Dmrt基因在水生生物中的研究进展
2016-11-12李法君付春鹏罗永巨
李法君 付春鹏 罗永巨
(1. 潍坊科技学院,寿光 262700; 2. 广西水产科学研究院,南宁 530021)
Dmrt基因在水生生物中的研究进展
李法君1付春鹏1罗永巨2
(1. 潍坊科技学院,寿光 262700; 2. 广西水产科学研究院,南宁 530021)
Dmrt(Doble-sex and Mab-3 Relatated Transcription factor)是指同果蝇Dsx基因和线虫Mab-3基因同源的基因家族,其家族成员的主要特征是所编码的多肽链中都包含一个具有 DNA 结合能力的保守基序-DM结构域。Dmrt基因是从无脊椎动物到脊椎动物都存在的古老基因,功能涉及性别决定与分化、许多组织和器官的形成及相关功能的维持等多方面。文章综述了近来Dmrt基因在水生生物中的研究进展; 按从低等到高等的顺序,梳理了水产动物Dmrt基因的功能; 分析了Dmrt1作为性别决定基因的历程; 进而对Dmrt基因在水生生物的研究热点做了展望。
Dmrt基因;Dsx基因;性腺;甲壳动物;鱼类;水生生物
Dmrt(Doble-sex and Mab-3 Relatated Transcription factor)是指与果蝇(Drosophila melanogaste)的性别决定基因Doublesex(Dsx)和秀丽隐杆线虫(Caenorhabditis elegans)的性别决定基因Maleabnormal-3(Mab-3)同源的基因[1—3],最早发现于果蝇中[4]。不同的Dmrt基因构成一个基因家族,该家族成员的主要特征是所编码的多肽链都包含一个能与DNA结合的保守基序-DM(Doublesex and Mab-3)结构域,该结构域在不同进化类型的生物中具有相当的保守性[5]。Dmrt基因是一类转录调控因子,该因子以锌指结构与目的DNA序列结合,通过调节目的基因的转录参与发育调节过程。研究表明,在整个脊椎动物和无脊椎动物中,只有性别决定基因Dmrt才具有进化保守性,也是参与性别决定最古老的发育基因家族[6]。 Dmrt基因的功能主要体现在性别决定、性腺分化、许多组织和器官的形成及相关功能的维持等多方面[3,7,8]。近来在水生生物,特别是甲壳动物中,有关Dmrt基因的研究已经取得了众多成果。而截止目前为止,还缺乏对其进行却进行全面总结的报道。鉴于此,本文对Dmrt基因在水生生物中的研究进行了综述,并探讨了Dmrt1基因及与性别决定的关系,以期为以后更好地研究水生生物Dmrt基因提供参考。
1 Dmrt基因家族的结构特征及种类
Dmrt基因家族成员的主要特征是其编码的多肽链中含有与DNA结合的保守区域-DM结构域。DM结构域中的锌指结构含有6个半胱氨酸(C)和2个组氨酸(H),这8个保守的氨基酸形成与Zn离子结合的两个位点(CCHC和HCCC)。DM结构域的羧基端有一个可识别DNA的α-螺旋,用以稳定DNADM结构域结合体[3]。从无脊椎动物到脊椎动物,Dmrt家族成员编码的DM结构域多肽序列高度保守,而在DM结构域外的序列相似性却很低。
不同物种的Dmrt基因家族成员不尽相同,在哺乳类、鸟类、爬行类、两栖类、鱼类中,Dmrt基因家族的种类一般为8种(Dmrt1-8)[9,10]、4种(Dmrt1-4)[11]、6种(Dmrt1-6)[12]、5种(Dmrt1-5)[13]、5种(Dmrt1-5)[14,15]。在无脊椎动物中,Dmrt基因家族成员数目因物种而异。
2 水生生物Dmrt基因的功能
2.1腔肠动物和扁形动物
目前为止,动物界中仅低等的海绵动物没有发现Dmrt基因[16,17],从腔肠动物开始检测到Dmrt基因的存在(表1)。鹿角珊瑚(Acropora millepora)为雌雄同体的生物,一般在春季产卵之前分化产生雌雄生殖细胞,在此过程中鹿角珊瑚的AmDM1基因表达水平会明显升高,暗示AmDM1基因可能参与了雌雄生殖细胞的分化过程[18]; 最新的研究表明,在海葵(Nematostella vectensis)中发现11个Dmrt基因(Nv-DMRT A-Nv-DMRT K),7个基因在雌雄个体间存在表达差异,其中Nv-DMRT B,F,G,H,I,K在雄性中的表达量明显高于雌性,而Nv-DMRT E在雌性中的表达量要高于雄性。进一步的分析表明,Nv-DMRT基因在海葵雌雄配子的发生过程中发挥作用[19]。虽然扁形动物的真涡虫(Schmidtea mediterranea)为雌雄同体动物,但Smed-dmd-1基因在雄性生殖系统中特异性表达,而且Smed-dmd-1基因对于精巢和雄性附属生殖腺的发生、发展、维持及重新生成是必须的[20],被认定为性别决定基因[14]。同属扁形动物的曼氏血吸虫(Schistosoma mansoni)(雌雄异体)中,dmd-1基因在雄性个体的表达量要显著高于雌性,表现为明确的性别特异性表达[20]。
综上所述,腔肠动物Dmrt基因的功能主要体现在参与雌雄配子的分化过程,而在扁形动物中,Dmrt基因的功能则进一步升级为调控雄性性腺的发育(表1),参与性别决定。
2.2软体动物
于非非等[21]克隆了马氏珠母贝(Pinctada matensii)的3个Dmrt基因(Dmrt2,3,4),并预测Dmrt2基因可能在马氏珠母贝的性别分化过程中起重要作用; 进而,Yu等[22]进一步研究了Dmrt2基因的组织和时空表达,结果显示,Dmrt2基因主要在马氏珠母贝雄性生殖腺中表达,其转录水平在雄性生殖腺的起始阶段较低,在生殖腺的成熟阶段最高,此外在鳃中也检测到微量表达。研究表明,Dmrt2参与了马氏珠母贝精子的形成过程。冯政夫等[23]克隆了栉孔扇贝(Chlamys farreri)的Cf-dmrt4-like基因,该基因从受精卵到匍匐幼虫各发育时期均有表达,其中卵裂期表达量较高; 在不同发育时期的精巢中均有表达,以成熟期的精巢表达量最高; 在卵巢中未见表达。由此推测,栉孔扇贝Cf-dmrt4-like基因参与了个体的早期发育,并在两性成体中发挥着不同的作用。周丽青等[24]在虾夷扇贝(Patinopecten yessoensis)中克隆了PyDmrt3和PyDmrt4两个基因,并推测PyDmrt4 可能参与了调控雄性性别的形成过程。张娜等[25]在长牡蛎(Crassostrea gigas)中发现了CgDsx,CgDmrtA2两个基因,CgDsx 基因可能调控长牡蛎早期的胚胎发育,而CgDmrtA2基因可能参与了胚胎中后期的发育过程,与神经的形成相关。太平洋牡蛎(Crassostrea gigas)的Cg-DMl基因在雌雄个体中均有表达,但在雄性生殖腺的表达量明显高于雌性,表明Cg-DMl参与了太平洋牡蛎雄性性腺的发育过程[26]。黑蝶真珠蛤(Pinctada margaritifera)是雌雄同体雄性先成熟的物种,其中的性转机制尚不清楚,研究人员通过性腺转录组数据分析了其中可能的性别分化和决定基因。研究表明,pmarg-dmrt基因和其他两个基因可能参与了精卵巢的转化过程[27]。在耳鲍(Haliotis asinin)中,HADMRT1基因在雄性的精巢中特异性表达,表明HADMRT1在耳鲍的精巢发育过程中发挥作用[28]。
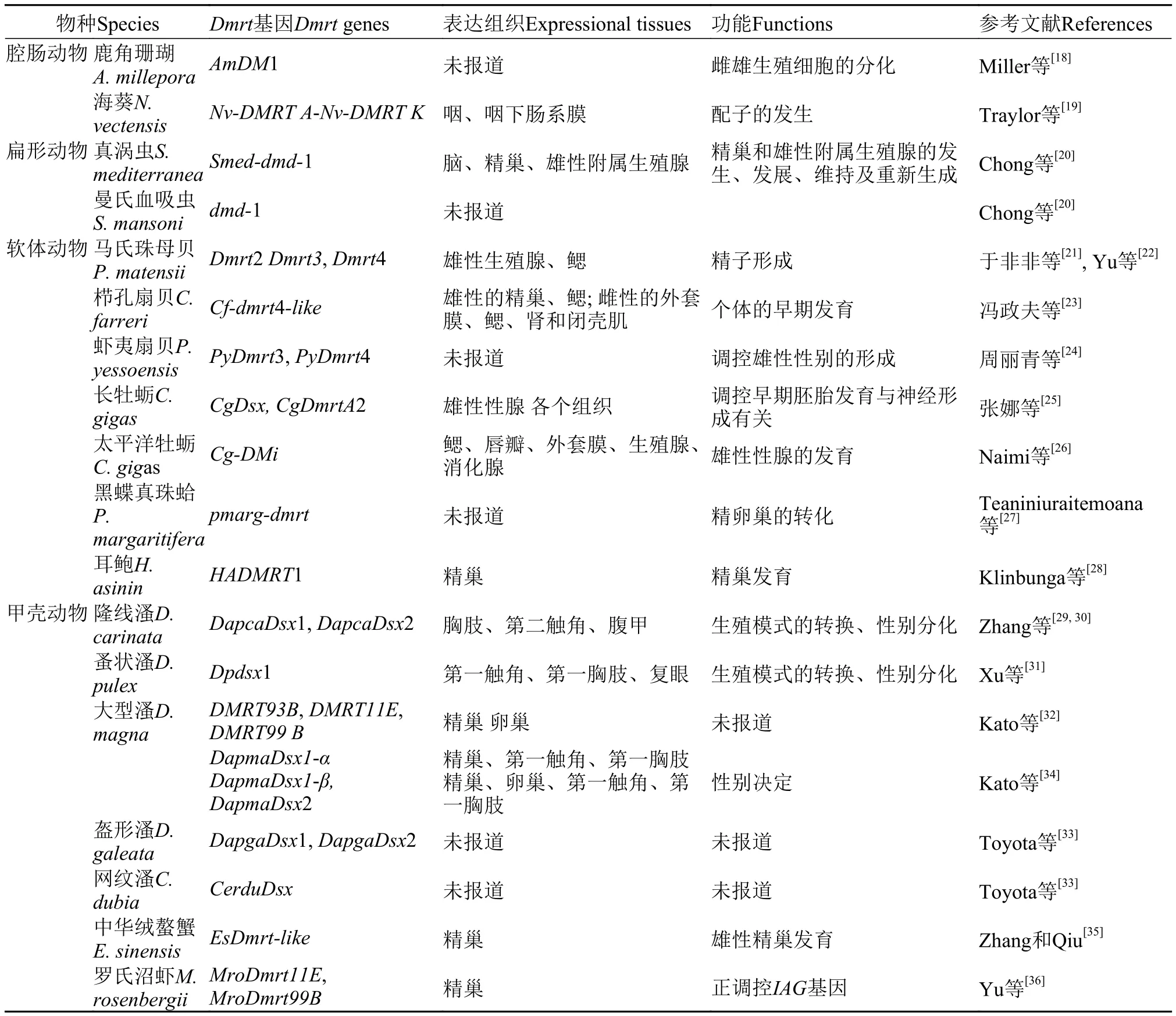
表1 Dmrt基因在水生无脊椎动物的研究汇总表Tab. 1 Summary of the study for Dmrt genes in the aquatic invertebrate
据此,软体动物的Dmrt基因主要在雄性的生殖腺及精子形成过程中发挥作用(表1)。
2.3甲壳动物
水蚤科动物在环境适宜的条件下多为雌性个体,行孤雌生殖,可以使种群大量繁殖; 在环境恶劣(光照周期缩短、食物短缺、群体密度增大、水质恶劣)的情况下,雌性个体所带的卵会产生雄性和雌性个体行有性生殖,产生休眠卵渡过恶劣环境,保证物种的持续性。由此可见,环境变化是诱导水蚤科动物生殖策略改变的原因,而此过程中所蕴含的性别决定和生殖策略改变的遗传机制也成为最近研究的热点。
