中国对虾蛋白磷酸酶1催化亚基β基因的克隆表达及特性分析*
2016-11-17何亮银唐小千绳秀珍战文斌
何亮银, 李 微, 唐小千, 邢 婧, 绳秀珍, 战文斌
(中国海洋大学水产动物病害与免疫实验室, 山东 青岛 266003)
中国对虾蛋白磷酸酶1催化亚基β基因的克隆表达及特性分析*
何亮银, 李 微, 唐小千, 邢 婧, 绳秀珍, 战文斌**
(中国海洋大学水产动物病害与免疫实验室, 山东 青岛 266003)
本论文利用RACE(Rapid-amplification of cDNA ends)技术克隆获得中国对虾(Fenneropenaeuschinensis)蛋白磷酸酶1催化亚基β(Protein phosphatase 1 catalytic subunit beta isoform,PP1β)基因cDNA序列全长。该基因全长1 214 bp, 包含一个987 bp的开放阅读框,编码328个氨基酸。同源性分析显示,中国对虾PP1β氨基酸序列与不同物种PP1β的相似性高达90%~91%,表现出高度保守性。多序列比对结果显示,不同物种PP1β均含有丝氨酸/苏氨酸特异性蛋白磷酸酶家族的特征基序GDxHG、GDxVDRG和GNHE。系统进化树分析显示,甲壳动物PP1β聚为一大支,中国对虾PP1β和凡纳滨对虾(Litopenaeusvannamei)聚为一小支。实时荧光定量PCR分析显示,PP1β在健康的中国对虾各组织中均有不同程度的表达,其中在性腺中表达最高,血细胞次之。白斑症病毒(White spot syndrome virus, WSSV)注射感染健康中国对虾后,血细胞和性腺中PP1β基因均呈上调表达,并在12 h达到峰值,且在血细胞中上调表达更显著。构建了中国对虾PP1β基因原核重组表达载体pET28a-PP1β,转化大肠杆菌后成功诱导表达重组PP1β蛋白(rPP1β),分子量为41 kDa。将亲和层析纯化的rPP1β免疫BALB/c小鼠制备抗血清,通过制备中国对虾血细胞滴片,应用间接免疫荧光法检测PP1β在血细胞中的分布情况,结果显示,中国对虾PP1β在血细胞的核区及细胞质内均有分布。本研究结果为进一步解析中国对虾PP1β与WSSV感染的相互关系提供了数据。
中国对虾;蛋白磷酸酶1;基因克隆;原核表达;免疫荧光
蛋白磷酸酶(Protein phosphatase)是控制蛋白质去磷酸化的关键酶,参与细胞内信号转导途径的调控,从而在生物体的生长发育、新陈代谢、细胞的分裂分化、细胞间通讯、基因表达、离子通道活性和免疫反应等多方面发挥重要作用[1-3]。根据蛋白磷酸酶作用底物的特异性可将其分为两大类:丝氨酸/苏氨酸蛋白磷酸酶和酪氨酸蛋白磷酸酶,其中,根据酶对底物的特异性、对抑制物的敏感程度差异和对不溶离子的需要,真核生物的丝氨酸/苏氨酸蛋白磷酸酶可分为蛋白磷酸酶1(Protein phosphatase 1,PP1),2A和2B三个主要亚型[4]。
在高等哺乳动物中已有较多报道显示,宿主细胞的蛋白磷酸酶可以和病毒之间存在密切联系,参与病毒的转录、复制和生命周期调控[5-8]。然而,目前在甲壳动物中蛋白磷酸酶与病毒间的互作研究十分有限,近年来有研究发现凡纳滨对虾(Litopenaeusvannamei)的一种蛋白磷酸酶被证实可以和白斑症病毒(White spot syndrome virus,WSSV)开放阅读框(ORF)427[9]和ORF403[10]编码蛋白相结合,该酶与已报道物种的PP1催化亚基β(PP1β)具有较高的同源性。中国对虾(Fenneropenaeuschinensis)广泛分布于中国黄、渤海,东海和南海也有少量分布,是中国北方沿海地区重要的海水养殖品种。对虾白斑病(White spot disease, WSD)自1990年代暴发以来,已成为全球甲壳动物养殖产业最主要的威胁,给对虾养殖产业造成了重大的经济损失[11]。本实验室的前期研究发现,中国对虾在感染WSSV后,其血细胞中的蛋白磷酸酶发生了显著上调表达[12],推测其可能与WSSV感染之间存在密切关系。
为进一步解析中国对虾蛋白磷酸酶在WSSV感染之间的关系,本研究克隆并分析了中国对虾PP1β基因,利用实时荧光定量PCR检测其在各组织中的表达情况以及对虾在感染WSSV后该基因的应答表达情况。同时,对中国对虾PP1β进行了原核重组表达,并制备了重组PP1β蛋白(rPP1β)的多克隆抗体,应用间接免疫荧光技术检测了PP1β在血细胞中的分布情况。
