What is the destiny of a threatened fish, Ptychobarbus chungtienensis, now that non-native weatherfishes have been introduced into Bita Lake, Shangri-La?
2016-11-15WanShengJIANGTaoQINWeiYingWANGYaPengZHAOShuSenSHUWeiHongSONGXiaoYongCHENJunXingYANG
Wan-Sheng JIANG, Tao QIN,2, Wei-Ying WANG, Ya-Peng ZHAO, Shu-Sen SHU,2, Wei-Hong SONG,Xiao-Yong CHEN,2,*, Jun-Xing YANG,*
1State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming Yunnan 650223, China
2Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Yezin Nay Pyi Taw 05282, Myanmar
3POWERCHINA Kunming Engineering Corporation Limited, Kunming Yunnan 650051, China
4Management Division of Yunnan Bitahai Nature Reserve, Shangri-La Yunnan 674400, China
What is the destiny of a threatened fish, Ptychobarbus chungtienensis, now that non-native weatherfishes have been introduced into Bita Lake, Shangri-La?
Wan-Sheng JIANG1, Tao QIN1,2, Wei-Ying WANG3, Ya-Peng ZHAO1, Shu-Sen SHU1,2, Wei-Hong SONG4,Xiao-Yong CHEN1,2,*, Jun-Xing YANG1,*
1State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming Yunnan 650223, China
2Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Yezin Nay Pyi Taw 05282, Myanmar
3POWERCHINA Kunming Engineering Corporation Limited, Kunming Yunnan 650051, China
4Management Division of Yunnan Bitahai Nature Reserve, Shangri-La Yunnan 674400, China
Biological invasion is a pervasive negative force of global change, especially in its effects on sensitive freshwater ecosystems. Even protected areas are usually not immune. Ptychobarbus chungtienensis is a threatened freshwater fish now almost confined to Bita Lake, in the Shangri-La region of Yunnan province, China. Its existence is threatened by the introduction of non-native weatherfishes (Misgurnus anguillicaudatus and Paramisgurnus dabryanus) by an unusual method known as ‘prayer animal release'. Periodic surveys revealed the ratio of invasive weatherfishes to P. chungtienensis has been increasing since the former species was first recorded from the lake in August, 2009. Ptychobarbus chungtienensis shows low genetic diversity in the relict Lake Bita population. Weatherfishes, however, have highly successful survival strategies. The degree of dietary overlap between the species is alarming and perhaps critical if food is found to be a limiting factor.
Biological invasion; Threatened fish;Prayer animal release; Genetic diversity; Dietary
lNTRODUCTlON
Biological invasions have become a pervasive agent of global change not only in homogenizing the earth's biota (Lodge, 1993;Simberloff et al., 2013), but also in challenging the conservation of biodiversity and natural resources (TEEB, 2010). Freshwater ecosystems are particularly sensitive to such invasions (Villéger et al., 2011), where invasive species become one of the major threats to the global freshwater biodiversity (Geist, 2011;Magurran, 2009). Although without enough attention, China is one of the countries suffering from serious biological invasions (Xu et al., 2006; Xing et al., 2016). When taking the intentional transplantation (Kang et al., 2013), escape of domestication stocks (Yang et al., 2011), and accompanied by other forms of introduction (Chen, 2010) into consideration, the biological invasion in the freshwater ecosystem in China is quite severe. Generally speaking,lacustrine ecosystem (lakes, ponds, etc.) are more sensitive than riverine ecosystem (rivers, streams, etc.), and the extinction, replacement of indigenous fish by introduced species is extremely common all over the lakes of China whether from plain lakes in the east (Chen et al., 2010), or plateau lakes in the west (Chen et al., 1998).1
Bita Lake is a plateau freshwater lake in the Yunnan Bitahai Nature Reserve, which is a major part of the Putatso National Park. It is also the core area of the Bitahai wetland, one of the Wetlands of International Importance (Ramsar sites in 2004),with an elevation of about 3 568 m a.s.l. and a catchment area 159 hm2(Yang & Ji, 2010) (Figure 1A-C). The fish fauna in Bita Lake is extremely simple, Ptychobarbus chungtienensis, one ofthe plateau schizothoracinae fishes (Cyprinidae), was recorded as the only fish species (Figure 1D), which was considered to be extraordinarily unusual in the evolution of species and the formation of lakes (Chen & Chen, 2002). Ptychobarbus chungtienensis is a threatened freshwater fish that has been listed as Endangered on the China Species Red List (Pan et al.,2010; Wang & Xie, 2004). It was originally recorded on the Zhongdian Plateau of the Jinsha River drainage (upper Yangtze) in China including Bita Lake, Napa Lake, Shudu Lake, the Naya,Xiaozhongdian and Geza Rivers1The population in Geza River was considered as a subspecies named P. chungtienensis gezaensis before with distinct morphological variations (Huang & Chen, 1986), but we now tentatively treated it as a distinct sister species P. gezaensis according to the regular practice in fish taxonomy that no more use of the category of subspecies.(Chen, 2010; Huang & Chen,1986). However, according to field surveys in recent years, it has been almost extirpated from its original localities, and only Bita Lake contains a relative large population (Jiang et al.,2013). The fact that the P. chungtienensis is endemic to Bita Lake and is the only native species present makes this lake ecosystem unique.