Zhang等[29,30]在隆线溞(Daphnia carinata)中相继研究了DM结构域的两个基因DapcaDsx1和DapcaDsx2,并且在雌性幼体、孤雌生殖的雌性、有性生殖雌性的早期阶段、有性生殖的雌性、休眠卵五个不同生殖阶段研究了DapcaDsx1和DapcaDsx2表达情况。结果这两个基因均显示出相似的变化规律,在有性生殖雌性的早期阶段表达量最高,且有性生殖阶段的表达量明显高于孤雌生殖阶段。DapcaDsx1和DapcaDsx2呈现明显的性别特异性表达,暗示它们可能在隆线溞由孤雌生殖向有性生殖阶段过渡的过程中发挥作用。蚤状溞(Daphnia pulex)中的研究表明,Dpdsx1基因在有性生殖雄性个体的第一触角、第一胸肢、复眼中的表达量最高,在行孤雌生殖和有性生殖的雌性个体中,上述三个组织中的表达量显著下降。且Dpdsx1基因在蚤状溞有性生殖的雄性阶段表达量最高,在雌性幼体阶段表达量最低。与隆线溞相似,蚤状溞Dpdsx1基因也主要在生殖模式的转换、性别分化过程中发挥作用[31]。Kato等[32]首先在大型溞(Daphnia magna)中克隆到三个Dmrt基因:DMRT11E,DMRT93B和DMRT99B,其中DMRT11E和DMRT99B在卵巢中的表达量要高于精巢,而DMRT93B则在雄性的精巢中特异性的表达。由于这三个基因在胚胎阶段没有表达,且不具有性别二态性表达,所以它们不是性别决定基因[33]。进而,Kato等[34]在大型溞中克隆得到两种Dsx 基因DapmaDsx1和DapmaDsx2,其中DapmaDsx1编码两种不同的亚型DapmaDsx1-α和DapmaDsx1-β。DapmaDsx1展现出明显的性别二态性表达,在雄性胚胎的形成过程中,DapmaDsx1表达量明显升高,而在雌性胚胎中则没有这种现象。进一步在雄性胚胎中沉默DapmaDsx1,可诱导其卵巢成熟,产生雌性特征; 相反,在雌性胚胎中异位表达Dapma Dsx1,可使其产生雄性特性。表明DapmaDsx1雄性性别决定中起着关键作用。因此,DapmaDsx1被认为是大型溞的性别决定基因[14]。Toyota等[33]进一步在蚤状溞、盔形溞(Daphnia galeata)、网纹溞(Ceriodaphnia dubia)中分别克隆了两个Dsx基因,在多刺裸腹水蚤(Moina macrocopa)中克隆了一个Dsx基因,这些基因均表现为明显的性别二态性表达,表明它们具有相似的功能。
近来,Dmrt基因在十足目(虾蟹类)中的研究也取得了相应进展。中华绒螯蟹(Eriocheir sinensis)中发现一个EsDmrt-like基因,EsDmrt-like在精巢中特异性表达,进一步的分析表明,EsDmrt-like主要在精巢中的支持细胞中表达,在成熟的精子中则没有检测到其表达,而支持细胞是为发育中的精子提供保护和营养的细胞,精子发育的各个阶段都是发生在支持细胞的表面。此结果表明在中华绒螯蟹中,EsDmrt-like是雄性精巢发育的关键因子[35]。罗氏沼虾(Macrobrachium rosenbergii)中发现两个Dmrt基因:MroDmrt11E和MroDmrt99B基因,这两个基因均在精巢中高度表达。通过RNA干扰分别沉默MroDmrt11E和MroDmrt99B基因,结果表明,MroDmrt11E正调控胰岛素样促雄腺激素(Insulinlike androgenic gland hormone,IAG)基因的表达[36]。
综上所述,甲壳动物Dmrt基因主要参与雄性精巢的发育过程,且在大型溞中Dsx1基因被认定为性别决定基因(表1)。
2.4鱼类
性别决定鱼类种类繁多,其性别决定方式和机制也呈现多样化。而Dmrt1基因作为鱼类的性别决定基因的证据来源于青鳉(Oryzias latipe)(XX/XY型,雌性同配、雄性异配)[37—40]。DMY(DM domain gene on the Y chromosome)基因位于青鳉Y染色体上的性别决定区域,是进化上相对年轻的基因,由位于常染色体的Dmrt1基因复制、转移到Y 染色体上而形成[41—43]。在雌性青鳉中过度表达DMY基因,可使雌性青鳉产生性逆转[37]; 相反,在雄性青鳉中沉默DMY基因或者使DMY基因产生突变,可使雄性个体逆转为雌性[44,45]。此外,青鳉的DMY基因只存在于Y染色体上,在X染色体上没有检测到它的同源基因,表达也只限于雄性的胚胎和成体精巢中的支持细胞,表明DMY基因对青鳉精巢的分化和功能维持至关重要[46,47]。然而DMY基因也仅见于青鳉,而其他鱼类却没有发现此基因[48],表明青鳉DMY基因的性别决定功能在鱼类中不具有普遍性[49]。Chen等[50]首次构建了比目鱼-半滑舌鳎(Cynoglossus semilaevis)(ZZ/ZW型,雄性同配、雌性异配)全基因组精细图谱,发现半滑舌鳎dmrt1基因是Z染色体连锁、雄性特异表达、精巢发育必不可少的关键基因,表现出性别决定基因的特性。
性腺发育自然界中鱼类存在雌雄异体和雌雄同体两大类,而后者又可分为精巢先成熟和卵巢先成熟两种类型。而Dmrt1基因在上述三种鱼类的性腺发育过程中均发挥重要作用。
雌雄异体的鱼类:在革胡子鲇(Clarias gariepinus)[51]、稀有鮈鲫(Gobiocypris rarus)[52]、尼罗罗非鱼(Oreochromis niloticus)[53]、牙鲆(Paralichthys olivaceus)[54,55]、红尾剑鱼(Xiphophorus maculatus)[56]、红鳍东方鲀(Takifugu rubripes)[57]、奥利亚罗非鱼(O. aurea)[58]、异育银鲫(Carassius auratus gibelio)[59]等鱼类中,Dmrt1基因在精巢中检测到特异性表达(表2)。在湖鲟(Acipenser fulvescens)[60]、斑马鱼(Danio rerio)[61]、大西洋鳕(Gadus morhua)[62]、银汉鱼(Odontesthes bonari-ensis)[63]、虹鳟(Oncorhynchus mykiss)[64]、密西西比铲鲟(Scaphirhynchus platorynchus)[65]、南方大口鲶(Silurus meridionals)[66]、团头鲂(Megalobrama amblycephala)[67]、许氏平鲉(Sebastods schlege-lii)[68]、腋孔蟾鱼(Halobatrachus didactylus)[69]中,Dmrt1基因主要在精巢中表达,此外在卵巢中也检测到微量的表达(表2)。
雌雄同体的鱼类:在此类型的鱼类中,精巢和卵巢之间存在相关转化的阶段。而诱导精卵巢转化的遗传信号分子,很可能出现在胚胎或幼体之后,而Dmrt1基因同样在其中发挥作用。雌性先成熟的黑鲷(Acanthopagrus schlegeli)[70]、金头鲷(Sparus auratus)[71]在卵巢向精巢转化的过程中,Dmrt1基因表达量明显升高; 而在雄性先成熟的点带石斑鱼(Epinephlus coioides)[72]、细棘海猪鱼(Halichoeres tenuispinis)[73]、黄鳝(Monopterus albus)[74]中,在由精巢向卵巢转的过程中,Dmrt1基因表达量则呈现相反的表达趋势。
虽然Dmrt1基因对鱼类精巢功能的维持起着关键作用,但Dmrt家族的其他成员对已经分化的性腺的正常发育和功能维持也有重要作用。在青鳉中,Dmrt3基因在成体的精巢中就有所表达,而Dmrt2和Dmrt4基因在成体精巢和卵巢中均有所表达[75]。最新的研究表明,黄鳝的Dmrt2,3,4,5 基因在发育生殖细胞中均有表达[76]。在尼罗罗非鱼[77]、奥利亚罗非鱼[78]、斑马鱼[61]中也存在类似的现象。
其他功能Dmrt家族基因除了参与性别决定和性腺分化与维持之外,还在鱼类神经系统和感觉器官的发育[79,80]、体节形成(沉默dmrt2b可导致尾部弯曲和U型体节)[81—83]、以及其他器官形成与功能维持方面[67,75,82]发挥作用。
据此,Dmrt基因家族在鱼类中作用主要体现在性腺分化(表2)、性别决定、体节发育、各种器官的形成和维持等方面。
3 Dmrt1基因功能的思考