1 材料与方法
1.1 总RNA提取及cDNA第一链的合成
性成熟的健康海捕中国对虾成虾购自青岛水产品市场,均为雄虾,体长15~17 cm,经PCR检测为WSSV阴性[12];按照Zhong等所述方法[13],以血淋巴∶抗凝剂(27 mmol/L sodium citrate, 336 mmol/L NaCl, 115 mmol/L glucose, 9 mmol/L EDTA, pH=4.2)为1∶1的体积比从中国对虾围心腔抽取血淋巴,分离血细胞,使用Trizol法提取总RNA,1%琼脂糖凝胶电泳检测RNA的完整性,nano-drop测定其浓度和纯度,取2 μg总RNA经RT-PCR合成cDNA第一链。
1.2PP1β基因的全长克隆
根据凡纳滨对虾、印度跳蚁(Harpegnathossaltator)和佛罗里达弓背蚁(Camponotusfloridanus)的PP1β序列,设计简并引物PP1β-F1和PP1β-R1(见表1)扩增中国对虾PP1β保守序列,根据获得的PP1β保守基因片段设计3’和5’RACE特异性引物PP1β-3’1、PP1β-3’2、PP1β-5’1、PP1β-5’2(见表1),分别与SMART cDNA合成试剂盒(Takara,Japan)内的引物UPM和NUP联合使用扩增中国对虾PP1βcDNA的3'和5'端序列。将所得到的3个片段拼接,获得PP1βcDNA全长,设计引物PP1β-F2和PP1β-R2(见表1)进行基因全长的克隆验证。PCR扩增条件为:94 ℃ 5 min;94 ℃ 30 s,58 ℃ 40 s,72 ℃ 1 min 30 s,35个循环;72 ℃ 10 min。PCR产物经1%琼脂糖凝胶电泳检测,回收纯化,连接到pMD-19T载体,转化感受态细胞DH5α,利用含氨苄青霉素的LB培养平板进行筛选,PCR检测阳性菌落后,送上海桑尼生物技术有限公司测序。
1.3PP1β基因的生物信息学分析
利用ORF Finder分析中国对虾PP1β的开放阅读框(Open reading frame,ORF)并推导其氨基酸序列,Smart在线软件(http://smart.embl-heidelberg.de/)预测蛋白功能域,ProtParam tool (http://web.expasy.org/protparam/)分析蛋白等电点与分子量,NetOGlyc 3.1 Server (http:// www.cbs.dtu.dk/services/NetOGlyc-3.1/)和NetPhos 2.0 Server(http://www.cbs.dtu.dk/ services/NetPhos/)分析O-糖基化位点和磷酸化位点。使用BLAST程序进行PP1β的同源性比对,将不同物种的PP1β氨基酸序列通过Clustal X 2.0软件进行多序列比对分析,用MEGA 4.0软件进行系统发生和进化分析,采用邻位相连法构建系统进化树。
1.4 实时荧光定量PCR检测PP1βmRNA的组织分布情况
为检测PP1βmRNA在中国对虾各组织中的表达差异,随机选取3尾健康中国对虾,取其血细胞、心脏、肝胰腺、肠、性腺、淋巴器官、鳃和肌肉,分别提取各组织总RNA,反转录合成cDNA链,测定并调整cDNA浓度到一致,以此为模板,以PP1β-F3和PP1β-R3(见表1)为引物扩增目的片段,每个样品做3个平行,18S rRNA作为内参。PCR扩增条件为:95℃ 2min;95℃ 10s,58℃ 10s,72℃ 20s,45个循环。根据测得的Ct值,利用2-Ct法计算不同组织中PP1β基因的相对表达量。
1.5 实时荧光定量PCR分析PP1β基因对WSSV感染的应答表达
取冻存于-80℃患白斑综合征病毒病的中国对虾的鳃,按照Li等所述方法[12]制备WSSV粗提液并进行病毒浓度的测定,0.01mol/L无菌磷酸盐缓冲液(PBS, 137mmol/L NaCl, 2.7mmol/L KCl, 8.09 mmol/L Na2HPO4, 1.47mmol/L KH2PO4, pH=7.4)调整WSSV浓度至108拷贝/mL,实验对虾分为感染组和对照组,分别注射100μL WSSV 粗提液 (107拷贝)或PBS。各组于感染后0、6、12、24、36、48和72h随机抽取中国对虾6尾,收集血细胞和性腺,进行RNA提取和实时荧光定量PCR实验,具体方法同1.4。利用2-ΔΔCt法计算WSSV感染后对虾血细胞和性腺组织中PP1β基因的相对表达量的变化情况。
