Effect of Shade Treatment in Summer on the Expression of Genes Related toTheanine Biosynthesis in Tea Plants(Camellia sinensis)
2016-11-09CHENQiYUShuWeiJIANGXueMeiZHAOYingMENGXiangYuWANXiaoChun
CHEN Qi YU Shu-Wei JIANG Xue-Mei ZHAO Ying MENG Xiang-Yu WAN Xiao-Chun
(State Key Laboratory of Tea Plant Biology and Utilization,Anhui Agricultural University,Hefei 230036)
Author introduction:CHEN Qi(1980—),female,Ph.D.,Lecturer,Major in plant secondary metabolism research.
* Corresponding author:E-mail:xcwan@ahau.edu.cn
EffectofShadeTreatmentinSummerontheExpressionofGenesRelatedtoTheanineBiosynthesisinTeaPlants(Camelliasinensis)
CHEN Qi YU Shu-Wei JIANG Xue-Mei ZHAO Ying MENG Xiang-Yu WAN Xiao-Chun*
(State Key Laboratory of Tea Plant Biology and Utilization,Anhui Agricultural University,Hefei 230036)
Camelliasinensis;theanine synthetase;theanine metabolism pathway;shading;comparative expression
Tea(Camelliasinensis(L.) O. Kuntze) is an important commercial crop consumed worldwide, primarily as a beverage made from the processed leaves. Biological studies have demonstrated the health benefits of secondary metabolites of tea, including theanine, flavonoids and caffeine. As tea is a shade-loving plant, the tropical rainforests are its native habitat, and its photosynthetic apparatus is adapted to function with maximum capacity under shade[1]. The compounds present in the tea plant are obviously influenced by photosynthesis during their life cycle, so people usually use shading treatment to improve the quality of plucking leaves[2]. The leaves of tea plants grown in the shade contain high amounts of amino acids but low amounts of catechins[3]. The sweetness of tea leaves is attributed to amino acids, especially theanine, which has a taste that can be described as umami or broth[4], whereas catechins and caffeine contribute to the astringency. As an important quality factor, theanine is a non-protein amino acid that was first discovered in tea leaves by Sakato[5]. Theanine constitutes over 1% of the dry weight of green tea, which has been extensively studied in relation to food science and human nutrition.
Research on the effect of illumination conditions on theanine metabolism in the tea plant was initiated during the mid-twentieth century[3,6~8], with the main focus being the influence of spring shading. Deng reported that the content of theanine and total free amino acids in the shoots and roots of tea seedlings increased after shading treatment, based on which it was concluded that moderate shading could improve the quality and yield of spring tea[9]. However, no systematic analysis has focused on the influence of shade treatment on theanine synthesis during summertime in tea plants.
In our study, we studied the level of theanine synthetase in the tea plant all through the year, and examined the expression of some genes related to theanine biosynthesis under controlled and shade treatment in summer. We also investigated the trend in the concentration of the main free amino acids, especially theanine. The aim of the present study was to evaluate the effect of shading treatment on the protein expression patterns of theanine synthetase and theanine pathway genes in relation to the qualitative and quantitative composition in the tea leaves, and to correlate these effects with changes in theanine accumulation. Our study will be useful for further elucidation of the molecular mechanisms by which light influences the theanine biosynthetic pathways in the tea plant and therefore provide more information on the uses of summer tea.
1 Materials and methods
1.1 Plant materials and shading treatment
Tea plants(C.sinensis(L.) O.Kuntze cv. Shuchazao) were cultivated at the experimental tea plantation of Anhui Agricultural University(Dayangdian farm), Anhui, China(latitude: 31.86°N, longitude: 117.27°E, altitude: 20 m above mean sea level). Sunlight was sampled at five locations once a month, starting from the spring equinox(March 20) to October 2013(Because pruning of the branches started in November, sampling could not be conducted beyond this time point). During the period of measurement, the bud, 1stleaf, and 2ndleaf were collected and placed directly in liquid nitrogen and stored at -80℃ for protein detection(with three replicates). Three areas(1.5 m×1.5 m) in the tea field were randomly selected for the shade treatment. The scaffolding and black polypropylene fabric provided 80%±5% light reduction[8]; the scaffolding was 2 m high, 3 m long, and 3 m wide. The plants were grown under sunshine or shaded conditions for 3 weeks in summer, from 21 July to 11 August 2014. Treatment with natural light was considered as the control treatment, and was compared with the experimental shading treatments. During the period of shading, the average light intensity decreased from 35 670±1 029 lx to 5 098±388 lx for the samples taken at 8:00 AM, at which time the average air temperature was 33.2±2.6℃. After shading treatment, young leaves(1stleaf and 2ndleaf), old leaves(4thleaf and 5thleaf) and stems(young stem) were collected and placed directly in liquid nitrogen and stored at -80℃ for western blot analysis, qPCR analysis and theanine analysis(with three replicates). All samples were examined in triplicate for both expression and quantitation analyses.