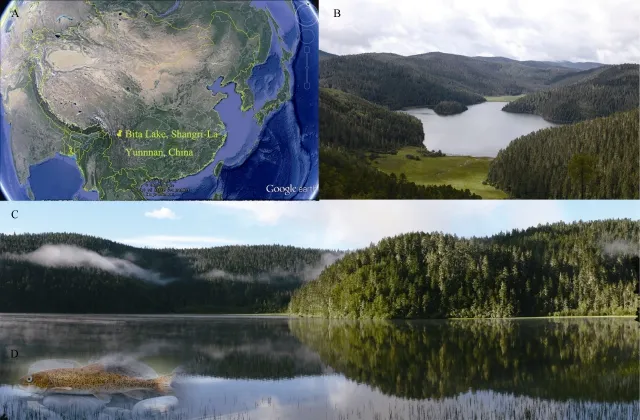
Figure 1 Bita Lake and the only native fish P. chungtiensis in itA: the location of Bita Lake (from the screenshot of Google Earth); B: the aerial view of Bita Lake from the Northern hillside; C: the nearby view of Bita Lake from the Northern lakeshore; D: the living photo of P. chungtiensis.
However, this special relationship of P. chungtienensis and Bita Lake has been interrupted by the introduction of non-native oriental weatherfishes species2These introduced oriental weatherfishes actually include two species with close relationships, similar morphology and ecological niches,Misgurnus anguillicaudatus and Paramisgurnus dabryanus. The two species were usually mixed in our samples so we simply refer to them as weatherfishes in this paper.at some point after 2009. The exotic species are generally threatening the native ones,particularly to those that have reduced populations with low genetic diversity (Magurran, 2009), and those in simple ecosystems with vulnerable trophic cascades (Estes et al.,2011). The situation in Bita Lake is possibly one example of this. From 2008 to 2013, we carried out periodic surveys of the fishes in Bita Lake. This paper aims to provide a dynamic assessment of P. chungtienensis and the introduced weatherfishes by successive monitoring data, and investigate the potential risks to this threatened species through its intrinsic genetic diversity and the extrinsic competition for food with weatherfishes.
MATERlALS AND METHODS
Acoustic surveys using scientific Echo sounders have increasingly become valuable and frequently-used methods in fish assemblages monitoring and fishery management(Fernandes, 2009). A portable Echo sounder HONDEX HE-51C operating at a frequency of 200 kHz was used during the surveys. Echo signals were recorded in the afternoon of eachinvestigating day along the same route. Four non-overlapping transects (BT1, 2, 3, 4) were chosen to cover the different depths, crossing the relative shallow areas in the east (<3 m),the deep areas in the mid-south (>7 m), and most areas with an average depth of 4 m. Echo recordings were cumulated at each transect, and the averages of each transect at different times each year were calculated to produce the curve of annual fluctuations. The recordings of the four transects from different times were statistically compared using the one-way ANOVA test (SPSS package). Fish samples were captured at night at the same day in three sites of the lake body using traps, and three surrounding inlet creeks using a backpack electro-shocker. The numbers of P. chungtienensis and weatherfishes in each sampling were recorded to see the changes of fish composition. Most of the fishes were released the next morning, and only a few samples were fixed by formalin to do the length-weight relationship, age determination, sex ratio and gonad analyses(Jiang et al., 2013), and conduct genetic diversity and dietary composition analyses in this study.