1.6PP1β的原核表达与纯化
设计分别带有酶切位点KpnI和Hind Ⅲ的原核表达用引物PP1β-F4和PP1β-R4(见表1),扩增PP1β编码基因全长。对扩增片段和pET-28a(+)载体分别进行双酶切,切胶纯化,以T4 DNA连接酶连接目的片段和载体,构建重组表达质粒pET-28a-PP1β,转化至大肠杆菌BL21(DE3)感受态细胞,随后将阳性菌株进行测序确认。
挑取阳性重组菌株的单菌落接种至LB液体培养基中(含50μg/mL卡那霉素),37℃,220r/min振荡培养,培养菌液的OD600为0.6时,向菌液中加入1.0mmol/L的异丙基-β-D-硫代半乳糖苷(IPTG)诱导大肠杆菌表达目的蛋白,继续振荡培养4h后,离心收集菌体,加PBS超声破碎30min,离心取沉淀,利用SDS-PAGE检测目的蛋白表达情况,以未诱导的菌体总蛋白为对照。将重组菌大量培养,离心破碎收集菌体,参照Qiagen镍琼脂糖亲和层析柱蛋白纯化操作步骤,在变性条件下纯化以包涵体形式表达的融合蛋白,尿素梯度透析复性,SDS-PAGE 电泳检测重组PP1β蛋白(r PP1β)纯化结果。
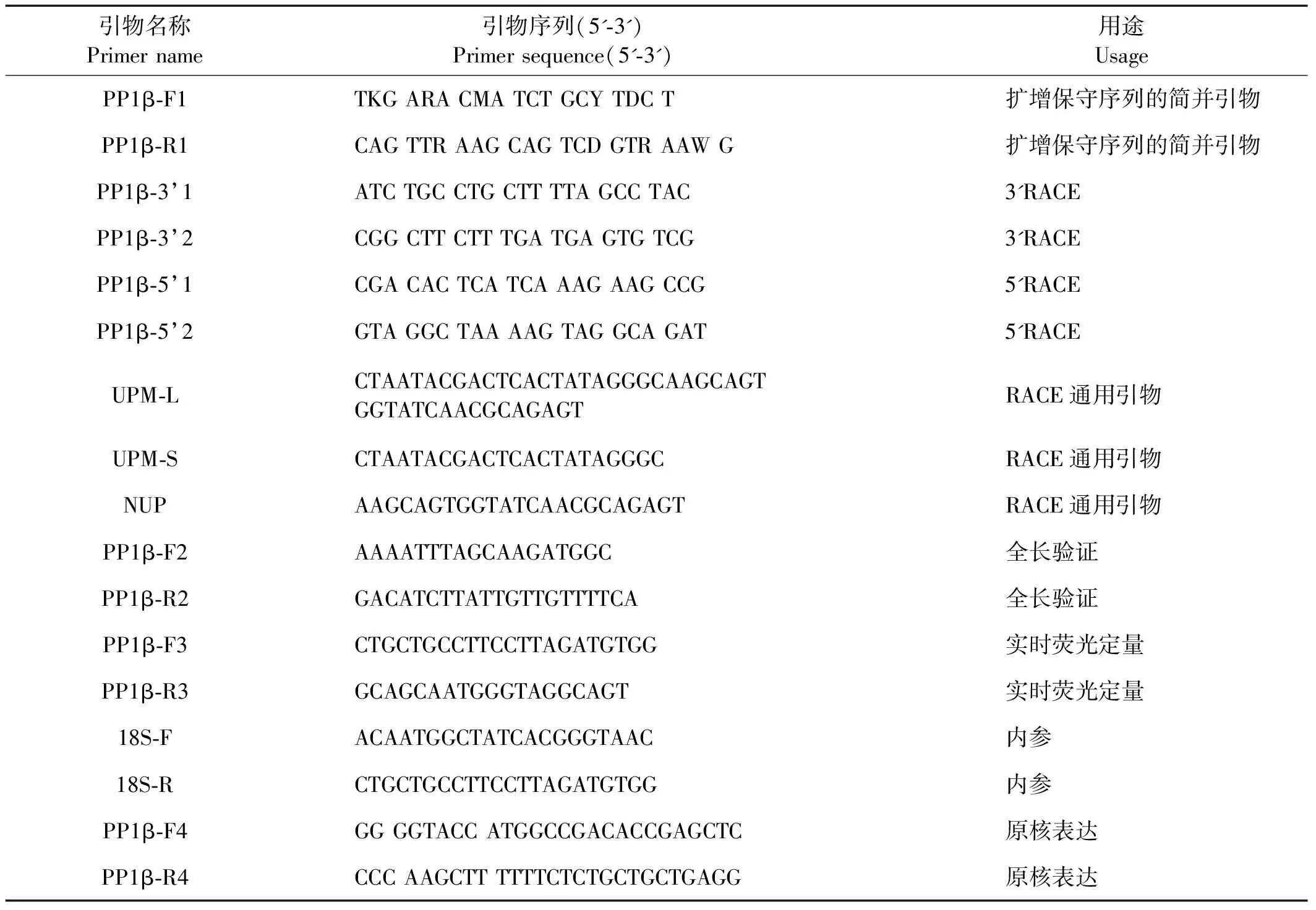
表1 中国对虾 PP1β cDNA全长扩增、实时荧光定量PCR和原核表达所用引物
1.7 鼠抗rPP1β血清的制备
取纯化复性后的rPP1β,按照Tang等所述方法[14]分4次免疫购自山东大学实验动物中心的SPF级BLAB/c小鼠。第3次加强免疫后第7天心脏一次性采血,室温倾斜放置2h,置于4℃过夜;次日5 000g离心20min得抗血清。间接ELISA方法测得鼠抗rPP1β血清效价。同时,参考Tang等报道的方法[15]利用Western blotting检测制备鼠抗血清的特异性。
1.8 间接免疫荧光实验(IIFA)检测PP1β在血细胞上的定位
用吸有4 ℃预冷抗凝剂的注射器从WSSV感染12 h后的中国对虾围心腔抽取血淋巴液,按抗凝剂与血淋巴3∶1 比例混匀,800g离心20min,弃上清;沉淀用抗凝剂重悬,800g离心20min,弃上清;沉淀用PBS重悬,滴于干净载玻片上,室温沉降1h,丙酮固定。以制备的鼠抗rPP1β血清为第一抗体,滴加在上述制备的血细胞滴片上,37℃湿盒中孵育45min,以正常鼠血清为阴性对照;PBST(PBS containing 0.05% Tween 20)洗3次,每次5min;以FITC标记的羊抗鼠IgG(Sigma,1∶256)为第二抗体,滴加在血细胞滴片上,37℃湿盒中孵育45min;PBST洗3次,每次5min;甘油封片,荧光显微镜下观察。
1.9 数据处理
所得数据使用SPSS 19.0进行统计分析,采用单因子方差分析及Duncan多重比较处理,以P<0.05作为差异显著水平。
2 结果
2.1 中国对虾PP1β基因序列分析
中国对虾PP1βcDNA全长及相应的氨基酸序列如图1所示。中国对虾PP1β完整的cDNA全长1 214 bp(GenBank登录号:KF773851),包含一个987 bp的开放阅读框,编码328个氨基酸。PP1β编码蛋白由PP2Ac结构域和低复杂度区域组成,其理论等电点为5.68,理论分子量为37.6 kDa,氨基酸序列中包含14个磷酸化位点和1个O-糖基化位点。成熟肽中Leu所占比例最高(11.9%),其次是Asp(7.3%)和Gly(7.3%)。5’非编码区13 bp,3’非编码区214 bp,其中包含1个终止密码子,1个多聚腺苷酸加尾信号(AATAAA)。

(下划线:PP2Ac结构域;阴影:低复杂度区域;方框:多聚腺苷酸加尾信号(AATAAA)。PP2Ac domain was shown with underlines, and low complexity region was shaded in grey. The polyadenylation signals(AATAAA)in the 3’-UTR were also boxed.)