1.2SDS-polyacrylamidegelelectrophoresisandwesternanalysis
Western blot analysis was performed as described by Tian[10]. The method for extraction of total soluble proteins was similar to that of a previous report[11], but was modified as follows. Samples(about 200 mg) were ground in liquid nitrogen. An extraction buffer(50 mmol·L-1Tris[pH 7.5], 20 mmol·L-1KCl, and 13 mmol·L-1dithiothreitol[DTT]) was added in a 1∶5 ratio(plant tissues[mg]∶buffer[mL]). After homogenization, the sample was re-extracted using 20 μL phenylmethanesulfonylfluoride(PMSF) and 40 μL nonylphenoxypoly(ethyleneoxy) ethanol(NP-40). The supernatant was collected and precipitated with 3-5 volumes of 10%(w/v) trichloroacetic acid in cold(-20℃) acetone for 2-4 h. After centrifugation, the precipitate was washed with 0.07% DTT(w/v) in cold(-20℃) 80% acetone. The protein samples were dried under vacuum, stored at -20℃, or re-suspended in a rehydration buffer(7 mol·L-1urea, 2 mol·L-1thiourea, 0.4%[w/v] 3-[{3-cholamidopropyl}-dimethylammonio]-1-propane[CHAPS], 60 mmol·L-1DTT, and 0.4%[w/v] PMSF) and kept at room temperature for 2 h. After the centrifugation, the supernatant was collected and stored at -80℃ until use. The total amount of protein was measured with a Bradford protein assay kit(Bio-Rad Laboratories) using bovine serum albumin as the standard. Proteins were separated by SDS-PAGE and detected using the standard western blotting method. Polyclonal antibodies against theanine synthetase(TS, DD410895) and glutamine synthetase(GS, AB115184) were made by us, and the specificity of the antibody preparation was also tested by western blot analysis[12].
1.3Expressionvalidationbyquantitativereal-timePCR
Total RNA was extracted by the modified CTAB method[13]. RNA integrity was confirmed using the Agilent 2100 Bioanalyzer with a minimum integrity value of 8. Double-stranded cDNA was prepared by reverse transcription of 4 μg purified mRNA in a 20 μL reaction solution using the Super SMART PCR cDNA Synthesis Kit(Clontech, Palo Alto, USA) following the manufacturer’s instructions. Real-time quantitative reverse transcription-PCR(qRT-PCR) was conducted to quantify the transcript levels of 9 representative genes(TS1,TS2,GS1,GS2,GOGAT,GDH,NiR,ADC,GAD1), which were selected for gene expression analysis from our previous transcriptome library[13]and GeneBank. All the primers used for the qRT-PCR analysis are listed in Table 1. The relative expression was normalized to that of the housekeeping geneGADPH(XM002263109) and calculated using the formula 2-Δ(ΔCp). qRT-PCR was performed using a Light Cycler_ 480 SYBR Green I Master kit(Promega) on an Opticon-2 qRT-PCR machine(CFX96 Touch, Bio-Rad).
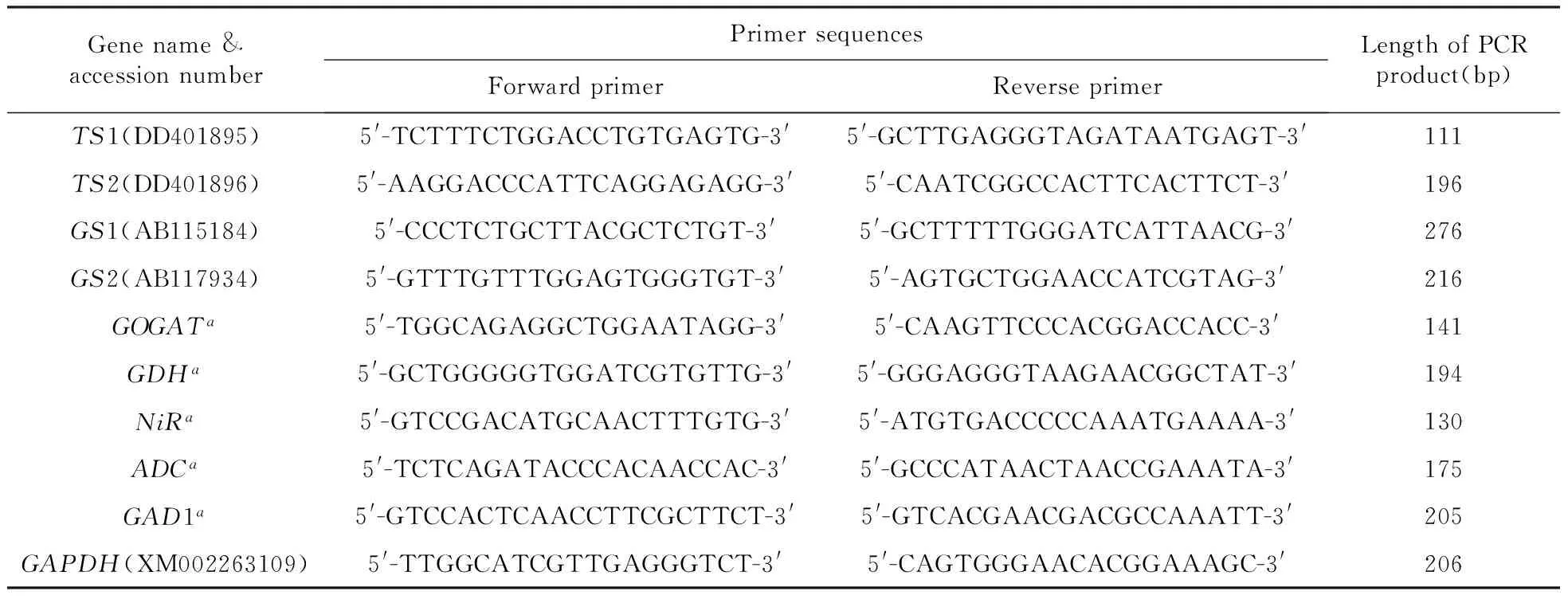
Table 1 Sequence of primers used to amplify the genes involved in theanine biosynthesis in C.sinensis(L.) Kuntze
Note:a. ESTs from our cDNA library.
