固定化微绿球藻去除+4-N、34--P效果的研究
2016-11-12梁晶晶蒋霞敏江茂旺张泽凌韩庆喜
梁晶晶 蒋霞敏 江茂旺 张泽凌 韩庆喜
(宁波大学海洋学院,宁波 315211)
梁晶晶蒋霞敏江茂旺张泽凌韩庆喜
(宁波大学海洋学院,宁波 315211)
为了探究固定化微绿球藻(Nannochloropsis oculata)去除污水中NH+4-N、PO34--P的效果,采用海藻酸钠固定化包埋技术进行实验。开展了固定化藻球大小、藻细胞包埋密度、藻球投放质量及充气培养条件对NH+4-N、PO3--P去除效果的单因子试验研究。结果表明,固定化藻球大小、藻细胞包埋密度、藻球投放质
微绿球藻;固定化;NH4+-N;PO34--P;去除率
微藻是一类原始且分布广泛的营光合自养、异养或兼养的低等植物,一般要借助显微镜才能观察到其细胞结构形态[1]; 其生长迅速,环境适应能力强,产量高效,含有许多陆地生物所缺乏的特殊生物活性物质,如EPA、DHA、虾青素等[2—5]。微藻固定化技术始于20世纪80年代,在环境领域主要应用于废水处理和生物监测,具有藻细胞密度高、反应速度快、固液分离效果好、运行稳定性高等[6,7]特点。固定化藻类处理废水不仅反应速度快、去除效率高,还可以实现废水资源的再生利用:一方面,经过处理的废水可作为工农业用水而重新加以利用; 另一方面,处理后的固定化藻细胞易于收获,进行加工生产,不仅避免产生二次污染,还可以加以综合利用。影响固定化微藻生长和NH+4-N、PO34--P去除效率的因素可分外部因素和内部因素,内部因素主要是藻种本身生理特征的差异造成; 外部因素主要包括温度、光照、营养条件、藻球规格大小、藻细胞包埋量、藻球用量等方面[8]。
微绿球藻(Nannochloropsis oculata)是一种较常见的单细胞海洋微藻,其细胞壁极薄且富含EPA,广泛应用于虾类、蟹类、贝类等的育苗及轮虫、卤虫等培养。蒋霞敏[9]研究表明微绿球藻具有环境适应能力强,繁殖速度快,且不易老化污染的优点,能吸收水中氮磷等营养物质供给自身生长需要。关于固定化微绿球藻研究鲜有报道,仅见郑莲等[10]和黄翔鹄等[11]将固定化微绿球藻引入对虾养殖水体,能有效降低水体中氨氮、亚硝酸氮等有害因子的浓度,同时能抑制弧菌的生长,提高水中溶解氧含量,使水体长时间保持较好的动态平衡状态。本文以微绿球藻为藻种,采用海藻酸钠包埋进行固定化,进行了不同藻球规格,藻细胞密度,藻球投放质量以及培养条件对固定化微绿球藻生长、NH+4-N和PO34--P净化效果的试验,为固定化微绿球藻应用于污水处理提供一定的理论依据。
1 材料与方法
1.1材料与仪器
试验藻种微绿球藻来自宁波大学海洋学院饵料生物实验室。培养液配方采用改良的宁波大学3#母液(表1),加入量母液与海水体积比为1 :1 000。培养用水采用象山港天然海水,经沙滤、暗沉淀、脱脂棉过滤和烧开冷却; 藻种置于GXZ智能型光照培养箱(宁波江南仪器厂)培养,培养条件:温度(25±1)℃,盐度25,光照强度80 μmol/(m2·s),pH 7.86,光暗周期12h :12h,不充气。为防止使用实际污水中的某些化学物干扰测定结果,本实验采用了人工模拟污水,即在缺氮3#母液内加一定量的NH4Cl配制成人工污水备用(NH+4-N含量17 m g/L)。
藻球制作方法:将处于指数生长期的微绿球藻离心浓缩(4000 r/min,10min)两次,取一定体积的藻细胞浓缩液与预先灭菌的5%海藻酸钠溶液按照1 :1均匀混合,形成海藻酸钠和微藻的混合液; 用注射器吸取混合液,并套上20#针头,在预冷的2% CaCl2溶液距离液面20 cm处,滴入混合液即形成直径(3.5 mm)的小球,藻球在CaCl2溶液中静置3h后取出,用消毒海水洗涤2—3次备用。
脱固定化方法:在测定固定化藻细胞密度时需先脱固定化,将固定化藻球放入盛有一定量3%的柠檬酸三钠(Na3C6H5O7)化解液中,摇动,直至固定化藻球完全溶解成悬浮状,再计数测定藻细胞密度。
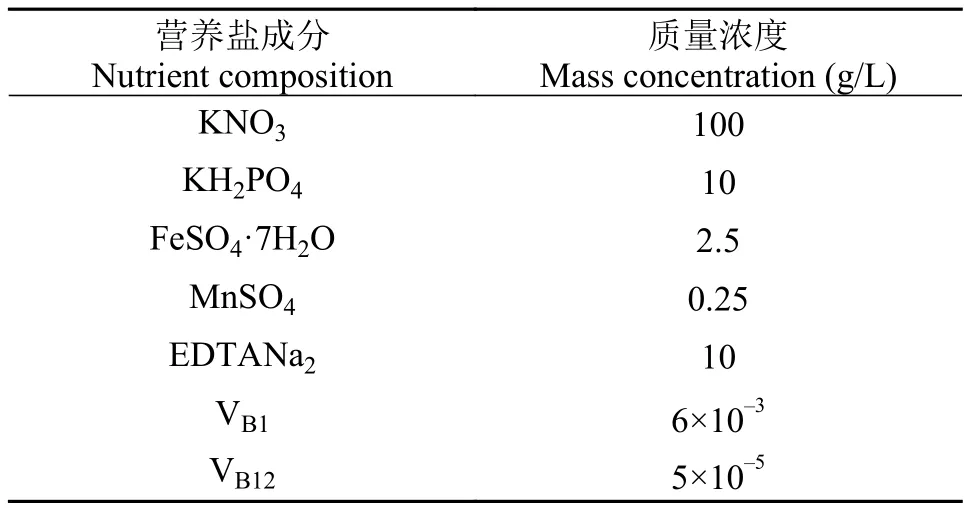
表1 改良的宁波大学3#母液配方Tab. 1 Composition of modified 3# culture medium
1.2试验方法
1.3检测方法
相对增长率 K 值,计算公式为:K=(lg Nt-lg N0)/T,式中,N0为培养的起始浓度,Nt为培养 t 时间后的浓度,t为培养时间。
