信号通路关键下游分子4E-BP1发现在MBM组中4E-BP1的基因表达量显著的上调,这在一定程度上表明肉骨粉替代组能够抑制TOR的活性。这也与在豆粕替代鱼粉在大菱鲆上的研究相[22]。因此,基于以上结果推测肉骨粉替代鱼粉后可能能够通过AAR通路影响TOR信号通路的活性。
4 结论
综上所述,本研究表明肉骨粉替代45%鱼粉蛋白显著上调大菱鲆肌肉和肠道中AAR信号通路中相关基因表达,当添加晶体氨基酸至鱼粉组水平时,可以在一定程度上改善这一现象。肉骨粉替代鱼粉也可一定程度上通过AAR信号通路进一步实现了对TOR信号通路的调控作用。这些研究结果,将有利于我们深入理解肉食性海水鱼类对不同蛋白源响应机制,为开发新型蛋白源提供理论依据。
[1] Gatlin D M, Barrows F T, Brown P, et al. Expanding the utilization of sustainable plant products in aquafeeds:A review[J]. Aquaculture Research, 2007, 38(6): 551-579.
[2] 曹霞, 曹瑾玲. 鱼粉的研究现状及展望[J]. 农产品加工: 创新版 (中), 2011(4): 69-75.
Cao X, Cao J. Research status and prospects of fish powder [J]. Agricultural Products Processing, Innovation, 2011(4): 69-75.
[3] Wang Y, Guo J , Bureau D P, et al. Replacement of fish meal by rendered animal protein ingredients in feeds for cuneate drum (Nibeamiichthioides)[J]. Aquaculture, 2006, 252(2): 476-83.
[4] 刘运正, 何艮, 麦康森, 等. 新型复合动植物蛋白源部分替代鱼粉对大菱鲆幼鱼生长和肉质的影响[J]. 中国海洋大学学报 (自然科学版), 2016, 46(1): 33-39.
Liu Y Z, He G, Mai K S, et al. Effect of partial replacement of dietary fishmeal with an animal and plant protein mixture on the growth performance and muscle texyure of juvenile turbot (ScophthalmusmaximusL.) [J]. Periodical of Ocean University of China(Natural Science), 2016, 46(1): 33-39.
[5] Kikuchi K, Sato T, Furuta T, et al. Use of meat and bone meal as a protein source in the diet of juvenile Japanese flounder[J]. Fisheries Science, 1997, 63(1): 29-32.
[6] Zhang S, Xie S, Zhu X, et al. Meat and bone meal replacement in diets for juvenile gibel carp (Carassiusauratusgibelio): Effects on growth performance, phosphorus and nitrogen loading[J]. Aquaculture Nutrition, 2006, 12(5): 353-362.
[7] Hardy R W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal[J]. Aquaculture Research, 2010, 41(5): 770-776.
[8] Bunpo P, Dudley A, Cundiff J K, et al. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase[J]. Journal of Biological Chemistry, 2009, 284(47): 32742-34279.
[9] Paddonjones D, Sheffieldmoore M, Zhang X J, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly[J]. American Journal of Physiology Endocrinology & Metabolism, 2004, 286(3): 321-328.
[10] Dudek S M, Semenkovich C F. Essential amino acids regulate fatty acid synthase expression through an uncharged transfer RNA-dependent mechanism[J]. Journal of Biological Chemistry, 1995, 270(49): 29323-29329.
[11] Dever T E, Feng L, Wek R C, et al. Phosphorylation of initiation factor 2α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast[J]. Cell, 1992, 68(3): 585-596.
[12] Su N, Kilberg M S. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene[J]. Journal of Biological Chemistry, 2008, 283(50): 35106-35117.
[13] Jin H O, Seo S K, Woo S H, et al. Activating transcription factor 4 and CCAAT/enhancer-binding protein-β negatively regulate the mammalian target of rapamycin via Redd1 expression in response to oxidative and endoplasmic reticulum stress[J]. Free Radical Biology and Medicine, 2009, 46(8): 1158-1167.
[14] Kilberg M S, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation[J]. Trends in Endocrinology & Metabolism, 2009, 20(9): 436-443.
[15] Jiang H Y, Wek S A, McGrath B C, et al. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response[J]. Molecular and Cellular Biology, 2004, 24(3): 1365-1377.
[16] Chaveroux C, Jousse C, Cherasse Y, et al. Identification of a novel amino acid response pathway triggering ATF2 phosphorylation in mammals[J]. Molecular & Cellular Biology, 2009, 29(29): 6515-6526.
[17] Kilberg M, Pan Y X, Chen H, et al. Nutritional control of gene expression: how mammalian cells respond to amino acid limitation[J]. Annual Review of Nutrition, 2005, (25): 59-85.