1.4 Endogenous amino acid analysis
Amino acids were extracted and analyzed according to the method reported by Tsushida and Takeo[14], with a slight modification as follows. The fresh material was dried at a temperature of 120℃ until it had completely dried. It was then ground into flour, and the powdered dry samples(0.1 g) were extracted in 18 mL of distilled boiling water and heated for 45 min at 100℃. The homogenates were kept at room temperature and adjusted to 20 mL with distilled water. The pH of the samples was adjusted to 8.0 with 50 mmol·L-1of borate buffer before amino acid analysis. The extract was centrifuged at 13 000 r·min-1for 10 min at 4℃ and finally filtered through a 0.22 μm Millipore filter before HPLC analysis. Amino acids, including theanine, were separated and analyzed using an HPLC system with a fluorescence detector adapted for free amino acid analysis[15]. The amino acid standards were obtained from Sigma(St. Louis, MO, USA).
2 Results and discussion
2.1 Effect of sunlight on TS
The development of tea leaves is highly correlated with environmental factors such as sunlight, air temperature, and nutrition. However, the accumulation of theanine shows an inverse correlation with the amount of sunshine; therefore, people usually use the shading method to increase the content of theanine in picking leaves of tea. We studied the protein expression ofCsTS from March to October using immune blotting analysis.CsTS expression showed a similar pattern in the bud and the first leaf during different stages of leaf development. The findings indicated thatCsTS expression was inhibited by sunlight(Fig.1).CsTS expression in the bud gradually increased in spring and then significantly deceased in summer. Moreover, in the first leaf, high expression was observed in autumn and spring, with low expression in summer.
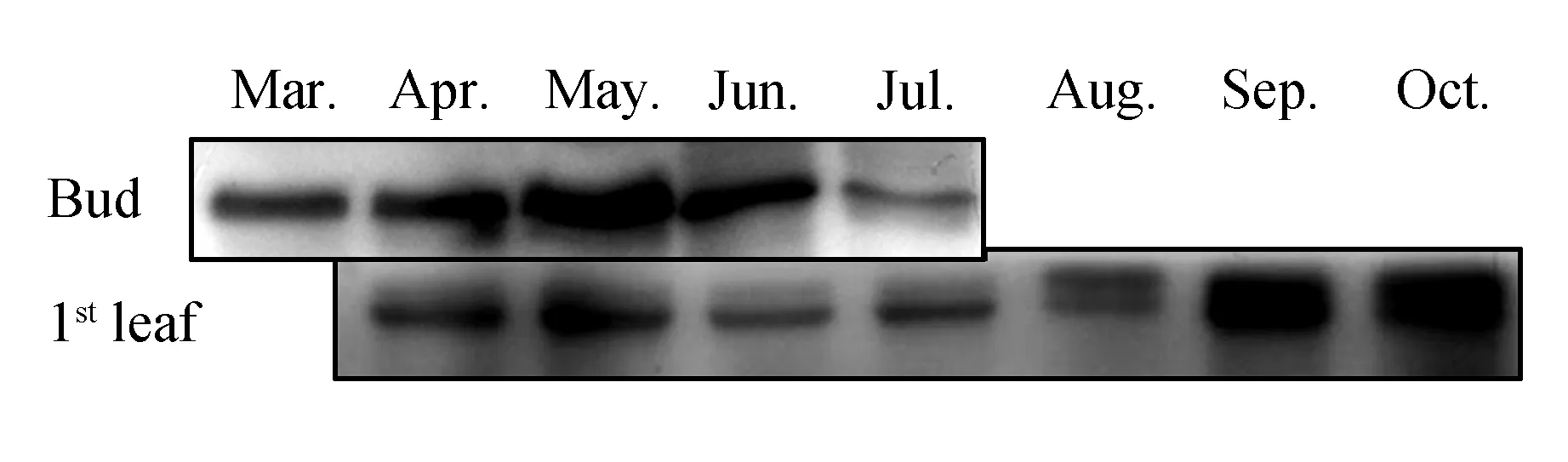
Fig.1 Western blot analysis of TS protein expression in the bud and the first leaf of tea plants(field planting) during different months
2.2 Expression of TS during shading treatment
We comparedCsTS andCsGS expression in different tissues of the aboveground parts in August 2013(Fig.2). TheCsGS protein showed high accumulation in every tissue, but the content ofCsTS protein in these tissues was obviously lower thanCsGS. Subsequently, protein expression of bothCsTS andCsGS in different aboveground parts of tea plants(field planting) was analyzed during shading treatment over 3 days. TheCsGS protein level obviously changed in every sample, and theCsTS protein level in old leaves and stems was also not influenced by shading treatment. However, in young leaves, theCsGS protein level obviously increased after shading treatment(Fig.3). Considering that it was difficult to extract proteins from the old leaves, we deduced that the protein samples had undergone partial degradation, but the part that had not degraded could still combine with antibodies.