The genetic diversity of the mitochondrial cytochrome b (Cyt b) gene sequence of P. chungtienensis was analyzed. Total DNA of 81 specimens from Bita Lake were purified from alcohol-preserved fin by the standard methods (Sambrook et al.,1989). The following cycling conditions were used in polymerase chain reaction (PCR): an initial denaturing step at 95 °C for 4 min; 38 cycles of denaturing at 94 °C for 1 min,annealing at 52 °C for 1 min, and an extension at 72 °C for 1 min;with a final extension step of 72 °C for 10 min. Amplification primers of Cyt b were adopted from other Cyprinidae species as L14724 (5′-GACTTGAAAAACCACCGTTG-3′) and H15915(5′-CTCCGATCTCCGGATTACAAGAC-3′) (Xiao et al., 2001). All sequences have been deposited in GenBank (Accession No:KJ841795-KJ841875). Three sequences from GenBank(Accession No: FJ601043, AY463507, and AY463508, the last one from Xiaozhongdian River and the others from Bita Lake)were also downloaded and included in all of the analyses. One sequence of P. gezaensis (historically considered a subspecies)from the Geza River (GenBank Accession No. AY463506) was used as the outgroup to calculate the genetic distance and haplotype variations. Sequences were aligned with ClustalW(Thompson et al., 1994) and then calculated the pairwise distance (p-distance) in MEGA 6 (Tamura et al., 2013). The number of distinct haplotypes, haplotype diversity (h) (Nei, 1987)and nucleotide diversity (π) (Nei & Tajima, 1981) were calculated using DnaSP version 5.0 (Librado & Rozas, 2009) to estimate the standard indices of genetic diversity. A Median-Joining (MJ) approach (Bandelt et al., 1999) was performed to visualize relationships among haplotypes in Network v.4.6.1.2(http://www.fluxus-engineering.com/).
Dietary composition of P. chungtienensis and the weatherfishes was analyzed to compare their food niche relationships and degree of dietary overlap. Stomachs of all fishes collected were excised and fixed in a 10% formalin solution to avoid further digestion, and the total length (TL) and body weight (BW) were recorded. Prey items were identified to the lowest possible taxonomic level and enumerated based on their main physical characteristics under a microscope. All individuals and segments of each prey category were sorted dry and weighed jointly to the nearest 0.001 g. The percentage by number (%N), percentage by weight (%W), percentage frequency of occurrence (%F.O) and index of relative importance (IRI) were recorded to quantify the diet composition,and the overlap formula of Pianka (1973) and a modified PS index of Shorygin (1952) were used to assess the diet overlaps. These equations used to quantify the diet composition and assess the dietary overlap were listed below.
Percentage by number (N%)=(number (n) of individuals of a prey category/total number (n) of individuals among all prey categories)×100 (1)
Percentage by weight (W%)=(weight (g) of individuals of a prey category/total weight (g) of individuals among all prey categories)×100 (2)
Percentage frequency of occurrence (F.O%)=(number (n) of stomachs containing a prey taxon/total number (n) of stomachs containing prey)×100 (Bowen, 1996; Hyslop, 1980) (3)
IRI =%F.O (%N + %W) (Pinkas et al., 1971) (4)
where IRI is the index of relative importance, %N, %W and %F.O are these defined at (1, 2, 3).

where Ojkis the overlap index of Pianka which indicated the niche overlap of species j and k, Pijand Pikare the numerical proportions of the food item i used by the species j and k respectively.