在低等(腔肠动物和扁形动物)和较低等(软体动物)的无脊椎动物中,现有的研究表明,Dmrt1基因“主管”配子分化与性腺发育。众所周知,性别决定和性腺分化是紧密联系的,“主管”性腺分化的基因随着生物的进化,很可能升级为性别决定基因。在涡虫中,Dmrt1基因作为性别决定基因已初见端倪[20]; 在节肢动物门中其性别决定地位得以巩固,达到高峰。例如在果蝇中,sxl(sex-lethal)-tra(transformer)-dsx的性别决定通路已经得以论证[39,40,85];甲壳纲的水蚤科中Dsx基因也是其性别决定基因[34];十足目(虾蟹类)中存在特殊腺体-促雄腺,其转录的IAG是十足目的性别决定基因[86—90],而Dmrt则是IAG基因的上游调控基因,正调控IAG的表达[36]。此时,Dmrt基因仍在十足目的性别决定通路中发挥作用,但其性别决定地位的作用已经开始弱化。根据Kopp的理论:在生物的进化过程中,性别决定机制也保持较快的进化速率,以至于性别决定的“主导”基因很少能在这个机制的“顶端”保存较长的时间[40]。例如,sxl和tra这两个基因仅是昆虫性别决定基因,在昆虫之外的物种中虽然存在,但其作用却不是性别决定; 再如,SRY基因仅在哺乳动物中行使特异的性别决定功能,在非哺乳的脊椎动物中,SRY基因就不存在。因此,(鱼类)青鳉的DMY基因作为性别决定基因也仅仅是个别现象,是由于Dmrt1基因复制产生的“副作用”,这可能与青鳉特殊的进化地位有关[15]; 半滑舌鳎中Dmrt1基因除了在Z染色体上,在W染色体上也存在一个假基因化拷贝(Pseudogenized copy),在性逆转的雄鱼(ZW型)中这个假基因化拷贝被激活,通过剂量补偿效益达到与正常雄鱼相同的表达水平[50]。但是以上现象在鱼类不存在普遍性。并且研究表明,在所有发现Dmrt1基因的鱼类中,Dmrt1基因无一例外地在鱼类的性腺发育中发挥作用[39]。至此,Dmrt1作为性别决定基因的地位进一步削弱。虽然在两栖类、爬行类、鸟类等非哺乳的脊椎动物中,Dmrt1和Sox9基因决定性别分化[6]。但Dmrt1基因作为性别决定基因也仅见于非洲爪蟾(Xenopus laevis)[91]和原鸡(Gallus gallus)[92]两个物种。而且研究表明,Dmrt1不可能是鸟类性别决定的开关基因[93]。在哺乳类脊椎生物中,SRY基因是其真正的性别决定基因[6]; 进而纵观无脊椎动物到脊椎动物的各个物种,不难发现,调控性腺(或配子)的分化与发育是Dmrt1基因的主导功能。例如,人类的Dmrt1基因是第一个在脊椎动物中得以鉴定的DM结构域基因,是在人类睾丸中发现的,定位于人类常染色体(9p24.3)上。该区域的缺失会导致睾丸发育异常,然而到目前为止没有发现该基因的点突变影响人类的性别决定[94]。
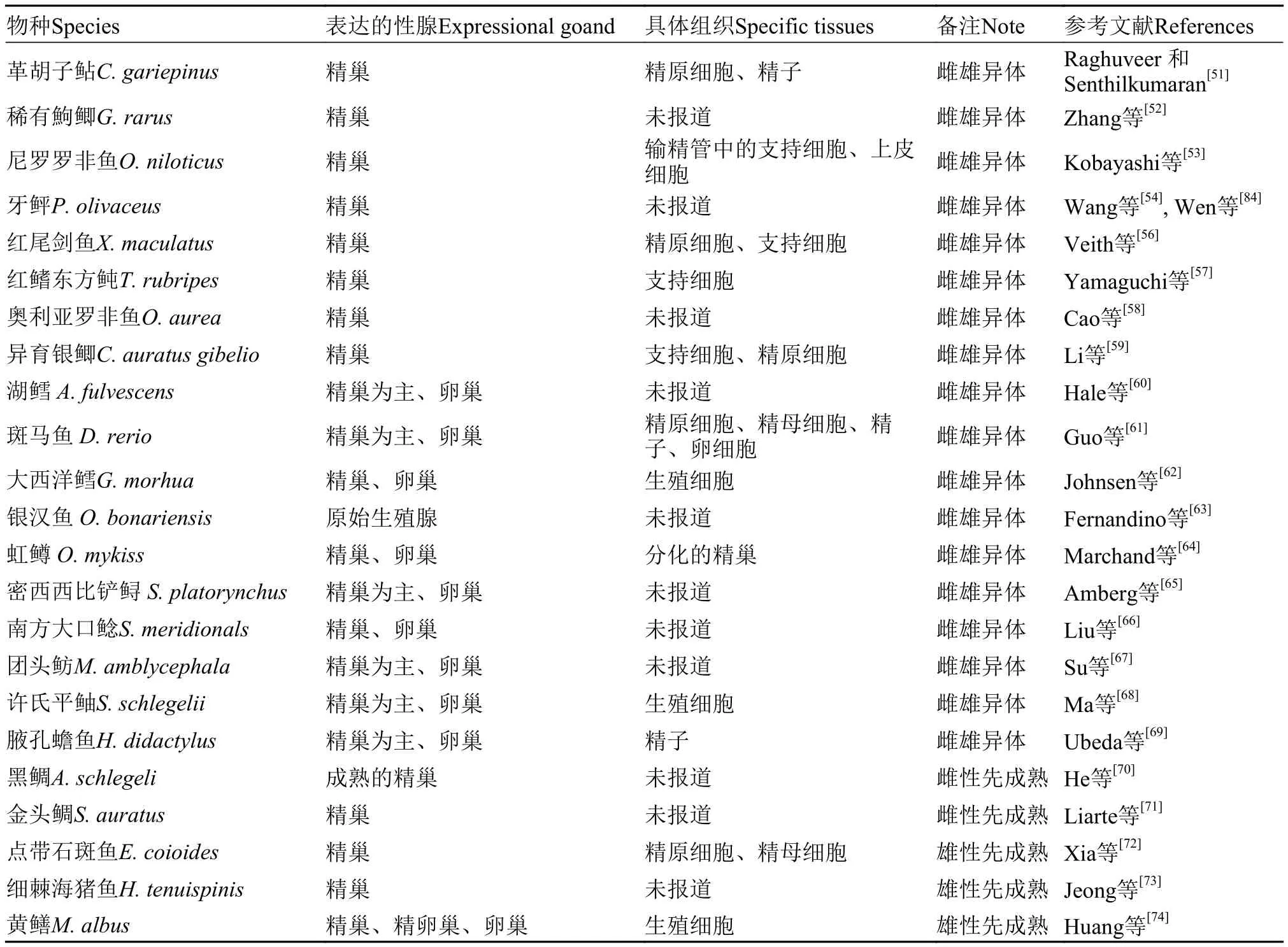
表2 Dmrt1基因在鱼类性腺中的表达Tab. 2 Expression of Dmrt1 genes in fish gonad
作为一个在无脊椎动物和脊椎动物中均广泛存在的古老基因,结构相对保守的Dmrt基因很可能在功能上也具有相当的保守性。如上所述,从低等的腔肠动物到高等的哺乳动物,Dmrt1基因的功能更多地是体现在性腺分化,特别是雄性性腺分化及精子成熟方面。而Dmrt1作为性别决定基因,现有的研究成果表明,最早出现于涡虫,在节肢动物中其性别决定基因地位达到高峰,并且在甲壳纲中性别决定基因地位已经开始弱化。在众多的脊椎动物中,Dmrt1作为性别决定基因地位已接近“尾声”。仅在个别物种-青鳉、半滑舌鳎、非洲爪蟾、原鸡中是性别决定基因。
4 展望
虽然有关Dmrt家族基因的研究在水生生物中取得了较大的进展,但尚有许多工作需要深入开展。从低等的腔肠动物到高等的脊椎动物-鱼类,几乎都存在雌雄同体的生物,在这些生物中,Dmrt基因无一例外地都是促进雄性配子或雄性生殖腺的发育,可见其功能的保守性,Dmrt多肽作为转录调节因子,众多的研究也为揭示其蕴含的分子机制提供了坚实的基础; 但每个物种具体的调控机制,特别是以Dmrt基因作为性别决定基因的物种,它们之间是否存在相似的调控网络,还需进一步研究; 在虾蟹类中,有关Dmrt家族基因的研究也仅见于河蟹和罗氏沼虾,其他十足目物种的Dmrt序列及功能还未见报道; 有趣的是,半滑舌鳎的研究还发现,半滑舌鳎的性染色体并不和已知性染色体的鱼类共祖先,而是和鸡的ZW染色体共起源,揭示半滑舌鳎和鸡性染色体的趋同进化现象[50]。而在半滑舌鳎和鸡中,Dmrt1都是性别决定基因。这提醒我们,在性染色体与这两个物种趋同进化的其他物种中,Dmrt1是否也是性别决定基因,值得深入探索。
[1]Zarkower D. Establishing sexual dimorphism:conservation amidst diversity [J]? Nature Reviews Genetics,2001,2(3):175—185
[2]Burtis K C,Baker B S. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides [J]. Cell,1989,56(6):997—1010
[3]Zhu L,Wilken J,Phillips N,U,et al. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers [J]. Genes & Development,2000,14(14):1750—1764
[4]Hildreth P E. Doublesex,a recessive gene that tranforms both males and females of drosophila into intersexes [J]. Genetics,1965,51(4):659—678
[5]Erdman S E,Burtis K C. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain [J]. The EMBO Journal,1993,12(2):527—535
[6]Cheng Z H. The function and trait of Dmrt gene [J]. Journal of Anhui Agricultural Agricultural,2006,34(5):835—836 [程子华. Dmrt基因的功能和特点. 安徽农业科学,2006,34(5):835—836]
[7]Ellegren H. Hens,cocks and avian sex determination. A quest for genes on Z or W [J]? Embo Reports,2001,2(3):192—196
[8]Kim S,Kettlewell J R,Anderson R C,et al. Sexually dimorphic expression of multiple doublesex-related genes in the embryonic mouse gonad [J]. Gene Expression Patterns,2003,3(1):77—82
[9]Ottolenghi C,Fellous M,Barbieri M,et al. Novel paralogy relations among human chromosomes support a link between the phylogeny of doublesex-related genes and the evolution of sex determination [J]. Genomics,2002,79(3):333—343
[10]Hong C S,Park B Y,Saint J P. The function of Dmrt genes in vertebrate development:it is not just about sex[J]. Developmental Biology,2007,310(1):1—9
[11]Wang H,Wang T R,Yuan J,et al. Advances in Dmrt gene family of vertebrates [J]. Guizhou Agricultural Science,2012,40(5):148—152 [汪海,王婷茹,袁静,等. 脊椎动物Dmrt基因的研究进展. 贵州农业科学,2012,40(5):148—152]