[18] Dever T E, Hinnebusch A G. GCN2 Whets the Appetite for Amino Acids[J]. Molecular Cell, 2005, 18(2): 141-142.
[19] Deval C, Chaveroux CMaurin A C, Cherasse Y, et al. Amino acid limitation regulates the expression of genes involved in several specific biological processes through GCN2-dependent and GCN2-independent pathways[J]. Febs Journal, 2009, 276(3): 707-718.
[20] Ye J, Kumanova M, Hart L S, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation[J]. The EMBO Journal, 2010, 29(12): 2082-2096.
[21] 许丹丹, 何艮. 氨基酸感知与代谢调控的研究进展[J]. 动物营养学报, 2015, 27(2): 342-351.
Xu D D, He G. Advances in Amino Acid Sensing and Metabolic regulation[J]. Chinese Journal of Animal Nutrition, 2015, 27(2): 342-351.
[22] Xu D, He G, Mai K, et al. Postprandial nutrient-sensing and metabolic responses after partial dietary fishmeal replacement by soyabean meal in turbot (ScophthalmusmaximusL.)[J]. British Journal of Nutrition, 2016, 115(3): 379-388.
[23] Wang Z, Mai K, Xu W, et al. Dietary methionine level influences growth and lipid metabolism via GCN2 pathway in cobia (Rachycentroncanadum)[J]. Aquaculture, 2016, 454: 148-156.
[24] Fournier V, Huelvan C, Desbruyeres E. Incorporation of a mixture of plant feedstuffs as substitute for fish meal in diets of juvenile turbot (Psettamaxima)[J]. Aquaculture, 2004, 236(1-4): 451-465.
[25] Wang Q, He G, Mai K, et al. Fishmeal replacement by mixed plant proteins and maggot meal on growth performance, target of rapamycin signalling and metabolism in juvenile turbot (ScophthalmusmaximusL.)[J]. Aquaculture Nutrition, 2015, 22:752-758. doi: 10. 1111/anu. 12296.
[26] Liu Y, He G, Wang Q, et al. Hydroxyproline supplementation on the performances of high plant protein source based diets in turbot (ScophthalmusmaximusL.)[J]. Aquaculture, 2014, 433: 476-480.
[27] Cai Z, Feng S, Xiang X, et al. Effects of dietary phospholipid on lipase activity, antioxidant capacity and lipid metabolism-related gene expression in large yellow croaker larvae (Larimichthyscrocea) [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2016, 201: 46-52.
[28] Yuan Y, Li S, Zhang L, et al. Influence of dietary lipid on growth performance and some lipogenesis-related gene expression of tongue sole (Cynoglossussemilaevis) larvae [J/OL]. Aquaculture Research, 2016. http://xueshu.baidu.com/s?wd=paperuri%3A%28d78d3930175de9b3932c1bc0f518b283%29&filter=sc_long_sign&tn=SE_xueshusource_2kduw22v&sc_vurl=http%3A%2F%2Fonlinelibrary.wiley.com%2Fdoi%2F10.1111%2Fare.12921%2Fabstract&ie=utf-8&sc_us=1426607 0529549240273. DOI:10.1111/are.12921.
[29] Seiliez I, Panserat S, Lansard M, et al. Dietary carbohydrate-to-protein ratio affects TOR signaling and metabolism-related gene expression in the liver and muscle of rainbow trout after a single meal[J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2011, 300(3): 733-743.
[30] Ravindran R, Loebbermann J, Nakaya H I, et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation[J]. Nature, 2016, 531(7595): 523-527.
[31] Mente E, Deguara S, Santos M B, et al. White muscle free amino acid concentrations following feeding a maize gluten dietary protein in Atlantic salmon (SalmosalarL.)[J]. Aquaculture, 2003, 225(1): 133-147.
[32] Furné M, García-Gallego M, Hidalgo M C, et al. Effect of dietary macronutrient proportion on intermediate metabolism and oxidative status in sturgeon (Acipensernaccarii) and trout (Oncorhynchusmykiss) [J]. Comparative Study Fish Physiology & Biochemistry, 2016, 42:1237-1248.
[33] 李明珠. 皱纹盘鲍 Δ5 脂肪酸去饱和酶研究[D]. 青岛: 中国海洋大学, 2013.
Li M Z. Research of Δ5 Fatty Acyl Desaturase in Abalone,HaliotisdiscushannaiIno[D]. Qingdao: Ocean University of China, 2013.
[34] Xu D, He G, Mai K, et al. Effect of fish meal replacement by plant protein blend onamino acid concentration, transportation, and metabolismin juvenile turbot (ScophthalmusmaximusL.) [J]. Aquaculture Nutrition, 2016,(待刊).
[35] Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25(4): 402-408.