标准曲线方程为:y=5.8899x-0.0094,R2= 0.9992。
标准曲线方程为:y=2.7418x-0.0034,R2=0.999
氮磷的去除率R按下式计算:R=(C0-Ct)/Ct× 100%,式中:R表示去除率(%); C0和Ct分别表示各种形态氮磷的初始浓度和各取样时段浓度(mg/L)。
1.4数据统计与分析
数据、图表用Excel进行处理,数据分析运用SPSS 17.0统计分析软件进行相关分析,并采用Duncan多重比较,差异显著水平为P<0.05。
2 结果
藻球直径大小对藻细胞生长速率(K值)影响显著(P<0.05),随着固定化藻球直径增大K值呈先升高后降低趋势,在藻球直径3.5 mm时,K值最大(0.332±0.002),显著高于(除藻球直径3.0 mm外)其他各组(图1)。
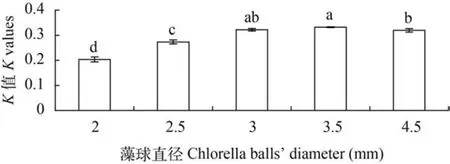
图1 不同藻球直径对微绿球藻生长的影响Fig. 1 Effects of different size of immobilized algal balls on the growth of Nannochloropsis oculata同行数据肩标无字母或相同字母表示差异不显著(P>0.05),不同小写字母表示差异显著(P<0.05); 下图同In the same rowzvalucs with no letter or the same letter superscripts mean no significant difference(P>0.05),while with different small letter superscripts mean significant difference(P<0.05); The same applies below
不同藻细胞包埋密度对K值影响显著(P<0.05),随着藻细胞包埋密度增加,K值逐渐降低,在包埋密度100×104cells/ball时K值最大(0.330±0.033)(图3)。
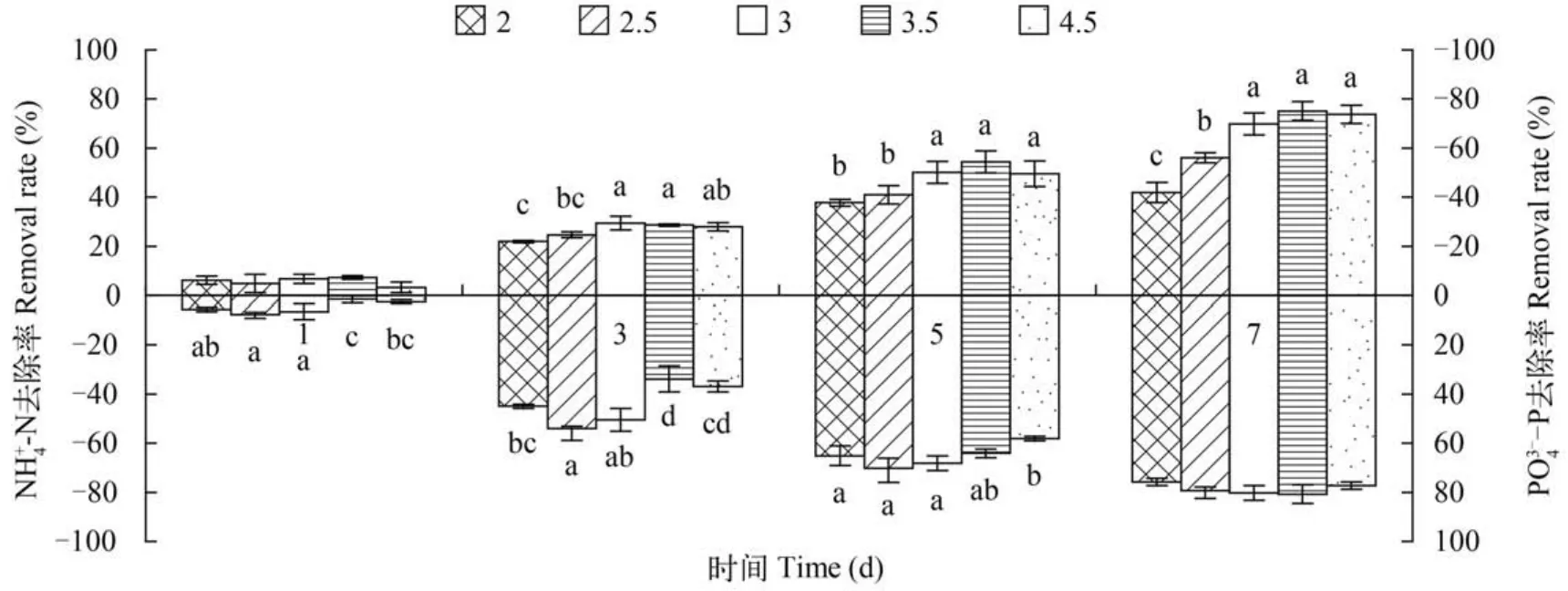
图2 不同藻球直径对微绿球藻NH+4-N, 34--P去除效果的影响Fig. 2 Effects of different size of immobilized algal balls on theN H+4-N, 34--P removal efficiency by Nannochloropsis oculata图例单位:mm Legend unit:mm
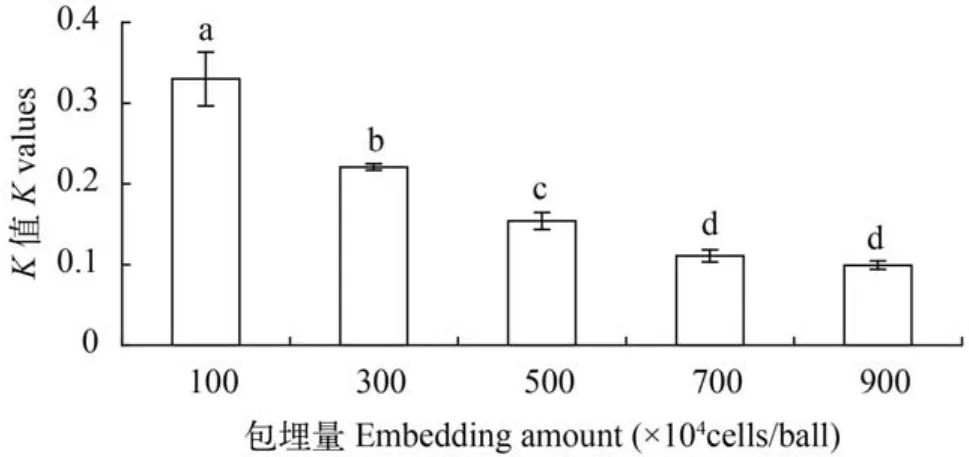
图3 不同藻细胞包埋密度对固定化微绿球藻生长的影响Fig. 3 Effects of different density of algal cells embedded on growth of immobilized Nannochloropsis oculata
水体中藻球不同投放量对藻细胞生长速率(K值)影响显著(P<0.05),随着藻球用量的增加K值下降(图5)。在藻球投放质量为10 g/L时K值达最大为(0.301±0.021)。
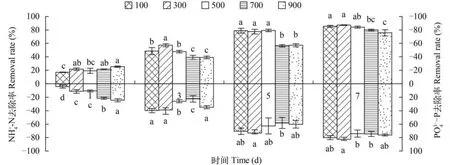
图4 不同藻细胞包埋密度对固定化微绿球藻NH+4-N,34--P去除率的影响Fig. 4 Effects of different density of algal cells embedded on immobilized Nannochloropsis oculataNH+4-N, 34--P removal efficiency图例单位为:×104cells/ball Legend unit:×104cells/ball
+表2 不同藻细胞密度第1天和单位密度吸收NH+4-N效果对比Tab. 2 Contrast ofN H4-N absorption of Nannochloropsis oculata in different densities at first day and unit density

+表2 不同藻细胞密度第1天和单位密度吸收NH+4-N效果对比Tab. 2 Contrast ofN H4-N absorption of Nannochloropsis oculata in different densities at first day and unit density