[12]Wang Z,Miyake T,Edwards S V,et al. Tuatara(Sphenodon) genomics:BAC library construction,sequence survey,and application to the DMRT gene family [J]. Journal of Heredity,2006,97(6):541—548
[13]Bewick A J,Anderson D W,Evans B J. Evolution of the closely related,sex-related genes dmw and dmrt1 in african clawed frogs(Xenopus) [J]. Evolution,2011,65(3):698—712
[14]Picard A L,Cosseau C,Mouahid G,et al. The roles of Dmrt(Double sex/Male-abnormal-3 Related Transcription factor) genes in sex determination and differentiation mechanisms:Ubiquity and diversity across the animal kingdom [J]. Comptes Rendus Biologies,2015,338(7):451—462
[15]Cao J L,Chen J J,Gan X,et al. Advances of Dmrt gene for fish [J]. Journal of Guangdong Ocean University,2011,31(1):94—98 [曹谨玲,陈剑杰,甘西,等. DMRT基因的研究进展. 广东海洋大学学报,2011,31(1):94—98]
[16]Bellefroid E J,Leclère L,Saulnier A,et al. Expanding roles for the evolutionarily conserved Dmrt sex transcriptional regulators during embryogenesis [J]. Cellular & Molecular Life Sciences,2013,70(20):3829—3845
[17]Wexler J R,Plachetzki D C,Kopp A. Pan-metazoan phylogeny of the DMRT gene family:a framework forfunctional studies [J]. Development Genes and Evolution,2014,224(3):175—181
[18]Miller S W,Hayward D C,Bunch T A,et al. A DM domain protein from a coral,Acropora millepora,homologous to proteins important for sex determination [J]. Evolution & Development,2003,5(3):251—258
[19]Traylor N G,Kane E G,Sombatsaphay V,et al. Sex-specific and developmental expression of Dmrt genes in the starlet sea anemone,Nematostella vectensis [J]. Evolutionary Developmental Biology,2015,6(1):13—19
[20]Chong T,Collins J J,Brubacher J L,et al. A sex-specific transcription factor controls male identity in a simultaneous hermaphrodite [J]. Nature Communications,2013,4:1814—1826
[21]Yu F F,Zhou L,Wang M F,et al. Cloning and sequence analysis of three DM domain in Pinctada matensii [J]. Journal of Agricultural Biotechnology,2007,15(5):905—906 [于非非,周莉,王梅芳,等. 马氏珠母贝(Pinctada matensii)3个DM结构域的克隆及序列分析.农业生物技术学报,2007,15(5):905—906]
[22]Yu F F,Wang M F,Zhou L,et al. Molecular cloning and expression characterization of dmrt2 in akoya pearl oysters,Pinctada martensii [J]. Journal of Shellfish Research,2011,30(2):247—254
[23]Feng Z F,Shao M Y,Sun D P,et al. Cloning,characterization and expression analysis of Cf-dmrt4-like gene in Chlamys farreri [J]. Journal of Fishery Scicnece of China,2010,17(5):930—940 [冯政夫,邵明瑜,孙大鹏,等. 栉孔扇贝Cf-dmrt4-like基因的克隆、序列特征及表达分析. 中国水产科学,2010,17(5):930—940]
[24]Zhou L Q,Yang A G,Wang Y Q,et al. Sequence analysis of DM domain in two Dmrt genes of three sex types of Patinopecten yessoensis [J]. Marine Sciences,2015,39(3):19—25 [周丽青,杨爱国,王清印,等. 虾夷扇贝不同性别类型2个Dmrt基因DM结构域分析. 海洋科学,2015,39(3):19—25]
[25]Zhang N,Huang W,Xu F,et al. Expression of two Dmrt family genes in the pacific oyster Crassostrea gigas [J]. Oceanologia et Limnologia Sinica,2015,46(3):717—724 [张娜,黄雯,许飞,等. 长牡蛎(Crassostrea gigas)两个Dmrt基因家族的时空表达. 海洋与湖沼,2015,46(3):717—724]
[26]Naimi A,Martinez A S,Specq M L,et al. Identification and expression of a factor of the DM family in the oyster Crassostrea gigas [J]. Comparative Biochemistry and Physiology Part A Molecular & Integrative Physiology,2009,152(2):189—196
[27]Teaniniuraitemoana V,Huvet A,Levy P,et al. Gonad transcriptome analysis of pearl oyster Pinctada margaritifera:identification of potential sex differentiation and sex determining genes [J]. BMC Genomics,2014,15(1):1—20
[28]Klinbunga S,Amparyup P,Khamnamtong B,et al. Isolation and characterization of testis-specific DMRT1 in the tropical abalone(Haliotis asinina) [J]. Biochemical Genetics,2009,47(1—2):66—79