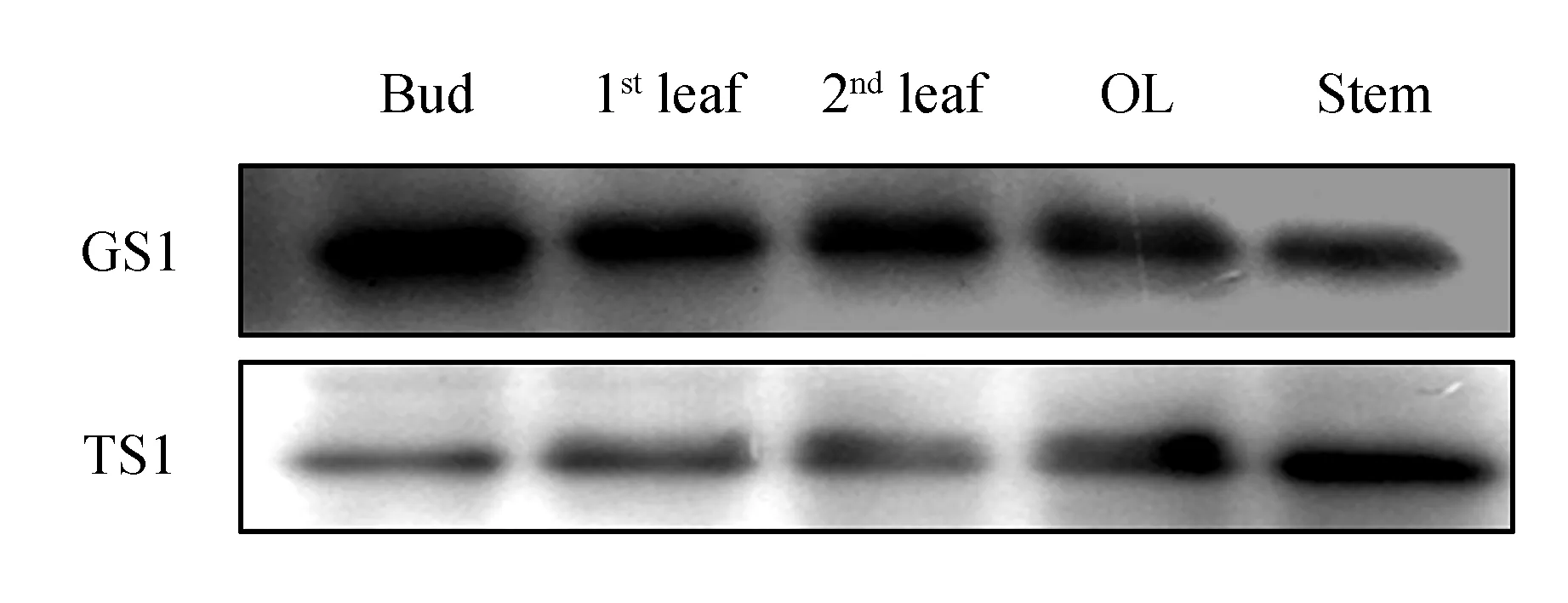
Fig.2 Western blot analysis of protein expression in different tissues of tea plants in August 2013 OL. Older leaves(4th/5th leaves); Stem. Soft stem at the top
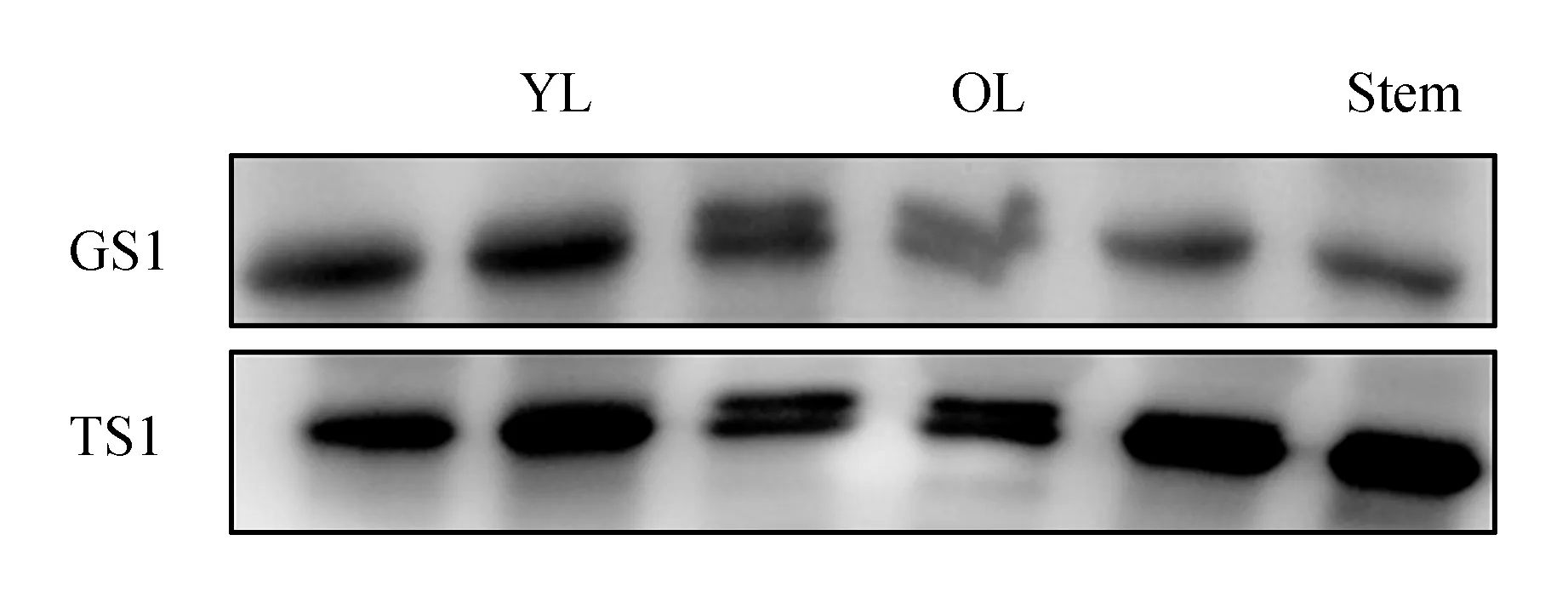
Fig.3 Western blot analysis of protein expression in different tissues of tea plants(field planting) during shading treatment at 3 days YL. Young leaves(1st/2nd leaves); OL. Older leaves(4th/5th leaves); Stem. Soft stem at the top The arrows indicate the shading treatment samples.