初始密度Preliminary density(×104cells/ball)最终密度Final density(×104cells/ball)100 16.99±0.43c 0.169±0.004 3.98±2.28d 0.039±0.023a 1110 300 21.49±1.89ab 0.071±0.006 10.94±3.11c 0.036±0.010b 1137.5 500 19.24±3.77bc 0.038±0.007 10.45±1.49c 0.021±0.003c 1762.5 700 21.49±0.43ab 0.030±0.001 21.39±1.49b 0.028±0.002cd 2025 900 25.24±1.15a 0.028±0.001 24.38±2.28a 0.027±0.002d 2037.5第1天去-N率Assimilation ratio at first day(%)NH+4单位密度去-N率Assimilation ratio at unit density(%/104cells)NH+4第1天去-P率Assimilation ratio at first day(%)3-4单位密度去-P率Assimilation ratio at unit density(%/104cells)3-4
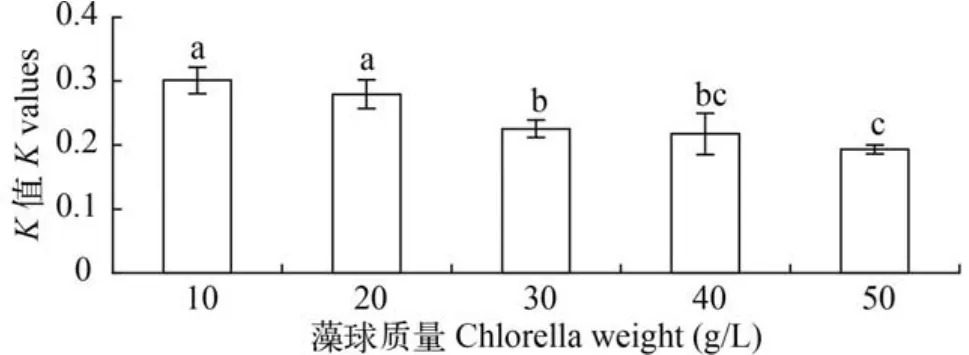
图5 不同投放质量对固定化微绿球藻生长的影响Fig. 5 Effects of different dosages of algae balls on immobilized Nannochloropsis oculata growth
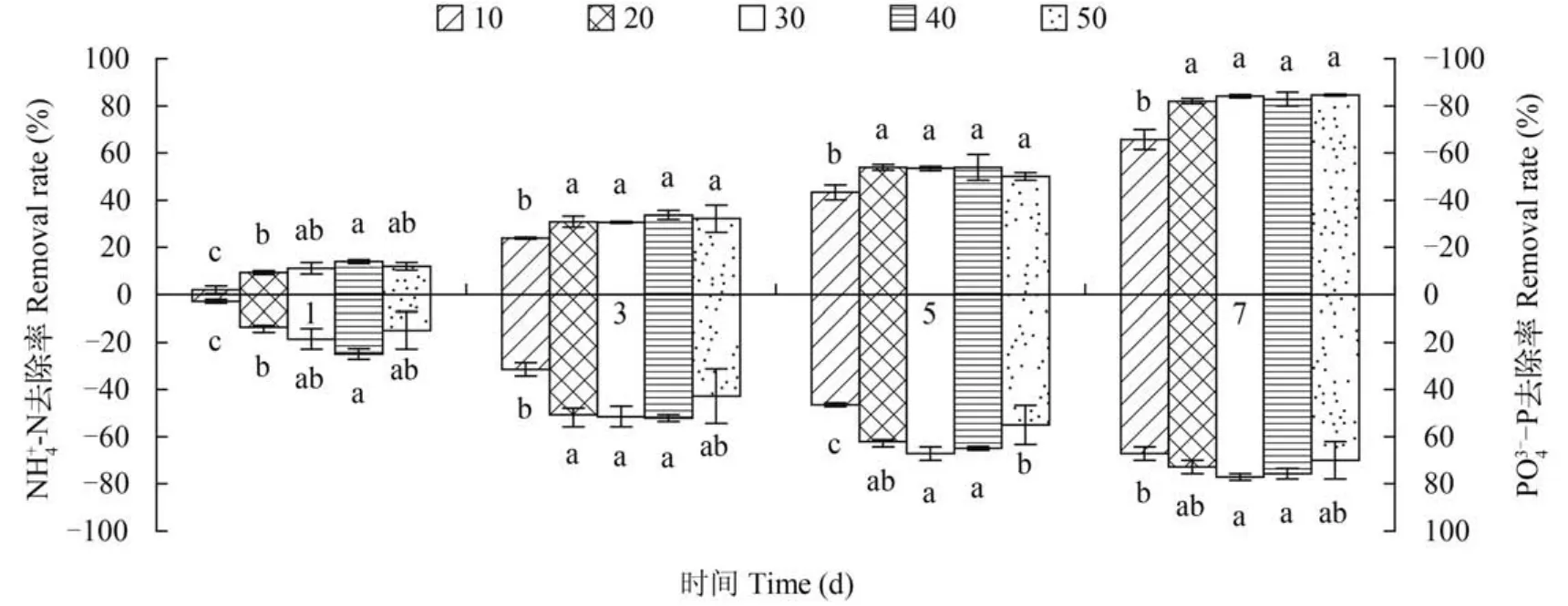
图6 不同投放质量对固定化微绿球藻NH+-N, 34--P去除率的影响4Fig. 6 Effects of different dosages of algae balls on immobilized Nannochloropsis OculataN H+-N, 34--P removal efficiency4图例单位:g/L Legend unit:g/L
充气培养对藻细胞生长速率(K值)影响显著(P<0.05),充气培养条件下藻细胞K值为(0.306±0.006);不充气组K值为(0.177±0.010),显著低于充气组(图7)。
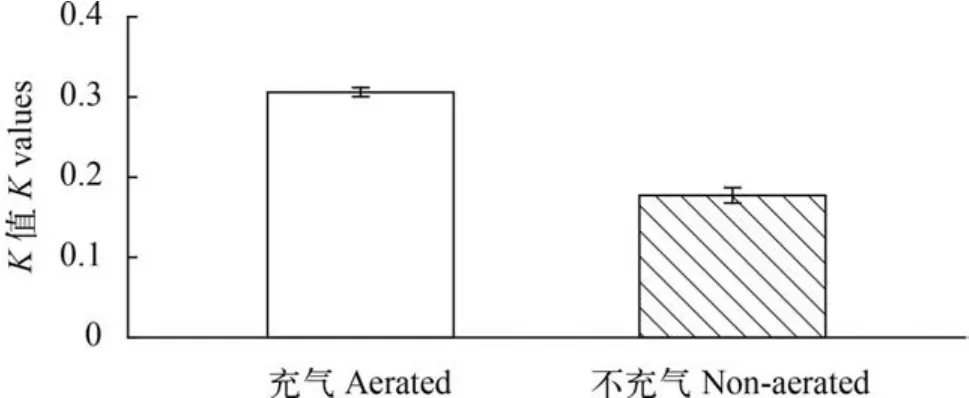
图7 充气培养对固定化微绿球藻生长的影响Fig. 7 Effects of aerated cultures on immobilized Nannochloropsis Oculata growth
充气培养对藻细胞氮磷去除率影响显著影响显著(P<0.05)。第7天充气组去除率为(85.93±0.45)%显著高于不充气组(49.32±0.45)%。充气培养对藻细胞氮磷去除率影响显著影响显著(P<0.05)。第7天充气组去除率为(66.66±5.00)%显著高于不充气组(46.29±2.12)%(图8)。

图8 充气培养对固定化微绿球藻NH+-N, 34--P去除率的4影响Fig. 8 Effects of aerated cultures on immobilized Nannochloropsis OculataN H+-N, 34--P removal efficiency4
3 讨论
固定化藻球的制作工艺对污水的处理密切相关,其中藻球直径大小对藻细胞生长速率(K值)影响差异显著(P<0.05),本研究表明随着固定化藻球直径增大K值呈先升高后降低趋势,在藻球直径3.5 mm时K值最大(0.332±0.002)。随着固定化藻球直径增大,NH+4-N和PO34--P去除率以藻球直径3.5 mm组效果最佳,这与袁冰等[12]的研究结果一致,在一定直径范围内藻球生长及NH+4-N和PO34--P去除率均是先升高后降低的趋势; 这与杨海波等[13]的随着藻球直径越大藻球生长量越大结果不同,可能是藻球越小,制备过程交联程度越大,藻球结构越致密,传质性能受到影响; 随着藻球直径增大,藻细胞在藻球中分布不均匀,外部藻细胞对藻球内部有一定遮蔽作用,同时随着藻球直径增大,藻球通透性逐渐降低,藻球内细胞营养不足,生长环境较差等,对藻细胞的增值生长及氮磷等营养物质的吸收有一定阻碍作用。