图1 中国对虾PP1βcDNA及推导的氨基酸序列
Fig.1 Full length cDNA sequence and deduced amino acid sequences ofF.chinensisPP1β
2.2 中国对虾PP1β与其他物种的同源性分析
将中国对虾PP1β的氨基酸序列通过BLAST在线分析显示,该序列与不同物种的PP1β序列的相似度高达90%~91%,表现出高度保守性。需要指出的是,中国对虾PP1β氨基酸序列与GenBank中一条C端缺失的凡纳滨对虾PP1β序列完全一致,相似性为100%。多序列比对结果显示,不同物种的PP1β均含有丝氨酸/苏氨酸特异性蛋白磷酸酶家族的特征基序GDxHG、GDxVDRG和GNHE(见图2)。构建的系统进化树显示,中国对虾PP1β与凡纳滨对虾聚为一小支,二者与其他甲壳动物PP1β聚为一大支(见图3)。
2.3PP1β基因在各组织中的表达
荧光定量PCR检测结果显示,PP1βmRNA在健康中国对虾8种组织中均有不同程度的表达,其中在性腺中的表达量最高,其次是血细胞,然后依次为淋巴器官、肠、鳃、心脏和肝胰腺,在肌肉中的表达量最低(见图4)。
2.4 WSSV感染后中国对虾血细胞和性腺中PP1β基因的表达变化
实时荧光定量PCR检测结果显示,在WSSV感染后中国对虾后血细胞和性腺中的PP1β基因均呈显著性上调表达,在感染后12 h,2个组织中PP1β表达量均达到峰值,其中血细胞中该基因的上调倍数显著大于性腺中,为(2.71±0.16)倍。峰值过后,PP1β基因的相对表达量在2个组织中均呈现逐渐降低的趋势但仍高于对照组水平,而与血细胞相比,性腺中的下调较为平缓,2种组织中该基因的相对表达量均于感染后72 h恢复至接近对照组水平(见图5)。
2.5 中国对虾PP1β基因的原核表达与抗体特异性分析
SDS-PAGE图谱显示,经诱导的含pET28a-PP1β表达质粒的大肠杆菌全蛋白中出现了一条分子量为41 kDa的特异性条带(见图6,泳道2),与理论预测值相符,而未经诱导的大肠杆菌没有出现相应的条带(见图6,泳道1)。重组蛋白经镍琼脂糖亲和层析纯化后获得了条带单一的高纯度rPP1β(见图6,泳道3)。应用制备的鼠抗rPP1β多克隆抗体结合Western blotting技术分析显示,多抗能特异性识别分子量为41 kDa的条带(见图6,泳道4),而阴性对照组未见条带(见图6,泳道5),表明制备的鼠抗rPP1β多克隆抗体特异性良好。

(序列比对所用PP1β物种来源及相应GenBank登录号如下:太平洋牡蛎(C.gigas), EKC31188;凡纳滨对虾(L.vannamei), AAT37505;印度跳蚁(H.saltator),EFN76901;佛罗里达弓背蚁(C.floridanus),EFN68092;斑马鱼(D.rerio),NP_001004527;热带爪蟾(X.tropicalis),CAJ81891;原鸡(G.gallus),NP_990453;褐家鼠(R.norvegicus),NP_037197;牛(B.taurus),NP_001029825;人(H.sapiens),NP_996759; “”:相同的氨基酸;“:”和“.”:相似的氨基酸;蓝色字体:PP2Ac结构域;阴影:丝氨酸/苏氨酸特异性蛋白磷酸酶家族的特征基序GDxHG,GDxVDRG和GNHE。