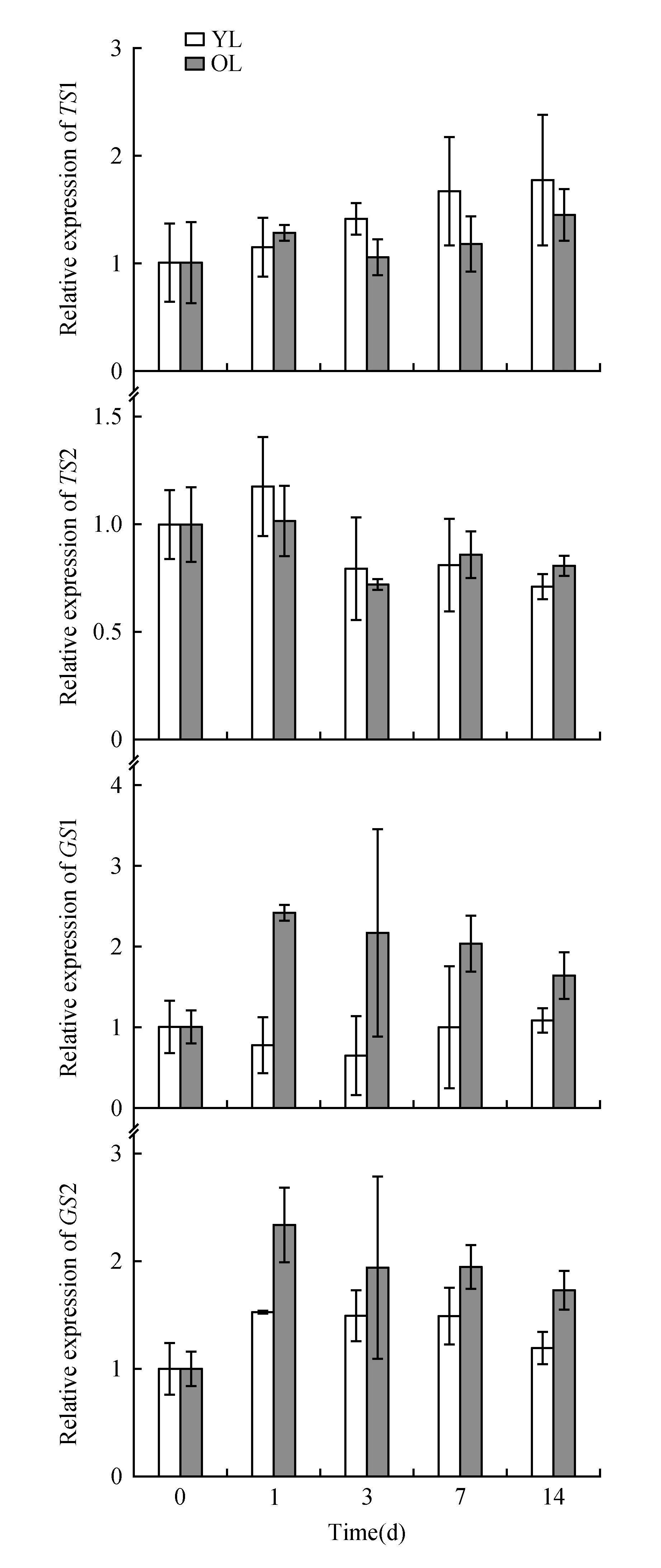
Fig.4 qRT-PCR analysis of TS and GS gene family expression in the young leaves(YL) and older leaves(OL) of tea plants(field planting)
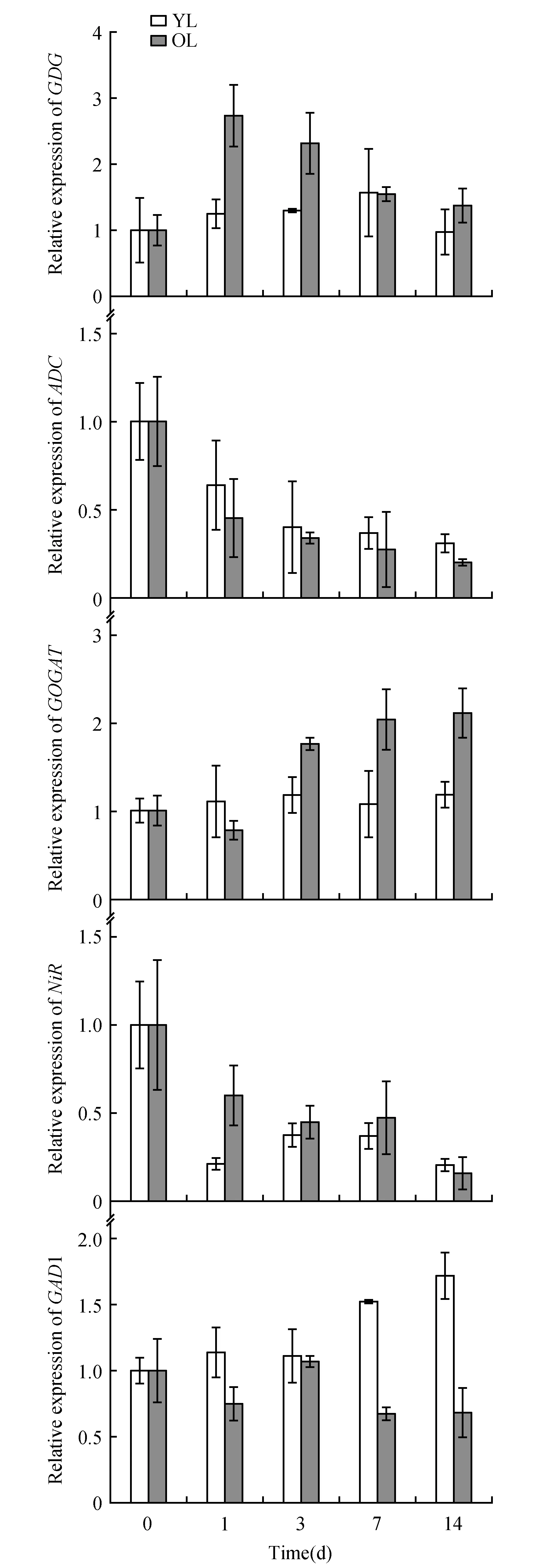
Fig.5 qRT-PCR analysis of genes involved in the theanine biosynthesis pathway in young leaves(YL) and older leaves(OL) of tea plants(field planting)
2.3Expressionanalysisoftheaninebiosynthesispathwaygenes
The expression patterns of a theanine biosynthesis gene and its related upstream pathway genes(TS1,TS2,GS1,GS2,GDH,GOGAT,ADC,NiRandGAD1) were examined by qRT-PCR in sunlight-exposed and shaded leaves. The housekeeping geneGAPDHwas used as the reference gene for the qRT-PCR analysis. The expression levels of these genes differed between sunlight-exposed leaves and shaded leaves, as shown in Fig.4 and Fig.5. Under shade, the expression level of one of the theanine synthetase isogenes(TS1) in the young leaves gradually increased compared with that in the old leaves; these results are consistent with the TS1 protein expression pattern(Fig.3). The expression level of the other theanine synthetase isogene,TS2, showed no correlation with the amount of sunshine. However, the expression ofGS1 andGS2 in the old leaves was significantly higher than that in the young leaves during shade treatment(Fig.4); these results differ slightly from those of GS1 protein expression, which is probably related to the protein degradation that occurs in the older leaves.
In our study, the genes involved in the conversion of ammonia to nitrogen showed higher expression levels in old shaded leaves than in young shaded leaves. Among these genes,GDH,GSandGOGATshowed less than three-fold increase in expression, which suggests that these genes were expressed at higher levels in the old shaded leaves. Genes involved in nitric nitrogen assimilation, such as NiR, showed an obvious decrease in their expression level, both in young and old shaded leaves. Among the other genes related to theanine and nitrogen metabolism, the expression pattern ofADCwas similar to that ofNiR, and the expression pattern ofGADwas similar to that ofTS1(Fig.5).
2.4Changesintheleveloftheanineandthemainaminoacidsinducedbyshadetreatment