水中投放固定化藻球的多寡直接影响污水处理效果,本实验结果表明投放不同质量藻球对固定化藻生长及NH+4-N和PO34--P去除效果影响差异显著(P<0.05)。藻球投放量大,生长速率减小,NH+4-N和PO34--P去除速率却增加。这与高鹏等[17]的观点相吻合,固定化微藻投入量的增加,能一定效果提高NH+4-N和PO34--P去除速率,但是随着固定化藻球用量达到一定值后,NH+4-N和PO34--P去除速率并不会相应提高,这种情况会造成藻球的浪费,在实际应用中,藻球与污水应有一个最佳比例值,这样既能缩短污水净化时间,又能节约成本。这是由于污水中随着藻球投放量加大,有效藻细胞数目也多,参与反应的藻细胞数目多,对氮磷吸收的量大大增加,所以在短时间内去除NH+4-N和PO3--P的效果就
4好; 但藻球用量过多,不但藻球之间相互遮挡,对光照利用效率降低,光合作用降低,细胞生长速率也相应降低[16]; 而且藻球用量过大,制作藻球的成本就大大增加,处理污水就会得不丧失。
充气与否对固定化藻去氮除磷密切相关,本实验结果表明,充气培养对固定化微绿球藻生长速率(K值)及氮磷去除速率影响差异显著(P<0.05)。在相同培养时间,充气培养条件下固定化微绿球藻生长速率和氮磷去除率均高于不充气条件。韩婷婷等[18]研究充气培养能显著提高半页马尾藻(Sargassum hemiphyllum)生长速率及活性磷吸收效果; 滕怀丽等[19]研究表明,充气培养能显著提高盐藻(Dunaliella salina)的生长速率以及其对培养液中氮的吸收速率; 这与本实验观点一致,充气培养能显著提高藻的生长速率和氮磷去除效果。Svensen等[20]发现轻微的搅动促进浮游植物的生长,生长率高于完全静止培养的浮游植物。海藻光合生长消耗水体中大量的无机盐并释放大量的氧,水体pH 升高,CO2等碳源供应不足引起藻体的最大光合作用能力下降[21,22]。充气培养条件下促进空气中CO2进入藻类培养水体[23],向水体中及时补充藻体光合生长所需的无机碳源,调节水体pH,促进藻体光合固碳,从而加速藻体的生长[24]。同时,充气加快藻体表面周围的营养盐交换,促进海藻同化吸收 N、P 营养盐,为藻体光合作用和生长提供有利的条件。
4 结论
本实验对固定化微绿球藻不同规格、不同藻细胞包埋密度、不同藻球投放质量和充气培养进行了NH+4-N、PO34--P去除效果优化研究,得到最优条件为:固定化微绿球藻应进行充气培养,藻球规格3.5 mm、藻细胞包埋密度100×104cells/ball、藻球投放量30 g/L。
[1]Zhou W Z,Huo S H,Zhu S N,et al. Microalgae immobilization and application on resources reclamation [J]. Renewable Energy Resources,2011,29(4):90—94 [周卫征,霍书豪,朱顺妮,等. 微藻固定化技术及其在资源化中应用. 可再生能源,2011,29(4):90—94]
[2]Teresa M M,Martins A A,Caetano N S. Microalgae for biodiesel production and other applications:A review [J]. Renewable and Sustainable Energy Reviews,2010,14(1):217—232
[3]Xia S,Wan L L,Li A,et al. Research and development of commercial biomass products and bioactive compounds of microalgae [J]. Natural Product Research and Development,2014,26(1):463—469 [夏嵩,万凌琳,李爱,等.微藻生物质产品和生物活性物质的研究与开发. 天然产物研究与开发,2014,26(1):463—469]