[29]Zhang M,Li H,Liu A,et al. Cloning,expression and cellular localization of the Doublesex gene in the water flea,Daphnia carinata,during different developmental stages[J]. Gene,2014,550(2):185—192
[30]Zhang M,Ma C,Lv W,et al. Molecular cloning,characterization and expression analysis of a Doublesex gene from Daphnia carinata(Crustacea:Cladocera) during different reproductive stages [J]. Genetics and Molecular Research,2015,14(2):5930—5942
[31]Xu S L,Zhou W,Chen P,et al. Identification and expression analysis of a doublesex1 gene in Daphnia pulex during different reproductive stages [J]. Development Genes and Evolution,2014,224(3):147—157
[32]Kato Y,Kobayashi K,Oda S,et al. Molecular cloning and sexually dimorphic expression of DM-domain genes in Daphnia magna [J]. Genomics,2008,91(1):94—101
[33]Toyota K,Kato Y,Sato M,et al. Molecular cloning of doublesex genes of four cladocera(water flea) species [J]. BMC Genomics,2013,14(1):239—252
[34]Kato Y,Kobayashi K,Watanabe H,et al. Environmental sex determination in the branchiopod crustacean Daphnia magna:deep conservation of a Doublesex gene in the sexdetermining pathway [J]. PLoS Genetics,2011,7(3):e1001345
[35]Zhang E F,Qiu G F. A novel Dmrt gene is specifically expressed in the testis of Chinese mitten crab,Eriocheir sinensis [J]. Development Genes and Evolution,2010,220(5—6):151—159
[36]Yu Y Q,Ma W M,Zeng Q G,et al. Molecular cloning and sexually dimorphic expression of two dmrt genes in the giant freshwater prawn,Macrobra-chium rosenbergii[J]. Agricultural Research,2014,3(2):181—191
[37]Matsuda M,Shinomiya A,Kinoshita M,et al. DMY gene induces male development in genetically female(XX)medaka fish [J]. Proceedings of the National Academy of Sciences,2007,104(10):3865—3870
[38]Matsuda M. Sex determination in the teleost medaka,Oryzias latipes [J]. Annual Review of Genetics,2005,39:293—307
[39]Matson C K,Zarkower D. Sex and the singular DM domain:insights into sexual regulation,evolution and plasticity [J]. Nature Reviews Genetics,2012,13(3):163—174
[40]Kopp A. Dmrt genes in the development and evolution of sexual dimorphism [J]. Trends in Genetics,2012,28(4):175—184
[41]Matsuda M,Nagahama Y,Shinomiya A,et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish [J]. Nature,2002,417(6888):559—563
[42]Kondo M,Hornung U,Nanda I,et al. Genomic organiza-tion of the sex-determining and adjacent regions of the sex chromosomes of medaka [J]. Genome Research,2006,16(7):815—826
[43]Indrajit N,Mariko K,Ute H,et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka,Oryzias latipes [J]. Proceedings of the National Academy of Science,2002,99(18):11778—11783
[44]Masuyama H,Yamada M,Kamei Y,et al. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka [J]. Chromosome Research,2012,20(1):163—176
[45]Paul B,Matsuda M,Lau E L,et al. Knock-down of DMY initiates female pathway in the genetic male medaka,Oryzias latipes [J]. Biochemical and Biophysical Research Communications,2006,351(4):815—819
[46]Kobayashi T,Matsuda M,Kajiura H,et al. Two DM domain genes,DMY and DMRT1,involved in testicular differentiation and development in the medaka,Oryzias latipes [J]. Developmental Dynamics,2004,231(3):518—526
[47]Matsuda M,Sato T,Toyazaki Y,et al. Oryzias curvinotus has DMY,a gene that is required for male development in the medaka,O. latipes [J]. Zoological Science,2003,20(2):159—161
[48]Kondo M,Nanda I,Hornung U,et al. Absence of the candidate male sex-determining gene dmrt1b(Y) of medaka from other fish species [J]. Current Biology,2003,13(5):416—420