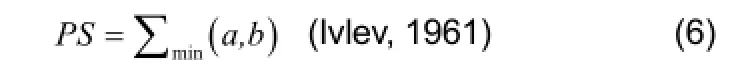
where a and b are the percentages by number of the prey common to the two predators, and the percentage similarity (PS) is a summation of the smaller of the values of a and b for each prey. The index ranges from 0 (no overlap) to 100 (complete overlap).
RESUTLS
Population dynamics of P. chungtienensis and weatherfishes
Eight effective Echo sounder surveys were made between 2009 and 2012. The fluctuations of mean numbers each year in each transects can be seen in Figure 2A. Generally, the first two transects (BT1 & 2) recorded more individuals than that in the last two transects (BT3 & 4). It might be a result of the different characters of specific length and depth of each transect. These recordings of transects were not significantly different among different years (from 2009 to 2012, P=0.270), which shows no significant change in the quantity of fish in these years. A total of 12 samples were used to compare the population dynamics of P. chungtienensis and weatherfishes can be seen in Figure 2B. The catch ratios of weatherfishes to P. chungtienensis varied from 0 (no weatherfishes) before August 2009 to a maximum of 16.2 in August 2012.
Genetic diversity of P. chungtienensis
A 1 140 base pair fragment of Cyt b sequence was amplified,with six variable sites and three parsimony-informative sites.
The overall p-distance within P. chungtienensis was 0.001, and the mean distance between P. chungtienensis and P. gezaensis was 0.017. Seven unique haplotypes were identified in all 84 sequences. The h and π values inferred from individuals of P. chungtienensis were 0.666 and 0.000 81, respectively. The Median-Joining network revealed a simple haplotype relationship for P. chungtienensis (Figure 3). There were at least 20 mutations isolating P. chungtienensis and its sister species P. gezaensis. Haplotype 1 (H1) was a relatively ancestral haplotype of P. chungtienensis, because it was shared by individuals from Bita Lake and the Xiaozhongdian River. Another comparable haplotype, H2, was shared by 37 individuals, and all other haplotypes radiated from H2 by only one or two mutations.
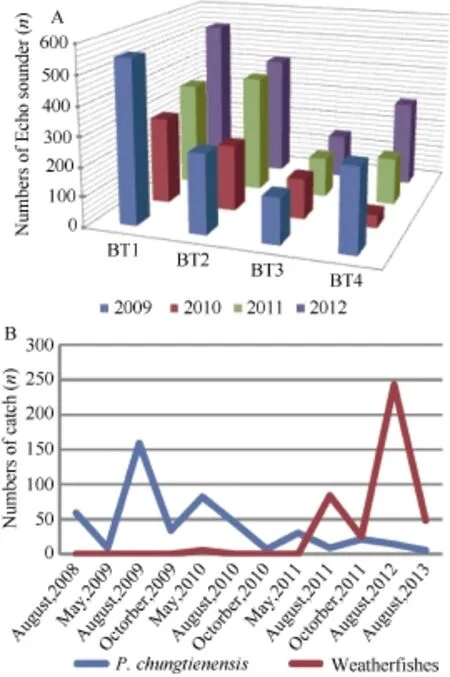
Figure 2 Field surveys of P. chungtiensis and weatherfishes in Bita LakeA: annual fluctuations in fish numbers (n) from Echo sounder recordings in four transects from 2009 to 2012; B: catch numbers (n) of P. chungtienensis and weatherfishes from 2008 to 2013.