[4]He S S,Gao B Y,Lei X Q,et al. Effects of initial nitrogen supply on the growth,morphology and lipid accumulation of oleaginous microalga Eustigmatos vischeri(eustigmatophyceae) [J]. Acta Hydrobilogica Sinica,2015,39(3):574—582 [何思思,高保燕,雷学青,等. 初始硝酸钠浓度对魏氏真眼点藻的生长、形态和油脂积累的影响. 水生生物学报,2015,39(3):574—582]
[5]Li A F,Liu R,Liu X J,et al. Effects of carbon sources on growth and fatty acid composition of Pinguiococcus pyrenoidosus CCMP [J]. Acta Hydrobiologica Sinica,2009,33(3):461—467 [李爱芬,刘然,刘晓娟,等. 碳源对粉核油球藻生长和脂肪酸组成特性的影响. 水生生物学报,2009,33(3):461—467]
[6]Naessens M,Leclerc J C,Tran-Minh C. Fiber optic biosensor using Chlorella vulgaris for determination of toxic compounds [J]. Ecotoxicology and Environmental Safety,2000,46(2):181—185
[7]Li H,Li L,Zhang F Y. Research on biological immobilization technology in the treatment of nitrogencontained wastewater [J]. Industrial Safety and Environmental Protection,2004,30(6):18—20 [李哗,李凌,张发有. 生物固定化技术在含氮废水处理中的研究. 工业安全与环保,2004,30(6):18—20]
[8]Touchette B W,Burkholder. Review of nitrogen and phosphorus metabolism in seagraasses [J]. Experimental Marine Biology and Ecology,2000,250(1—2):133—167
[9]Jiang X M. Effects of temperatures,light intensities and nitrogen concenrations on the growth and fatty acid compositions of Nannochloropsis oculata [J]. Marine Sciences,2002,26(8):9—12 [蒋霞敏. 温度、光照、氮含量对微绿球藻生长及脂肪酸组成的影响. 海洋科学,2002,26(8):9—12]
[10]Zheng L,Huang X H,Liu C W,et al. Immobilization of Nannochloirs oculata in water quality control in shrimp mariculture [J]. Marine Sciences,2005,29(6):4—8 [郑莲,黄翔鹄,刘楚吾,等. 微绿球藻固定化培养及其对对虾养殖水质调控. 海洋科学,2005,29(6):4—8]
[11]Huang X H,Li C L,Zheng L,et al. Effects of the immoblized microalgae on the quantity dynamics of vibrio in theshrump ponds [J]. Acta Hydrobiologica Sinica,2005,29(6):684—688 [黄翔鹄,李长玲,郑莲,等. 固定化微藻对虾池弧菌数量动态的影响. 水生生物学报,2005,29(6):684—688]