[49]Zheng Y,Wang Z Z,Chen J Z. Progresses on the study of sex differentiation genes in fish [J]. Acta Hydrobiologica Sinica,2015,39(4):798—810 [郑尧,王在照,陈家长. 调控鱼类性腺分化基因的研究进展. 水生生物学报,2015,39(4):798—810]
[50]Chen S,Zhang G,Shao C,et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle[J]. Nature Genetics,2014,46(3):253—260
[51]Raghuveer K,Senthilkumaran B. Identification of multiple dmrt1s in catfish:localization,dimorphic expression pattern,changes during testicular cycle and after methyltestosterone treatment [J]. Journal of Molecular Endocrinology,2009,42(5):437—448
[52]Zhang X,Zha J,Wang Z. Influences of 4-nonylphenol on doublesex and mab-3-related transcription factor 1 gene expression and vitellogenin mRNA induction of adult rare minnow(Gobiocypris rarus) [J]. Environmental Toxicology and Chemistry,2008,27(1):196—205
[53]Kobayashi T,Kajiura H,Guan G,et al. Sexual dimorphic expression of DMRT1 and Sox9 a during gonadal differentiation and hormone-induced sex reversal in the teleost fish Nile tilapia(Oreochromis niloticus) [J]. Developmental Dynamics,2008,237(1):297—306
[54]Wang D S,Zhou L Y,Kobayashi T,et al. Doublesex-and Mab-3-related transcription factor-1 repression of aromatase transcription,a possible mechanism favoring the male pathway in tilapia [J]. Endocrinology,2010,151(3):1331—1340
[55]Wen A,You F,Sun P,et al. Sexually dimorphic gene expression patterns during gonadal differentiation in olive flounder,Paralichthys olivaceus [J]. 2015,DOI:10.1163/15707563—00002470
[56]Veith A M,Schäfer M,Klüver N,et al. Tissue-specific expression of dmrt genes in embryos and adults of the platyfish Xiphophorus maculatus [J]. Zebrafish,2006,3(3):325—337
[57]Yamaguchi A,Lee K H,Fujimoto H,et al. Expression of the DMRT gene and its roles in early gonadal development of the Japanese pufferfish Takifugu rubripes [J]. Comparative Biochemistry and Physiology Part D∶ Genomics & Proteomics,2006,1(1):59—68
[58]Cao J,Cao Z,Wu T. Generation of antibodies against DMRT1 and DMRT4 of Oreochromis aurea and analysis of their expression profile in Oreochromis aurea tissues[J]. Journal of Genetics and Genomics,2007,34(6):497—509
[59]Li X Y,Li Z,Zhang X J,et al. Expression characterization of testicular DMRT1 in both Sertoli cells and spermatogenic cells of polyploid gibel carp [J]. Gene,2014,548(1):119—125
[60]Hale M C,Jackson J R,DeWoody J A. Discovery and evaluation of candidate sex-determining genes and xenobiotics in the gonads of lake sturgeon(Acipenser fulvescens) [J]. Genetica,2010,138(7):745—756
[61]Guo Y,Cheng H,Huang X,et al. Gene structure,multiple alternative splicing,and expression in gonads of zebrafish Dmrt1 [J]. Biochemical and Biophysical Research Communications,2005,330(3):950—957
[62]Johnsen H,Seppola M,Torgersen J S,et al. Sexually dimorphic expression of dmrt1 in immature and mature Atlantic cod(Gadus morhua L.) [J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology,2010,156(3):197—205
[63]Fernandino J,Hattori R,Shinoda T,et al. Dimorphic expression of dmrt1 and cyp19a1(ovarian aromatase) during early gonadal development in pejerrey,Odontesthes bonariensis [J]. Sexual Development,2008,2(6):316—324
[64]Marchand O,Govoroun M,D'Cotta H,et al.DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout,Oncorhynchus mykiss [J]. Biochimica et Biophysica Acta(BBA)-Gene Structure and Expression,2000,1493(1):180—187
[65]Amberg J J,Goforth R,Stefanavage T,et al. Sexually dimorphic gene expression in the gonad and liver of shovelnose sturgeon(Scaphirhynchus platorynchus) [J]. FishPhysiology and Biochemistry,2010,36(4):923—932