Diet composition and overlapping of P. chungtienensis and weatherfishes
A total of 105 individuals of P. chungtienensis (90-221 mm in TL and 11.9-134 g in BW) were used to investigate stomach contents, of which 92 (87.6%) individuals contained food. The sample of weatherfishes included 125 individuals (76-203 mm in TL and 3.8 to 74.5 g in BW) which 119 (95.2%) had food. The diet composition (listed by N%, W%, F.O% and IRI) of P. chungtienensis and weatherfishes in Bita Lake is given in Table 1. According to the IRI values for P. chungtienensis, algae(Spirogyra and Zygnema) were most important, followed in order by Trichoptera (Philopotamus), Amphipoda (Gammarus bitaensis), and aquatic plants. These four items together represented 97.6% of the food intake. For weatherfishes,the sequence was Amphipoda (Gammarus bitaensis), algae(Spirogyra and Zygnema), and Diptera (larvae of Chironomus). These three items comprised 95.7% of the relative dietary importance. The overlap index of Pianka between P. chungtienensis and weatherfishes was 0.69. According to a study of trophic relationships of fishes in an Ontario temperate lake (Keast, 1978), the Ojkvalue of 0.3 or less was insignificant,and one of 0.7 or more was considered high overlap of prey composition. The PS index of Shorygin between P. chungtienensis and weatherfishes was 79.16%, which is much greater than the critical point of 60% (obvious similarity of prey composition (Blaber & Bulman, 1987)). Both the overlap index of Pianka and a modified PS index of Shorygin suggested a high degree of dietary overlap between P. chungtienensis and weatherfishes.

Figure 3 Median-joining network of P. chungtienensis based on seven haplotypes (H1 to H7) of Cyt b gene from 84 individuals in Bita Lake and the Xiaozhongdian River, with comparing to its sister species P. gezaensis in the Geza River
DlSCUSSlON
Non-native species have been introduced into new ecosystems primarily through human activity, either deliberately or unintentionally (Gozlan et al., 2010). The approach of deliberately introductions is mainly from societal demands for fish products for food aquaculture (51%), ornamental fish (21%),sport fishing (12%) and fisheries (7%) (Gozlan, 2008). Nearly 8% of other introductions are accredited to accidental introductions, such as the escape from aquaculture installations,dispersal through ballast water, or introduced as a contaminant of a consignment of aquaculture species (Gozlan et al., 2010).
The exotic weatherfishes presence in Bita Lake occurred due to an unusual pathway of biological introduction - the practice as “prayer animal release”. Bita Lake is a protected area in the Bitahai Nature Reserve, where intentional introduction of exotic species is strictly forbidden. However, the majority of citizens inthe area practice Tibetan Buddhism, when releasing animals into the wild are thought to generate good karma in metempsychosis. Buddhist monks have played an important role in promoting conservation efforts around the world (Chong,2012). However, religious belief can also lead to unsustainable practices without sound ecological knowledge (Gong et al.,2012). The introduction of exotic weatherfishes into Bita Lake may be a negative example. Moreover, the active release of weatherfishes into the lake is sporadic and private, according to a conservation officer, as the believers usually use coat pockets or backpacks to transport the weatherfishes, and release them into the lake or the inflowing streams.