[12]Yuan B,Sun L Q,Hou S C,et al. Preparation of immobilized Chlorella and impact on N and P uptake [J]. Marine Environmental Science,2011,30(6):804—808 [袁冰,孙利芹,侯士昌,等. 固定化小球藻的制备及对N、P 吸收的影响. 海洋环境科学,2011,30(6):804—808]
[13]Yang H B,Yu Y,Zhang X H,et al. Study on immobilization culture of marine microalga Chlorella vulgaris [J]. Fisheries Science,2001,20(5):4—7 [杨海波,于媛,张欣华,等. 小球藻固定化培养的初步研究. 水产科学,2001,20(5):4—7]
[14]Mallick N,Rai L C. Influence of culture density,pH,organic acids and divalent cationson the removal of nutrients and metals by immobilized Anabaena doliolum and Chlorella vulgaris [J]. World Journal Microbiology & Biotechnology,1993,9(2):196—201
[15]Jimntnez-Perez M V,Sanchez-Castillo P,Romera O,et al.Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure [J]. Enzyme and Microbial Technology,2004,34(5):392—398
[16]Mao X X,Jiang X M,Qian P. Effect of immobilized Prochlorococcus culture onNH+4-N removal [J]. Chinese Journal of Ecology,2014,33(11):3075—3080 [毛欣欣,蒋霞敏,钱鹏. 原绿球藻固定化培养去除NH+4-N的效果. 生态学杂志,2014,33(11):3075—3080]
[17]Gao P. Study on purification for livestock waste water by immobilized Microystis aeruginsa [D]. Sichuan Agricultural University. 2011 [高鹏. 固定化铜绿微囊藻及其对畜禽废水的净化研究. 四川农业大学. 2011]
[18]Han T T,Fu G Q,Qi Z H,et al. Effects of aerated culture on growth,nutrient uptake,and biochemical composition in Sargassum hemiphyllum [J]. Journal of Fishery Sciences of China,2015,22(2):311—318 [韩婷婷,付贵权,齐占会,等. 充气培养对半页马尾藻生长、营养盐吸收和生化组成的影响. 中国水产科学,2015,22(2):311—318]
[19]Teng H L,Huang X X,Zhou H Q,et al. Effects of bubbling on growth,use of N and P and biochemical composition of the microalgae Dunaliella salina [J]. Journal of Fisheries of China,2010,34(6):942—948 [滕怀丽,黄旭雄,周洪琪,等. 充气方式对盐藻生长、细胞营养成分及氮磷营养盐利用的影响. 水产学报,2010,34(6):942—948]
[20]Svensen C,Egge J K,Stiansen J E. Can silicate and turbulence regulate the vertical flux of biogenic matter? A mesocosm study [J]. Marine Ecology Progress Series,2001,217:67—80