[66]Liu Z,Zhang Y,Wang D. Studies on feminization,sex determination,and differentiation of the Southern catfish,Silurus meridionalis-a review [J]. Fish Physiology and Biochemistry,2010,36(2):223—235
[67]Su L,Zhou F,Ding Z,et al. Transcriptional variants of Dmrt1 and expression of four Dmrt genes in the blunt snout bream,Megalobrama amblycephala [J]. Gene,2015,573(2):205—215
[68]Ma L,Wang W,Yang X,et al. Characterization of the Dmrt1 gene in the black rockfish Sebastes schlegeli revealed a remarkable sex-dimorphic expression [J]. Fish Physiology and Biochemistry,2014,40(4):1263—1274
[69]Úbeda M,Merlo M A,Ortiz J B,et al. Expression profiling of the sex-related gene Dmrt1 in adults of the Lusitanian toadfish Halobatrachus didactylus(Bloch and Schneider,1801) [J]. Gene,2014,535(2):255—265
[70]He C L,Du J L,Wu G C,et al. Differential Dmrt1 transcripts in gonads of the protandrous black porgy,Acanthopagrus schlegeli [J]. Cytogenetic and Genome Research,2003,101(3—4):309—313
[71]Liarte S,Chaves E,García A,et al. Testicular involution prior to sex change in gilthead seabream is characterized by a decrease in DMRT1 gene expression and by massive leukocyte infiltration [J]. Reproductive Biology and Endocrinoogyl,2007,5:20—35
[72]Xia W,Zhou L,Yao B,et al. Differential and spermatogenic cell-specific expression of DMRT1 during sex reversal in protogynous hermaphroditic groupers [J]. Molecular and Cellular Endocrinology,2007,263(1):156—172
[73]Jeong H B,Park J G,Park Y J,et al. Isolation and characterization of DMRT1 and its putative regulatory region in the protogynous wrasse,Halichoeres tenuispinis [J]. Gene,2009,438(1):8—16
[74]Huang X,Guo Y,Shui Y,et al. Multiple alternative splicing and differential expression of dmrt1 during gonad transformation of the rice field eel [J]. Biology of Reproduction,2005,73(5):1017—1024
[75]Kondo M,Froschauer A,Kitano A,et al. Molecular cloning and characterization of DMRT genes from the medaka Oryzias latipes and the platyfish Xiphophorus maculatus [J]. Gene,2002,295(2):213—222
[76]Sheng Y,Chen B,Zhang L,et al. Identification of Dmrt genes and their up-regulation during gonad transformation in the swamp eel(Monopterus albus) [J]. Molecular Biology Reports,2014,41(3):1237—1245
[77]Guan G,Kobayashi T,Nagahama Y. Sexually dimorphic expression of two types of DM(Doublesex/Mab-3)-domain genes in a teleost fish,the Tilapia(Oreochromis niloticus) [J]. Biochemical and Biophysical Research Communications,2000,272(3):662—666
[78]Cao J L,Yu J H,Cao Z M,et al. Temporal and spatial expression of DMO and DMT gene in Oreochromis aurea[J]. Journal of Fisheries of China,2007,31(2):129—136[曹谨玲,俞菊华,曹哲民,等. 奥利亚罗非鱼DMO和DMT基因的时空表达特征分析. 水产学报,2007,31(2):129—136]
[79]Wen A,You F,Tan X,et al. Expression pattern of dmrt4 from olive flounder(Paralichthys olivaceus) in adult gonads and during embryogenesis [J]. Fish Physiology and Biochemistry,2009,35(3):421—433
[80]Li Q,Zhou X,Guo Y,et al. Nuclear localization,DNA binding and restricted expression in neural and germ cells of zebrafish Dmrt3 [J]. Biology of the Cell,2008,100(8):453—463
[81]Winkler C,Hornung U,Kondo M,et al. Developmentally regulated and non-sex-specific expression of autosomal dmrt genes in embryos of the Medaka fish(Oryzias latipes) [J]. Mechanisms of Development,2004,121(7):997—1005
[82]Zhou X,Li Q,Lu H,et al. Fish specific duplication of Dmrt2:characterization of zebrafish Dmrt2b [J]. Biochimie,2008,90(6):878—887
[83]Liu S,Li Z,Gui J F. Fish-specific duplicated dmrt2b contributes to a divergent function through Hedgehog pathway and maintains left-right asymmetry establishment function [J]. PloS One,2009,4(9):e7261