GenBank accession number:C.gigas, EKC31188;L.vannamei, AAT37505;H.saltator,EFN76901;C.floridanus,EFN68092;D.rerio,NP_001004527;X.tropicali,CAJ81891;G.gallus,NP_990453;R.norvegicus,NP_037197;B.taurus,NP_001029825;H.sapiens,NP_996759. “” represents the same amino acid, “:” and “.” represent the similar amino acids. The PP2Ac domain was shown in blue font. The conserved catalytic domains which were specific for Ser/Thr phosphatases (GDxHG,GDxVDRG and GNHE) were shaded in gray.)
图2 中国对虾PP1β氨基酸序列与其他物种的多序列比对
Fig.2 Multiple alignment of the deduced amino acid sequences of PP1β fromF.chinensiswith those from other species
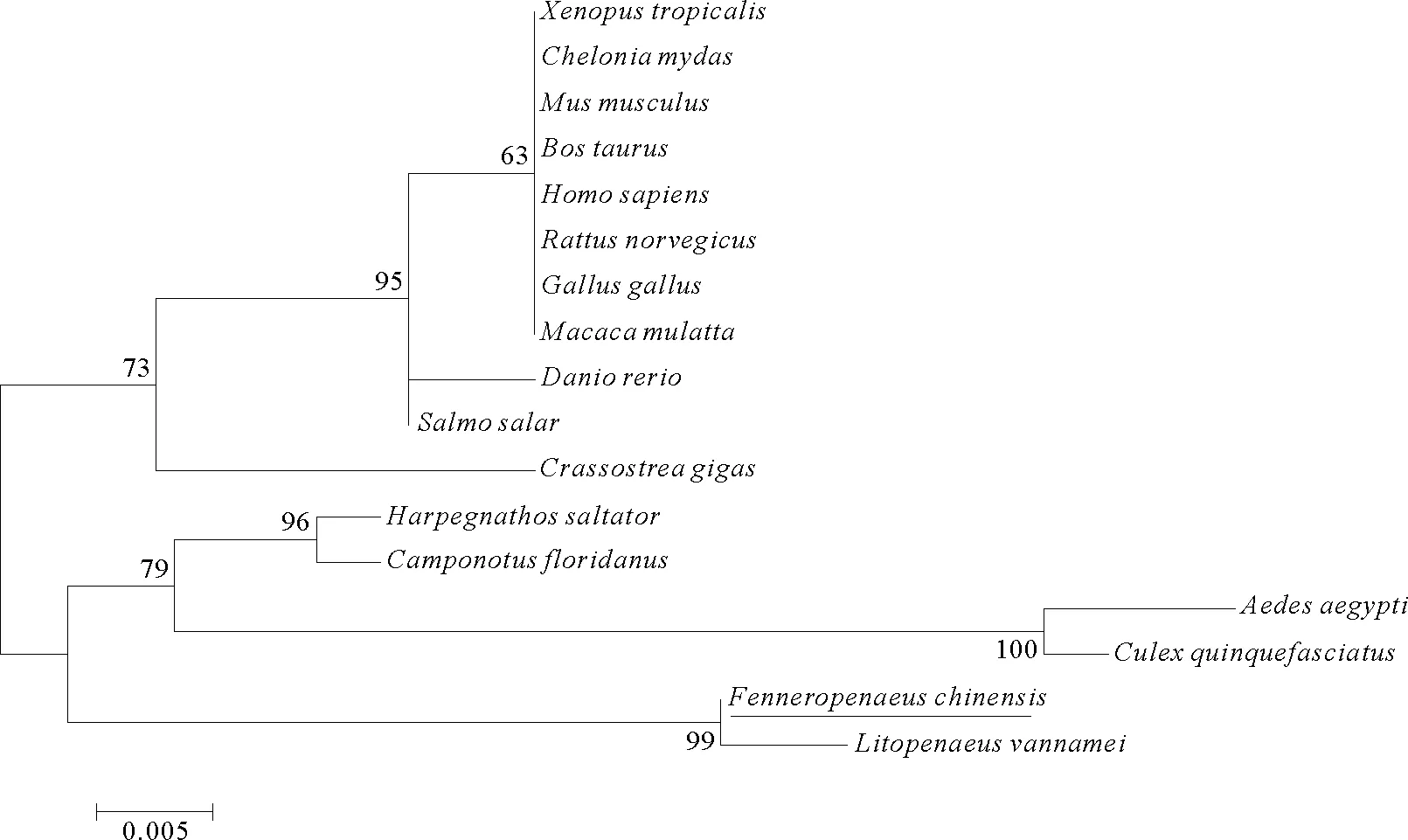
(系统进化树所用序列除图2中的11种之外,其他6种包括:猕猴(M.mulatta),NP_001247581;小家鼠(M.musculus),NP_766295;绿海龟(C.mydas),EMP24516;大西洋鲑(S.salar),ACN58678;埃及伊蚊(A.aegypti),XP_001663366;致倦库蚊(C.quinquefasciatus),XP_001843526。GenBank accession number (not including the ones in Figure 2):M.mulatta, NP_001247581;M.musculus, NP_766295;C.mydas, EMP24516;S.salar, ACN58678;A.aegypti, XP_001663366;C.quinquefasciatus, XP_001843526.)
图3 不同物种PP1β氨基酸序列系统进化树
Fig.3 Phylogenetic analysis of the deduced amino acid sequences ofF.chinensisPP1β
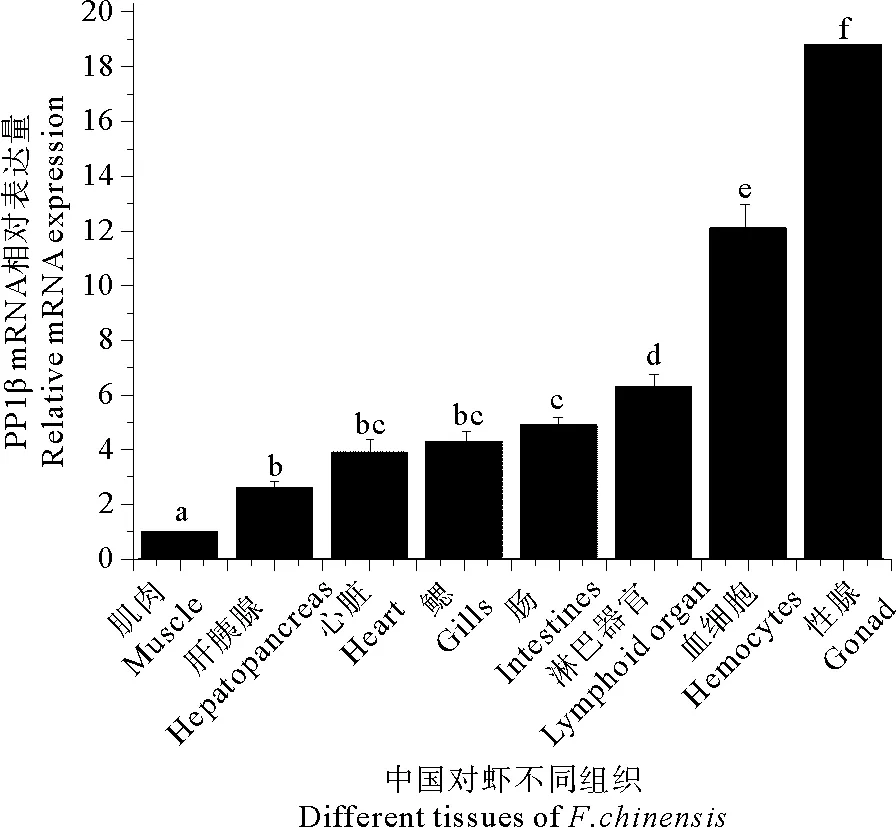
(小写字母表示各组织中PP1β mRNA相对表达量显著性差异水平(P<0.05)。Different letters indicates significant difference between different tissues (P<0.05).)