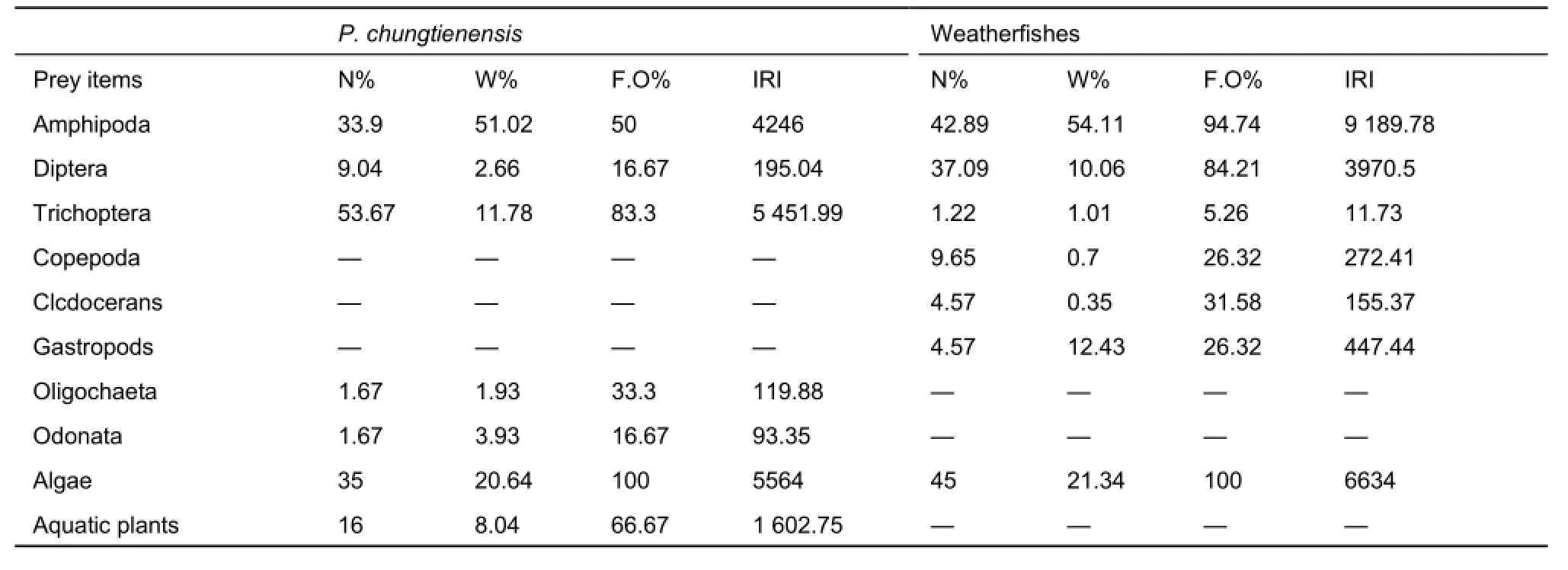
Table 1 Diet composition of P. chungtienensis and weatherfishes in Bita Lake
Although the annual fluctuation from Echo recordings shows little or no significant difference in fish amount between 2009 and 2012 in Bita Lake, the ratio of weatherfishes to P. chungtienensis by catch has been increasing since the former were first collected in August, 2009. Ptychobarbus chungtienensis is an endemic fish with a current distribution confined to Bita Lake, and its genetic diversity revealed in this study is very low when even comparing the h and π values to the pooled population of the flagship species of conservation,Chinese sturgeon Acipenser sinensis, one of the four Category I State protected fishes in China (h, 0.666 vs. 0.949; π, 0.00081 vs. 0.011) (Zhang et al., 2003). By contrast, weatherfishes thrive from the cold temperate to the subtropical regions in eastern Asia, and has been shown to expand its range very rapidly after introduction into new habitats throughout the world(Franch et al., 2008; United States Fish and Wildlife Service,2012). Another two lakes on the Zhongdian plateau, Shudu Lake and Napa Lake, were once populated by P. chungtienensis. However, when various non-native fishes including weatherfishes were introduced into these lakes, the P. chungtienensis no longer occurs in them now. Now weatherfishes have been introduced into Bita Lake. Given their very successful survival strategy (United States Fish and Wildlife Service, 2012), and the high degree of dietary overlap with P. chungtienensis that we have demonstrated above, we are deeply concerned about the future existence of P. chungtienensis.
In order to further preserve P. chungtienensis from its last habitat, first of all, more effective measures are imperative to prevent other introduced species from Bita Lake, which need wisdom to balance the existing conflict between conservation and religious faith. Furthermore, continuous periodic monitoring and comparative studies are indispensable to find if there is any other critical factors that will put the P. chungtienensis in more danger. Last but not the least, some pre-arranged plans are also necessary before it's too late, such as the timely launching of artificial breeding research of P. chungtienensis.
ACKNOWLEDGEMENTS
We appreciate to Guo-Hua YU, Li-Na DU, Xiao-Fu PAN, Shu-Wei LIU, Jian YANG, Xiao-Ai WANG for assisting in the field work. We thank Wen-Dong DING, Zhen-Shun MAO in helping to promote this work in Bita Lake and Dr. Richard WINTERBOTTOM for improving the writing.
Bandelt HJ, Forster P, Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution, 16(1): 37-48.
Blaber SJM, Bulman CM. 1987. Diets of fishes of the upper continental slope of eastern Tasmania: content, calorific values, dietary overlap and trophic relationships. Marine Biology, 95(3): 345-356.
Bowen SH. 1996. Quantitative description of the diet. In: Murphy BR, Willis DW. Fisheries Techniques. 2nd ed.Bethesda, Maryland: American Fisheries Society.