[21]Wu H Y,Gao K S,Du D H F. Short-term effects of solar ultraviolet radiation on the photochemical efficiency of Spirulina platensis in non-aerated and aerated cultures [J]. Acta Hydrobiologica Sinica,2005,29(6):673—677 [吴红艳,高坤山,渡辺辉夫. 静止和充气培养条件下短期紫外辐射对钝顶螺旋藻光化学效率的影响. 水生生物学报,2005,29(6):673—677]
[22]Xu J T,Gao K S. Co-effects of CO2and solar UVR on the growth and photosynthetic performance of the economic red macroalga Porphyra haitanensis [J]. Acta Oceanologica Sinica,2013,35(5):184—190 [徐军田,高坤山. CO2升高和阳光紫外线辐射对坛紫菜生长和光和特性的耦合效应. 海洋学报,2013,35(5):184—190]
[23]Rodríguez-Maroto J M,Jiménez C,Aguilera,et al. Air bubbling results in carbon loss during microalgal cultivation in bicarbonate-enriched media:experimental data and process modeling [J]. Aquacultural Engineering,2005,32(s3—4):493—508
[24]Zou D H. Effects of elevated atmospheric CO2on growth,photosynthesis and nitrogen metabolism in the economic brown seaweed,Hizikia fusiforme(Sargassaceae,Phaeophyta) [J]. Aquaculture,2005,250(3—4):726—735
STUDY ON REMOVAL RATE OF NH4+-N AND PO43--P BY IMMOBILIZED NANNOCHLOROPSIS OCULATA
LIANG Jing-Jing,JIANG Xia-Min,JIANG Mao-Wang,ZHANG Ze-Ling and HAN Qing-Xi
(School of Marine Sciences,Ningbo University,Ningbo 315211,China)
Microalgae Nannochloropsis oculata,immobilized with sodium alginate,was used to explore its removal efficiency ofNH+4-N andPO34--P from artificial sewage water. Algae ball size,cell densities,dosages of algae balls,and aeration cultured were applied in the single-factor. The results showed that all these conditions significant impact the removal of ofNH+4-N andPO34--P. The growth rate of K value achieved the highest value(0.332±0.002) when the diameter was 3.5 mm; the removal rate ofNH+4-N andPO34--P were the highest one at the diameter was 3.5 mm,which were(75.08±3.83)% and(80.80±3.81)%,respectively. The maximum of the growth K values was(0.330±0.033) with the