图4PP1βmRNA在中国对虾不同组织中的分布
Fig.4 Quantitative real-time RT-PCR analysis of tissues distribution ofPP1βtranscripts in healthyF.chinensis
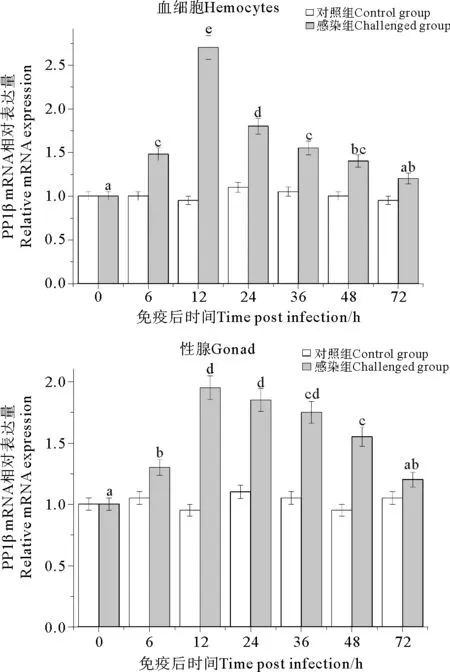
(小写字母表示感染组对虾PP1β基因表达量与对照组相比差异显著(P<0.05)。Different letters indicates significant difference between different tissues (P<0.05).)
图5 WSSV感染后中国对虾PP1β基因在血细胞和性腺中的时空表达分析
Fig.5 Expression profiles ofPP1βin hemocytes and gonad ofF.chinensispost WSSV infection
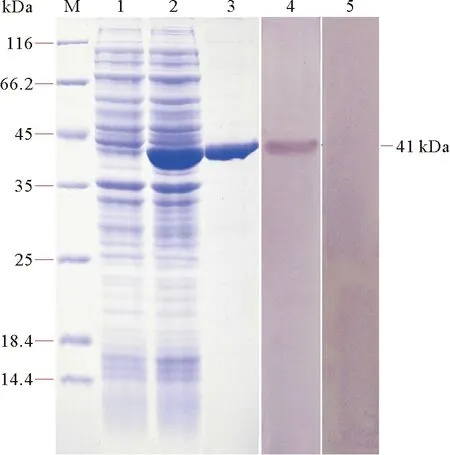
(M:标准分子量蛋白;1:未诱导的重组菌总蛋白;2:诱导后的重组菌总蛋白;3:纯化后的重组蛋白,4:鼠抗rPP1β血清与诱导后重组菌总蛋白反应;5:正常鼠血清与诱导后重组菌总蛋白反应。 M: Marker; 1: Total proteins from uninduced bacterial cell lysate; 2: Total proteins from induced bacterial cell lysate; 3: Purified recombinant protein; 4: Antisera against rPP1β reacted with induced bacterial cell proteins; 5: Negative control.)
图6 PP1β重组蛋白的SDS-PAGE及Western blotting分析
Fig.6 SDS-PAGE and Western blotting analysis of PP1β recombinant protein
2.6 中国对虾PP1β在血细胞上的定位
IIFA检测结果显示,鼠抗rPP1β血清能与中国对虾血细胞发生阳性反应,荧光显微镜下可见黄绿色荧光信号在血细胞的核区及细胞质内均有分布(见图 7B),而以正常小鼠血清作为对照时,未显示其与血细胞发生结合,血细胞中无黄绿色荧光信号,血细胞仅被伊文斯兰衬染为红色(见图 7A)。
3 讨论
PP1作为丝氨酸/苏氨酸蛋白磷酸酶家族中的一员,是一种高度保守的蛋白质,在真菌与哺乳动物间的同源性高达72%[16],本文克隆得到的中国对虾PP1β基因编码328个氨基酸,以其氨基酸序列进行BLAST比对,发现中国对虾PP1β蛋白与已报道的不同物种的PP1β蛋白序列相似度极高,为90%以上,这一结果证明了PP1β在不同物种间高度保守,与上述报道相一致。不同物种的PP1不仅在序列上极保守,而且在功能方面也具有一定的保守性,有研究指出,在真菌中由PP1突变而引起的表型变化在一定程度上能通过诱导外源哺乳动物的PP1基因从而得到缓解[17-18],由此本文推测,相较真菌而言与哺乳动物在进化上更为接近的对虾,其PP1分子也具有与高等动物相似的功能,参与到对虾生命活动调控的各个方面。
在哺乳动物中,蛋白磷酸酶参与生殖细胞的分化、精子移动和减数分裂等过程的调控,其在性腺组织中呈高丰度表达[19-20]。目前,无脊椎动物的蛋白磷酸酶是否具有上述功能还不清楚,但是有报道指出果蝇(Drosophilamelanogaster)体内存在多种特异性的蛋白磷酸酶(PpY-55A,PpN58A,PpD5,PpD6),这些酶在果蝇精巢中表达量极高[21-25]。本文以实时荧光定量PCR法检测到PP1βmRNA在对虾性腺中表达量最高,也与上述结论相符合,推测对虾性腺中的PP1β承担着与高等动物PP1β相似的功能。PP1β在中国对虾血细胞中表达量也比较高,并且在WSSV感染后,血细胞中PP1β基因的上调表达程度较性腺中更显著,这可能是由于血细胞是对虾免疫功能的主要承担者[26],PP1β参与了血细胞应答WSSV的反应所致。
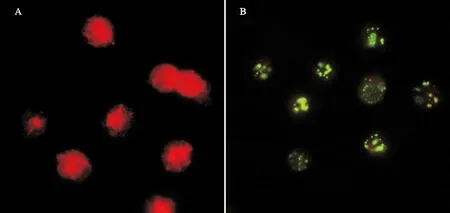
(A:血细胞与正常鼠血清反应;B:血细胞与鼠抗PP1β重组蛋白血清反应。A:Normal sera reacted with the haemocytes; B: Antisera against rPP1β reacted with the haemocytes.)