Chen JZ, Shen GM, Meng SL, Qu JH. 2010. Investigation and study on the aquaculture alien species in the lower reaches of Yangtze river. Chinese Agricultural Science Bulletin, 26(3): 315-319. (in Chinese)
Chen XY. 2010. Vertebrates-fishes. In: Yang L, Li H, Yang XJ. Yunnan Wetlands. Beijing: China Forestry Press, 1-596. (in Chinese)
Chen YR. Yang JX, Li ZY. 1998. The diversity and present status of fishes in Yunnan Province. Chinese Biodiversity, 6(4): 272-277. (in Chinese)
Chen ZM, Chen YR. 2003. The magic Chungtien schizothoracin. Discovery of Nature, (5): 67-70. (in Chinese)
Chong KY. 2012. Religiously protecting myanmar's environment. Science,337(6102): 1604-1605.
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ,Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M,Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA. 2011. Trophic downgrading of planet earth. Science, 333(6040): 301-306. Fernandes PG. 2009. Classification trees for species identification of fishschool echotraces. ICES Journal of Marine Science, 66(6): 1073-1080.
Franch N, Clavero M, Garrido M, Gaya N, López V, Pou-Rovira Q, Queral JM. 2008. On the establishment and range expansion of oriental weatherfish (Misgurnus anguillicaudatus) in NE Iberian Peninsula. Biological Invasions, 10(8): 1327-1331.
Geist J. 2011. Integrative freshwater ecology and biodiversity conservation. Ecological Indicators, 11(6): 1507-1516.
Gong BC, Hamer R, Meng XX, Meng QH, Feng JC, Xue DY. 2012. Limits to religious conservation efforts. Science, 338(6108): 740.
Gozlan RE. 2008. Introduction of non-native freshwater fish: is it all bad?. Fish and Fisheries, 9(1): 106-115.
Gozlan RE, Britton JR, Cowx I, Copp GH. 2010. Current knowledge on non-native freshwater fish introductions. Journal of Fish Biology, 76(4):751-786.
Huang SY, Chen YY. 1986. Phylogenetic relationships of Diptychus chungtienensis and D. kaznakovi, with special reference to the zoogeographical analysis. Acta Zootaxonomica Sinica, 11(1): 100-107. (in Chinese)
Hyslop EJ. 1980. Stomach contents analysis-a review of methods and their application. Journal of Fish Biology, 17(4): 411-429
Ivlev VS. 1961. Experimental Ecology of the Feeding of Fishes. New Haven:Yale University Press.
Jiang WS, Zhao YP, Wang WY, Yang JX, Chen XY. 2013. Length-weight relationship and biological data of a threatened fish, Ptychobarbus chungtienensis (Tsao, 1964) in Bita Lake of Shangri-La, Yunnan, China. Journal of Applied Ichthyology, 29(5): 1173-1176.
Kang B, Deng JM, Wang ZM, Zhang J. 2013. Transplantation of icefish(salangidae) in China: glory or disaster?. Reviews in Aquaculture, 7(1): 13-27. Keast A. 1978. Trophic and spatial interrelationships in the fish species of an Ontario temperate lake. Environmental Biology of Fishes, 3(1): 7-31.
Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics, 25(11): 1451-1452.
Lodge DM. 1993. Biological invasions: lessons for ecology. Trends in Ecology & Evolution, 8(4): 133-137.
Magurran AE. 2009. Threats to freshwater fish. Science, 325(5945): 1215-1216. Nei M. 1987. Molecular Evolutionary Genetics. New York: Columbia University Press.
Nei M, Tajima F. 1981. DNA polymorphism detectable by restriction endonucleases. Genetics, 97(1): 145-163.
Pan XF, Yang J, Chen XY, Yang JX. 2010. Threatened fishes of the world:Ptychobarbus chungtienensis Tsao 1964 (Cyprinidae). Environmental Biology of Fishes, 89(1): 1-2
Pianka ER. 1973. The structure of lizard communities. Annual Review of Ecology and Systematics, 4(1): 53-74.