To investigate whether the theanine biosynthesis pathway was affected by shade treatment in summer, the theanine and main amino acid components in leaves were extracted and quantified(Fig.6). The total amount of amino acids in the shaded tea leaves was remarkably increased by treatment after 7 days. Theanine, the most abundant amino acid in the tea plant, comprised a large percentage of the total free amino acids and was the biggest contributor to the increase in the total amount of amino acids. Glutamic acid is a direct substrate for theanine biosynthesis in the tea plant[16], and alanine can be converted to ethylamine, which acts as another substrate, through alanine decarboxylase[17]; therefore, changes in the levels of these amino acids during shade treatment were also investigated. The results show that both the glutamic acid and alanine content was decreased in shaded leaves. Furthermore, arginine, aspartate and some other amino acids showed the same trend as the total free amino acids in young leaves, which was also related to nitrogen transport and reserve. All these results implied that shade treatment is conducive to the accumulation of amino acids, mostly comprised of theanine, in tea plant leaves.
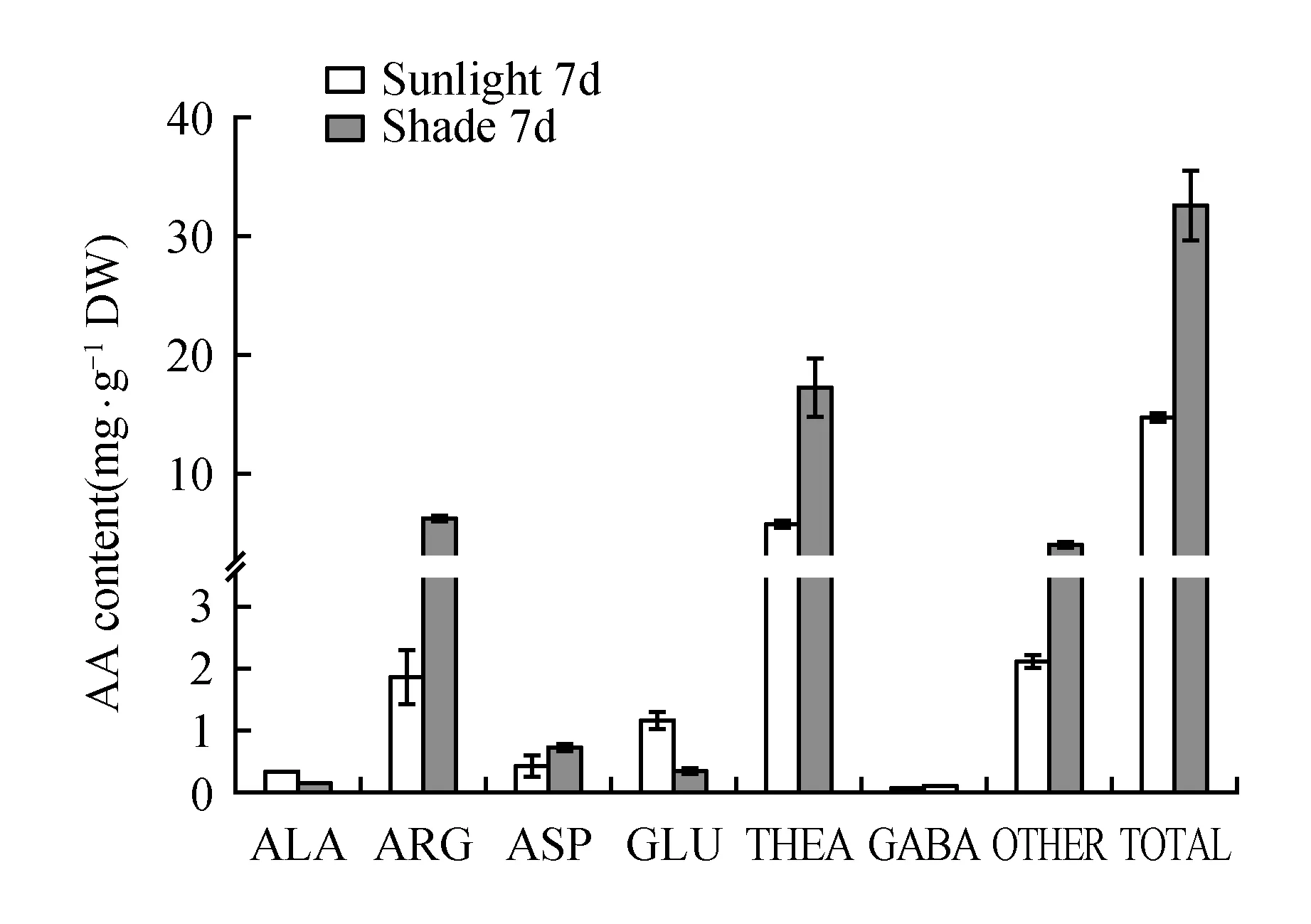
Fig.6 Concentrations of theanine and the other main amino acids in the young leaves of the tea plant during shading treatment and normal sunlight after 7 days of treatment
3 Discussion
3.1Relationshipbetweenaminoacidcontentandteaquality
Many studies have investigated the relationship between the taste of tea and metabolites, especially theanine, which is known to have an immense effect on the taste of tea, with a correlation coefficient of 0.989[18]. In summer, tea leaf contains a lower amount of amino acids and more catechins, so the leaves become more bitter and astringent. It is important to understand how the utilization of summer tea can be increased, as this would be useful with regard to tea production and tea research. In this paper, we investigated the effect of shade treatment on theanine biosynthesis in summer. Polyclonal antibodies with high sensitivity against TS and GS, obtained from rabbits, were utilized for immunoblotting analysis. We concluded that the expression patterns of the TS protein in the tea plant gradually increased in spring and autumn and decreased in summer, but the expression of the GS protein was maintained at high levels even in summer. Therefore, we deduced that theanine biosynthesis was greatly affected by sunlight. Then, during the summer shade period, we found that the TS protein levels increased in the young leaves. This implies that shade treatment may improve tea quality in summer. As reported before, spring shade is often used to enhance the quality of tea[19], because shade treatment is beneficial for theanine accumulation and decreases the flavonoid content[9,20]. Catechins are responsible for the bitterness and astringency of tea infusions(Kallithraka et al.,1997). Appropriate decreases in the catechin and anthocyanin concentrations are propitious for improving the flavor and quality of beverage made from tea leaves[21~22].