图7 PP1β在血细胞上的定位
Fig.7 Localization of PP1β in Chinese shrimp haemocytes by IIFA
在真核细胞内,几乎所有的信号转导过程都是通过由蛋白激酶和蛋白磷酸酶催化的磷酸化和去磷酸化作用来调节的。因此,蛋白磷酸酶被认为广泛分布于细胞的核区及细胞质内[27]。本文对PP1β重组蛋白进行了纯化,获得了纯度较高的目的蛋白,此纯化产物经简单的透析和冻干后,直接作为抗原免疫小鼠获得抗血清,免疫印迹实验证实制备的抗血清可与rPP1β发生特异性结合反应,显示其具有较好的特异性,应用间接免疫荧光实验检测发现PP1β存在于中国对虾血细胞的核区及细胞质内,该结果与上述观点相吻合,推测对虾PP1β与其他高等真核生物类似,在介导胞内信号转导等重要生物学过程中发挥着重要作用。
[1] Klumpp S, Krieglstein J. Serine/threonine protein phosphatases in apoptosis [J]. Current Opinion in Pharmacology, 2002, 2(4): 458-462.
[2] Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes[J]. Physiological Reviews, 2000, 80(1): 173-210.
[3] Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response[J]. Nature Reviews Immunology, 2005, 5(1): 43-57.
[4] 王柏婧, 谢秀杰, 魏群. Ⅰ 型蛋白磷酸酶研究进展[J]. 微生物学报, 2008, 48(2): 269-273.
Wang B J, Xie X J, Wei Q. Advances of protein phosphatase-1-A Review[J]. Acta Microbiologica Sinica, 2008, 48(2): 269-273.
[5] Tallóczy Z, Virgin H W, Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1[J]. Autophagy, 2006, 2(1):24-29.
[6] Ammosova T, Jerebtsova M, Beullens M, et al. Nuclear protein phosphatase-1 regulates HIV-1 transcription[J]. Journal of Biological Chemistry, 2003, 278(34): 32189-32194.
[7] Ilinykh P A, Tigabu B, Ivanov A, et al. Role of protein phosphatase 1 in dephosphorylation of Ebola virus VP30 protein and its targeting for the inhibition of viral transcription[J]. Journal of Biological Chemistry, 2014, 289(33): 22723-22738.
[8] Zhang F, Moon A, Childs K, et al. The African swine fever virus DP71L protein recruits the protein phosphatase 1 catalytic subunit to dephosphorylate eIF2α and inhibits CHOP induction but is dispensable for these activities during virus infection[J]. Journal of Virology, 2010, 84(20): 10681-10689.
[9] Lu L, Kwang J. Identification of a novel shrimp protein phosphatase and its association with latency-related ORF427 of white spot syndrome virus[J]. FEBS Lett, 2004, 577(1-2): 141-146.
[10] He F, Kwang J. Identification and characterization of a new E3 ubiquitin ligase in white spot syndrome virus involved in virus latency[J]. Virology Journal, 2008, 5: 151.
[11] Stentiford G D, Oidtmann B, Scott A, et al. Crustacean diseases in European legislation: Implications for importing and exporting nations[J]. Aquaculture, 2010, 306: 27-34.
[12] Li W, Tang X Q, Xing J, et al. Proteomic analysis of differently expressed proteins inFenneropenaeuschinensishemocytes upon white spot syndrome virus infection[J]. Plos One, 2014, 9(2): 89962.
[13] Zhong R J, Tang X Q, Zhan W B, et al. Expression kinetics of β-integrin in Chinese shrimp (Fenneropenaeuschinensis) hemocytes following infection with white spot syndrome virus[J]. Fish and Shellfish Immunology, 2013, 35: 539-545.
[14] 唐小千, 战文斌, 周丽, 等. 6种海洋致病性弧菌36 kDa外膜蛋白特性分析[J]. 中国海洋大学学报(自然科学版), 2009, 39(2):197-202.
Tang X Q, Zhan W B, Zhou L, et al. Characterization of 36 kDa Outer Membrane Proteins of Six PathogenicVibrioSpecies[J]. Periodical of Ocean University of China, 2009, 39(2):197-202.
[15] Tang X Q, Wang X L, Zhan W B. An integrin β subunit of Chinese shrimpFenneropenaeuschinensisinvolved in WSSV infection[J]. Aquaculture, 2012, 368: 1-9.
[16] Ceulemans H, Stalmans W, Bollen M. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution[J]. Bioessays, 2002, 24(4):371-381.
[17] Doonan J H, MacKintosh C, Osmani S, et al. A cDNA encoding rabbit muscle protein phosphatase 1 alpha complements the Aspergillus cell cycle mutation, bimG11[J]. Journal of Biological Chemistry, 1991, 266(28):18889-18894.
[18] Sangrador A, Andrés I, Eguiraun A, et al. Growth arrest of Schizosaccharomyces pombe following overexpression of mouse type 1 protein phosphatases[J]. Molecular and General Genetics, 1998, 259(5): 449-456.
[19] Nakamura K, Shima H, Watanabe M, et al. Molecular cloning and characterization of a novel dual-specificity protein phosphatase possibly involved in spermatogenesis[J]. Biochemical Journal, 1999, 344(3): 819-825.
[20] Smith G D, Wolf D P, Trautman K C, et al. Primate sperm contain protein phosphatase 1, a biochemical mediator of motility[J]. Biology of Reproduction, 1996, 54(3): 719-727.
[21] Arbeitman M N, Furlong E M, Imam F, et al. Gene expression during the life cycle ofDrosophilamelanogaster[J]. Science, 2002, 297: 2270-2275.
[22] Armstrong C G, Mann D J, Berndt N, et al.DrosophilaPPY, a novel male specific protein serine/threonine phosphatase localized in somatic cells of the testis[J]. Journal of Cell Science, 1995, 108: 3367-3375.
[23] Armstrong C G, Dombradi V, Mann D J, et al. Cloning of a novel testis specific protein serine/threonine phosphatase, PPN 58A, fromDrosophilamelanogaster[J]. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression, 1998, 1399: 234-238.