Pinkas L, Oliphant MS, Iverson LK. 1971. Food Habits of Albacore, Bluefin Tuna and Bonito in California Waters. California: California Department of Fish and Game, 152: 1-105.
Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press.
Shorygin AA. 1952. Feeding and feeding relationships of the Caspian Sea fishes Pischepromizdat. Moscow, 1-267. (in Russian)
Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J,Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P, Sousa R,Tabacchi E, Vilà M. 2013. Impacts of biological invasions: what's what and the way forward. Trends in Ecology & Evolution, 28(1): 58-66.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6:molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12): 2725-2729.
TEEB. 2010. The Economics of Ecosystems and Biodiversity: Ecological and Economic Foundations. London and Washington: Earthscan.
Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22(22): 4673-4680.
United States Fish and Wildlife Service. 2012[2014-05-20]. Oriental Weatherfish (Misgurnus anguillicaudatus) Ecological Risk Screening Summary. Web version. http://www.fws.gov/injuriouswildlife/pdf_files/Misgurnus_ anguillicaudatus_WEB_8-21-12.pdf.
Villéger S, Blanchet S, Beauchard O, Oberdorff T, Brosse S. 2011. Homogenization patterns of the world's freshwater fish faunas. Proceedings of the National Academy of Sciences of the United States of America,108(44): 18003-18008.
Wang S, Xie Y. 2004. China Species Red List Vol. 1. Red List. Beijing:Higher Education Press. (in Chinese)
Xiao WH, Zhang YP, Liu HZ. 2001. Molecular systematics of Xenocyprinae(Teleostei: Cyprinidae): taxonomy, biogeography, and coevolution of a special group restricted in East Asia. Molecular Phylogenetics and Evolution, 18(2): 163-173.
Xing YC, Zhang CG, Fan EY, Zhao YH. 2016. Freshwater fishes of China:species richness, endemism, threatened species and conservation. Diversity and Distributions, 22(3): 358-370.
Xu HG, Ding H, Li MY, Qiang S, Guo JY, Han ZM, Huang ZG, Sun HY, He SP, Wu HR, Wan FH. 2006. The distribution and economic losses of alien species invasion to China. Biological Invasions, 8(7): 1495-1500.
Yang B, Chen XY, Yang JX. 2011. Non-native carp of the genus Cyprinus in Lake Xingyun, China, as revealed by morphology and mitochondrial DNA analysis. Biological Invasions, 13(1): 105-114.
Yang XJ, Ji YH. 2010. Wetlands of international importance in Yunnan province. In: Yang L, Li H, Yang XJ. Yunnan Wetlands. Beijing: China Forestry Press, 1-596. (in Chinese)
Zhang SM, Wang DQ, Zhang YP. 2003. Mitochondrial DNA variation,effective female population size and population history of the endangered Chinese sturgeon, Acipenser sinensis. Conservation Genetics, 4(6): 673-683.
01 May 2016; Accepted: 10 July 2016
s: This study was supported by the National Key Technology R & D Program of China (2008BAC39B03), the Yunnan Provincial Science Technology Program (2009CD106/ 2010GA009/ 2013FB070), the Yunnan 2013 special funds for biodiversity conservation projects, and the fund of Southeast Asia Biodiversity Research Institute, Chinese Academy of Science (Y4ZK111B01)
s, E-mail: chenxy@mail.kiz.ac.cn, yangjx@mail. kiz.ac.cn
10.13918/j.issn.2095-8137.2016.5.275
杂志排行
Zoological Research的其它文章
- Lamprey: a model for vertebrate evolutionary research
- Evolution and phylogenetic application of the MC1R gene in the Cobitoidea (Teleostei: Cypriniformes)
- A new species of the genus Triplophysa (Cypriniformes:Nemacheilidae), Triplophysa daochengensis, from Sichuan Province, China
- A new cave species of the Genus Triplophysa from Yunnan, China
- Identification of candidate piRNAs in the gonads of Paralichthys olivaceus (Japanese flounder)
- Identification of Sphaeroma terebrans via morphology and the mitochondrial cytochrome c oxidase subunit I(COI) gene