3.2Effectsofshadetreatmentontheaninebiosynthesis
Plants are capable of undergoing physiological adjustments in response to a wide range of environmental stimuli. Tea plants have adapted to shade in their natural habitat, so sensitivity to strong light is expected. Shade can therefore mitigate the damage caused by photoinhibition in the tea plant[23]. In our study, we determined the expression level of genes related to theanine synthetase and the level of total free amino acids; the results illustrated that increase in theanine concentration may be a result of more protein synthesis and more substrate consumption. Changes in the level of glutamic acid and alanine, which are direct or indirect substrates for theanine biosynthesis, were decreased during shade treatment of the leaves, which indicate that they are involved in the accumulation of theanine.
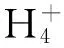
In conclusion, the expression of theanine pathway genes and changes in concentrations of all free amino acids were detected in leaves of adult tea trees grown under shade net in summer. Polyclonal antibodies with high sensitivity against TS and GS, obtained from rabbits, were utilized for immune blotting analysis. We concluded that the expression patterns of TS protein in tea leaves were inhibited in summer and increased after shading treatment. The content of theanine and the total free amino acids were also gradually increased during shading treatment. Therefore, the shade treatment in summer can effectively improve tea quality through activation of theanine biosynthesis or accumulation the free amino acids in tea leaves.
1.Janendra W A,Costal M D,Janaki M A,et al.Ecophysiology of tea[J].Braz J Plant Physiol,2007,19(4):299-332.
2.Sakai S.Recent studies and problems of photosynthesis of tea plant[J].JARQ,1975,9:101-106.
3.Ku K M,Choi J N,Kim J,et al.Metabolomics analysis reveals the compositional differences of shade grown tea(CamelliasinensisL.)[J].J Agric Food Chem,2009,58(1):418-426.
4.Yamaguchi S,Ninomiya K.Umami and food palatability[J].J Nutr,2000,130(4):921-926.
5.Sakato Y.The chemical constituents of tea.Ⅲ.A new amide theanine(in Japanese)[J].Nippon Nogeik Kaishi,1949,23:262-267.