density of 100×104cells/ball. The highestNH+4-N andPO34--P removal rate were(87.20±0.43)% and(82.58±1.72)%,respectively,at the group of 300×104cells/ball; however,100×104cells/ball was the optimal algal cell density based on unit algal cells removal ratio ofNH+4-N andPO34--P. The increased algae balls dosages decreased the growth rate of K values. 10 g/L group had the maximum K values(0.301±0.02) and 50 g/L had the minimum K values(0.193±0.01). The removal rates ofNH+4-N were(84.12±0.78)% and(84.63±0.45)% when the dosage was 30 g/L and 50 g/L,respectively. 30 g/L group had the highestPO34--P removal rate(77.13±1.43)%. Combined analyses revealed that 30 g/L was optimum for the dosages of algae balls. The K value,the removal ofNH+4-N andPO34--P were significantly(P<0.05) higher with aeration than non-aerated; K values were(0.306±0.006) and(0.177±0.010),respectively;NH+4-N removal rates were(85.93±0.45)% and(49.32±0.45)%,respectively;PO34--P removal rates were(66.66±5.00)% and(46.29±2.12)%,respectively. This study optimized the conditions of immobilized microalgae Nannochloropsis oculata:immobilized Nannochloropsis oculata should be aerated cultures; algae ball size was 3.5 mm; the algal cell density was 100×104cells/ball; and the dosage of algae balls was 30 g/L.
Nannochloropsis oculata; Immobilization;NH+4-N;PO34--P; Removal rate
Q142
A
1000-3207(2016)05-1033-08
10.7541/2016.134
2016-01-11;
2016-04-10
国家海洋公益项目(201305022); 浙江省公益项目(2015C3204); 宁波市科技项目(2015C10062)宁波市创新团队(2011B81003)资助 [Supported by the Nonprofit Research Project for the State Oceanic Administration(201305022); Nonprofit Research Project of Zhejiang Province(2015C3204); Project of Science and Technology of Ningbo City(2015C10062); the Innovative Group of Ningbo City(2011B81003)]
梁晶晶(1990—),女,河南开封人; 硕士; 主要研究方向为微藻生态学。E-mail:1245558982@qq.com
蒋霞敏(1957—),女,E-mail:jiangxiamin@nbu.edu.cn