[36] Yao C L, Kong P, Wang Z Y, et al. Molecular cloning and expression of MyD88 in large yellow croaker,Pseudosciaenacrocea[J]. Fish & Shellfish Immunology, 2009, 26(2): 249-255.
[37] Zhang P, McGrath BC, Reinert J, et al. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice[J]. Molecular and Cellular Biology, 2002, 22(19): 6681-6688.
[38] Wek S A, Zhu S, Wek R C. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids[J]. Molecular & Cellular Biology, 1995, 15(8): 4497-4506.
[39] Yamamoto T, Unuma T, Akiyama T. Postprandial changes in plasma free amino acid concentrations of rainbow trout fed diets containing different protein sources[J]. Fisheries Science, 1998, 64(3): 474-481.
[40] Schuhmacher A, Wax C, Gropp J M. Plasma amino acids in rainbow trout (Oncorhynchusmykiss) fed intact protein or a crystalline amino acid diet[J]. Aquaculture, 1997, 151(1): 15-28.
[41] Bd H J, Manning. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis[J]. Trends in Endocrinology & Metabolism, 2011, 22(3): 94-102.
[42] Ellisen L W. Growth control under stress: mTOR regulation through the REDD1-TSC pathway[J]. Cell Cycle, 2005, 4(11): 1500-1502.
[43] Brugarolas J, Lei K, Hurley R L, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex[J]. Genes & Development, 2004, 18(23): 2893-2904.
责任编辑 朱宝象
Influence of Fishmeal Replacement with Meat and Bone Meal on Amino Acid Response Signaling Pathway of Juvenile Turbot (ScophthalmusmaximusL.)
SONG Fei, XU Dan-Dan, ZHOU Hui-Hui, MAI Kang-Sen, XU Wei, HE Gen
(1.The Key Laboratory of Aquaculture Nutrition and Feeds, Ministry of Agriculture; 2.The Key Laboratory of Mariculture, Ministry of Education, Ocean University of China, Qingdao 266003, China)
The objective of this study was to evaluate the effect of fishmeal(FM) replacement with meat and bone meal (MBM) balanced with or without essential aminoacids (EAA) on the amino acid response (AAR) signaling pathway in juvenile turbot,ScophthalmusmaximusL. Fish (9.01±0.01)g were fed with three isonitrogenous and isoenergetic diets including 60% FM (FM diet); 45% FM replaced by MBM diet (MBM diet) and MBM diet supplemented with EAAs to match the EAA profile of FM diet (MBM+AA diet) for 30 days. Results showed that compared with the FM diet, MBM diet significantly up-regulated the gene expression peak levels of asparagine synthesis (ASNS), activating transcription factor3 (ATF3), activating transcription factor4(ATF4), CCAAT-enhance binding proteinhomology protein(CHOP), regulated in development and DNA damage responses 1 (REDD1) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1) in muscle. There were no difference between the MBM+AA diet and FM diet on the genes expression level of ASNS, ATF3, ATF4, CHOP, REDD1and 4E-BP1 at 2h after re-feeding. The MBM+AA diet and FM diet significantly down-regulated the expression of these genes in comparison with MBM diet. There was no significant difference on the expression of general control nonderepressible 2(GCN2) among the three diets. Similarly, in intestine, MBM diet significantly increased the gene expression peak level of GCN2, ASNS, ATF4, CHOP and 4E-BP1 in comparison with FM diet. Added EAA had no effect to decrease these genes expression level. In conclusion, 45% FM replaced by MBM diet up-regulated the gene expression level of the key regulators involved in AAR signaling pathway, and supplemented EAA had an effect to ameliorate MBM replacement deficiency to some extent.
turbot; meat and bone meal; fishmeal; amino acid response signaling pathway; key regulators; gene expression
国家自然科学基金项目(31222055,31572627);国家重点基础研究发展计划项目(2014CB138602)资助
2016-06-28;
2016-08-05
宋 飞(1989-),女,博士生,从事水产动物蛋白质代谢研究。E-mail: sophioe@163.com
** 通讯作者:E-mail:hegen@ouc.edu.cn
S963
A
1672-5174(2016)11-117-10
10.16441/j.cnki.hdxb.20160239
宋飞, 许丹丹, 周慧慧, 等. 肉骨粉部分替代鱼粉对大菱鲆幼鱼氨基酸应答信号通路的影响[J]. 中国海洋大学学报(自然科学版), 2016, 46(11): 117-126.
SONG Fei, XU Dan-Dan, ZHOU Hui-Hui, et al. Influence of fishmeal replacement with meat and bone meal on amino acid response signaling pathway of juvenile turbot (ScophthalmusmaximusL.)[J]. Periodical of Ocean University of China, 2016, 46(11): 117-126.
Supported by the National Nature Science Foundation of China (31222055,31572627); National Program on Key Basic Research Projects (2014CB138602)