[84]Bradley K M,Breyer J P,Melville D B,et al. An SNP-based linkage map for zebrafish reveals sex determination loci [J]. G3:Genes,Genomes,Genetics,2011,1(1):3—9
[85]Ma W J,Vavre F,Beukeboom L. Manipulation of arthropod sex determination by endosymbionts:diversity and molecular mechanisms [J]. Sexual Development,2014,8(1—3):59—73
[86]Lezer Y,Aflalo E D,Manor R,et al. On the safety of RNAi usage in aquaculture:The case of all-male prawn stocks generated through manipulation of the insulin-like androgenic gland hormone [J]. Aquaculture,2015,435:157—166
[87]Ventura T,Manor R,Aflalo E,et al. Timing sexual differentiation:full functional sex reversal achieved in Macrobrachium rosenbergii through silencing of a single insulin-like gene [J]. Biology of Reproduction,2012,86(3):90,1—6
[88]Ventura T,Rosen O,Sagi A. From the discovery of the crustacean androgenic gland to the insulin-like hormone in six decades [J]. General and comparative endocrinology,2011,173(3):381—388
[89]Rosen O,Manor R,Weil S,et al. A sexual shift induced by silencing of a single insulin-like gene in crayfish:ovarian upregulation and testicular degeneration [J]. PloS One,2010,5(12):e15281
[90]Ventura T,Sagi A. The insulin-like androgenic gland hormone in crustaceans:From a single gene silencing to a wide array of sexual manipulation-based biotechnologies[J]. Biotechnology Advances,2012,30(6):1543—1550
[91]Yoshimoto S,Okada E,Umemoto H,et al. A W-linked DM-domain gene,DM-W,participates in primary ovary development in Xenopus laevis [J]. Proceedings of the National Academy of Sciences,2008,105(7):2469—2474
[92]Smith C A,Roeszler K N,Ohnesorg T,et al. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken [J]. Nature,2009,461(7261):267—271
[93]Yang X L,Jiang H S,Yang N. Mechanism of avian sex determination and differentiation [J]. Hereditas,2012,34(4):407—411 [杨秀荣,蒋和生,杨宁. 鸟类性别决定与性别分化机制. 遗传,2012,34(4):407—411]
[94]Qiu X H. Research progress of sex determination [J]. Biological Technology World,2014,11:114 [丘晓花. 性别决定的研究进展. 生物技术世界,2014,11:114]
RESEARCH PROGRESS OF DMRT GENES IN HYDROBIONTES
LI Fa-Jun1,FU Chun-Peng1and LUO Yong-Ju2
(1. Weifang University of Science and Technology,Shouguang 262700,China; 2. Guangxi Academy of Fishery Sciences,Nanning 530021,China)
Dmrt is a gene family that is homologous to Doublesex in Drosophila melanogaste and Mab-3 in Caenorhabditis elegans. Dmrt genes encode a large family of transcription factors including a characteristic conservative zinc finger DNA-binding domain(i.e.,DM domain). This ancient gene family has been identified in animal groups ranging from invertebrates to vertebrates. Their biological functions include sex determination and differentiation,construction of tissues and organs,and maintenance related functions. This paper reviews the recent progress of Dmrt genes in hydrobionts with theirs functions in line of evolution from invertebrates to vertebrates. We analyzed the course of sex determining gene for Dmrt genes.We further presented prospects of their future research in hydrobionts.
Dmrt gene; Dsx gene; Gonad; Crustacean; Fish; Hydrobiontes
Q173
A
1000-3207(2016)05-1068-10
10.7541/2016.138
2015-10-08;
2016-01-05
国家现代农业产业技术体系专项(CARS-49); 广西十二五重大科技专项(桂科重14121004-2-2)资助 [Supported by China Agriculture Research System(No. CARS-49); Guangxi Science and Technology Research Program(No. 14121004-2-2)]
李法君(1976—),男,山东寿光人; 博士; 主要从事水产动物遗传育种研究。E-mail:lifajun1976@163.com
罗永巨(1967—),男,广西博白人,博士,研究员; 主要从事水产动物遗传育种研究。E-mail:lfylzc123@163.com