6.Takeuchi A,Matsumoto S,Hayatsu M.Effects of shading treatment on the expression of the genes for chalcone synthase and phenylalanine ammonia-lyase in tea plant(Camelliasinenesis)[J].Bull Natl Res Inst Veg,Ornam Plants Tea Series B,1995,8:1-9.
7.Akio M,Masaki T.Nitrate and oxalate contents of tea plants(CamelliasinensisL.) with special reference to types of green tea and effect of shading[J].Soil Sci Plant Nutr,2002,48(4):547-553.
8.Xiao R L,Wang J R,Shan W X,et al.Tea plantation environment and quality under different degrees of shading[J].Chinese J Eco-Agric,2007,15(6):6-11.
9.Deng W W,Fei Y,Wang S,et al.Effect of shade treatment on theanine biosynthesis inCamelliasinensisseedlings[J].Plant Growth Regul,2013,71(3):295-299.
10.Tian L,Kong W F,Pan Q H,et al.Expression of the chalcone synthase gene from grape and preparation of an anti-CHS antibody[J].Protein Expres Purif,2006,50(2):223-228.
11.Jani D,Meena L S,Rizwan-ul-Haq Q M,et al.Expression of cholera toxin B subunit in transgenic tomato plants[J].Transgenic Res,2002,11(5):447-454.
12.Deng W W,Wang S,Chen Q,et al.Effect of salt treatment on theanine biosynthesis inCamelliasinensisseedlings[J].Plant Physiol Bioch,2012,56(2):35-40.
13.Yang H,Shi C Y,Wei C L,et al.Deep Sequencing of theCamelliasinensistranscriptome revealed candidate genes for major metabolic pathways of tea-specific compounds[J].BMC Genomics,2011,12(1):131-150.
14.Tsushida T,Takeo T.Ethylamine content of fresh tea shoots and made tea determined by high performance liquid chromatography[J].J Sci Food Agric,1984,35(1):77-83.
15.Kotaniguchi H,Kawakatsu M,Toyo’oka T,et al.Automaticamino acid analysis utilizing 4-fluoro-7-nitrobenzo-2-oxa-1,3-diazole[J].J Chromatogr B,1987,420(3):141-145.
16.Takeo T.L-Alanine as a precursor of ethylamine inCamelliasinensis[J].Phytochem,1974,13(8):1401-1406.
17.Takeo T.L-Alanine decarboxylase inCamelliasinensis[J].Phytochem,1978,17(2):313-314.
18.Nakagawa M.Chemical components and taste of green tea[J].Jarq,1975,9(3):156-160.
19.Zhang W J,Lin C L,Xiong M M.Research progress in shading efficiency for tea plants[J].Fujian J Agr Sci,2007,22(4):457-460.
20.Wang Y S,Gao L P,Shan Y,et al.Influence of shade on flavonoid biosynthesis in tea(Camelliasinensis(L.) O.Kuntze)[J].Scientia Hortic,2012,141(1):7-16.
21.Yamamoto T,Juneja L R,Kim M.Chemistry and applications of green tea[M].Florida:CRC press,1997:23-36.
22.Scharbert S,Holzmann N,Hofmann T.Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse[J].J Agric Food Chem,2004,52(11):3498-3508.
23.Mohotti A J,Lawlor D W.Diurnal variation of photosynthesis and photo inhibition in tea:effects of irradiance and nitrogen supply during growth in the field[J].J Exp Bot.2002,53(367):313-322.
24.Lam H M,Coschigano K T,Oliveira I C,et al.The molecular-genetics of nitrogen assimilation into amino acids in higher plants[J].Annu Rev Plant biol,1996,47(1):569-593.
夏季遮阴对茶树茶氨酸合成及其代谢相关基因表达的影响
陈 琪 于淑伟 江雪梅 赵 颖 孟祥宇 宛晓春*
(安徽农业大学省部共建茶树生物学与资源利用国家重点实验室,合肥 230036)
为了研究夏季遮阴对茶树茶氨酸代谢途径的影响及对茶树内含物品质的改良作用,本研究以多年实生茶树为研究对象,利用Western blotting检测了夏季遮阴对茶树不同部位中茶氨酸合成酶(TS)、谷氨酰胺合成酶(GS)蛋白表达的影响,确定遮阴有利于嫩叶中TS的表达;随后利用实时荧光定量PCR检测方法探索了茶氨酸代谢途径中相关基因:TS、GS、谷氨酰胺α-酮戊二酸氨基转移酶(GOGAT)、谷氨酸脱氢酶(GDH)以及与氮素吸收和转化密切相关的亚硝酸还原酶(NiR)、精氨酸脱羧酶(ADC),在老、嫩叶中及不同遮阴时期的表达情况。结果表明遮阴有利于嫩叶中TS基因的表达,与叶部氨的再同化作用密切相关的GS、GOGAT和GDH基因均在老叶中有明显增加,而与硝基氮代谢相关的NiR、ADC基因表达在老叶与嫩叶中均明显下降。利用HPLC对遮阴后嫩叶中游离氨基酸含量的检测结果表明,遮阴明显促进茶叶部游离氨基酸总量的提高,其中贡献率最大是茶氨酸。本研究从分子水平上解析了遮阴促进茶叶部茶氨酸积累的调控机制,为今后夏秋茶栽培措施改良和品质改善提供理论基础。
茶树;茶氨酸合成酶;茶氨酸代谢;遮阴;相对表达
S571.1
A
10.7525/j.issn.1673-5102.2016.02.010
This research was supported by Natural Science Foundation of China(Grant No.31170649 and No.31500566);The Specialized Research Fund for the Doctoral Program of Higher Education of China(Grant No.20113418130001)
date:2015-09-10