[24] Chintapalli V R, Wang J, Dow J A. Using FlyAtlas to identify betterDrosophilamelanogastermodels of human disease[J]. Nature Genetics, 2007, 39: 715-720.
[25] Vibranovszki M D, Lopes H F, Karr T L, et al. Stagespecific expression profiling ofDrosophilaspermatogenesis suggests that meiotic sex chromosome inactivation drives genomic relocation of testis-expressed genes[J]. PLoS Genetic, 2009, 5: 1000731.
[26] Johansson M W, Keyser P, Sritunyalucksana K, et al. Crustacean haemocytes and haematopoiesis[J]. Aquaculture, 2000, 191: 45-52.
[27] Mao H, Rosenthal K S. An N-terminal arginine-rich cluster and a proline-alanine-threonine repeat region determine the cellular localization of the herpes simplex virus type 1 ICP34. 5 protein and its ligand, protein phosphatase 1[J]. Journal of Biological Chemistry, 2002, 277(13): 11423-11431.
责任编辑 朱宝象
Cloning, Expression and Characterization of Protein Phosphatase 1 Catalytic Subunit Beta Isoform in ShrimpFenneropenaeuschinensis
HE Liang-Yin, LI Wei, TANG Xiao-Qian, XING Jing, SHENG Xiu-Zhen, ZHAN Wen-Bin
(Laboratory of Pathology and Immunology of Aquatic Animals,Ocean University of China,Qingdao 266003,China)
Protein phosphatase 1 (PP1) of host cells was documented to play a crucial role in virus infection in mammals, participating in the transcription, replication and life cycle regulation of virus. PP1 in hemocytes ofFenneropenaeuschinensiswas up-regulated significantly after WSSV infection as was demonstrated in our previous work, which indicated that PP1 ofF.chinensiswas involved in WSSV infection. To further illustrate the role of PP1 in WSSV infection, in present work, protein phosphatase 1 catalytic subunit beta isoform (PP1β) gene ofF.chinensiswas cloned and sequenced by rapid amplification of cDNA ends approaches (RACE). The full-length cDNA sequence ofPP1βgene was 1,214 bp, and contained an open reading frame (ORF) of 987 bp that encoded for a polypeptide of 328 amino acids. Homology comparison showed that PP1β ofF.chinensisshared 90%~91% amino acids with thst of other species, indicating the high conservation ofPP1βgene. Multiple sequence alignment was performed using the ClustalW Multiple Alignment program. It was demonstrated that all amino acid sequences of PP1β from various species contained three conserved catalytic domains which were specific for Ser/Thr phosphatases, GDxHG,GDxVDRG and GNHE. A neighbor-joining (NJ) tree was constructed based on the protein sequences of PP1β from 17 species by the NJ algorithm using MEGA 4.0 software package and Clustal X using α-lactalbumin as outgroup. The result showed thatF.chinensiswas clustered withL.vannamei, and PP1β of crustaceans gathered in one branch. By quantitative real-time RT-PCR,PP1βgene mRNA was observed in all the eight tissues of healthyF.chinensis, with the high transcription level in gonad and hemocytes. The high transcription level ofPP1βgene in gonad suggested that PP1β possibly involved in germ cell differentiation and spermatogenesis as was reported in mammals. Moreover, the gene in the above two tissues was up-regulated after WSSV infection, with the peak value found at 12 h, while the fold change ofPP1βgene transcript abundance in hemocytes was higher than that in gonad. The higher transcription level ofPP1βgene observed in hemocytes suggested that PP1β might play an important role in resistance to WSSV infection. ThePP1βgene ORF was cloned into pET-28a expression plasmid and the recombinant plasmid was transformed intoE.coliBL21 (DE3). SDS-PAGE analysis showed that the molecular weight of recombinant protein was 41 kDa. Subsequently, rPP1β was purified by using affinity chromatography, and the polyclonal antibody against rPP1β was produced by immunizing mouse. The localization of PP1β inF.chinensishaemocytes was determined by indirect immunofluorescence assay (IIFA). The results showed that PP1β was synthesized in cell nucleus and cytoplasm, which implied that PP1β might mediate kinds of biological processes such as the signal transduction inF.chinensis. Overall, this study provided important data for illustrating the relationship betweenF.chinensisPP1β and WSSV infection.
Fenneropenaeuschinensis; protein phosphatase 1 catalytic subunit beta isoform; gene cloning; prokaryotic expression; immunofluorescence
国家重点基础研究发展规划项目 (2012CB114405);“泰山学者特聘专家”项目;青岛海洋科学与技术国家实验室鳌山科技创新计划项目(2015ASKJ01);山东省科技发展计划项目(2014GNC111015);山东省自主创新及成果转化专项项目(2014ZZCX06205)资助
2016-01-27;
2016-04-21
何亮银(1987-),男,博士生,主要从事水产动物病害与免疫学研究。
** 通讯作者: E-mail:wbzhan@ouc.edu.cn
S91
A
1672-5174(2016)11-073-09
10.16441/j.cnki.hdxb.20160024
何亮银, 李微, 唐小千, 等. 中国对虾蛋白磷酸酶1催化亚基β基因的克隆表达及特性分析[J]. 中国海洋大学学报(自然科学版), 2016, 46(11): 73-81.
HE Liang-Yin, LI Wei, TANG Xiao-Qian, et al. Cloning, expression and characterization of protein phosphatase 1 catalytic subunit beta isoform in shrimpFenneropenaeuschinensis[J]. Periodical of Ocean University of China, 2016, 46(11): 73-81.
Supported by National Basic Research Program of China(2012CB114405); “Taishan Scholar Program of Shangdong Province”; The Scientific and Technological Innovation Project Financially Supported by Qingdao National Laboratory for Marine Science and Technology(2015ASKJ01); Science and technology development project of Shandong Province(2014GNC111015); Independent innovation and achievement transformation project in Shandong Province(2014ZZCX06205)
