饲料及畜禽产品中霉菌毒素快速检测技术研究进展
2016-11-01董国良彭大鹏韩肖亚刘振利袁宗辉
董国良,彭大鹏,韩肖亚,刘振利,袁宗辉
(1.国家兽药残留基准实验室(华中农业大学)、农业部兽药残留检测重点实验室,武汉 430070;2.农业部畜禽产品质量安全风险评估实验室(武汉),武汉 430070;3.华中农业大学动物医学院,武汉 430070)
饲料及畜禽产品中霉菌毒素快速检测技术研究进展
董国良1,3,彭大鹏1,2,3*,韩肖亚1,3,刘振利1,2,3,袁宗辉1,2,3
(1.国家兽药残留基准实验室(华中农业大学)、农业部兽药残留检测重点实验室,武汉 430070;2.农业部畜禽产品质量安全风险评估实验室(武汉),武汉 430070;3.华中农业大学动物医学院,武汉 430070)
霉菌毒素是某些霉菌的次级代谢产物,在自然界中分布极为广泛,可污染动物饲料及畜禽产品,对动物和人类健康造成巨大危害。作者介绍了饲料与畜禽产品中常见的霉菌毒素及其对动物和人类的危害,总结了近年来快速检测技术在霉菌毒素检测方面所取得的进展,分析了霉菌毒素抗体制备的主要影响因素,并对霉菌毒素快速检测技术发展前景作出展望。
霉菌毒素;快速检测技术;抗体
霉菌毒素(mycotoxins)是一类低分子量化合物,是某些小型丝状真菌的次级代谢产物,在自然界中分布极为广泛。霉菌毒素能导致动物和人发生疾病甚至死亡,某些霉菌毒素具有细胞毒性、致癌、致突变或免疫抑制等毒性反应,能够对人或动物造成不同程度的毒害作用[1]。据联合国粮农组织调查分析,全世界每年约有25.0%的作物受到霉菌毒素污染[2]。截至目前,已知的霉菌毒素有300~400种[3]。
微量的霉菌毒素便可产生巨大毒害作用,而混合污染又是其重要特点。因此,快速、精确检测饲料及畜禽产品中霉菌毒素各组分,是保障动物和人类健康的重要手段。其分析程序通常包括三个使用标准:检测速度、所需技术技能水平以及能否提供定性或定量的结果[4]。常规化学及仪器检测技术耗时长、成本高、前处理复杂,难以满足现场、快速、大批量的检测需要。而目前残留检测技术的发展趋势,要求试剂消耗少、样品用量少、在线自动化、高通量地快速分析[5]。因此,快速检测技术(rapid detection technology)必将成为霉菌毒素残留检测研究的重点。
1 几种主要霉菌毒素及其危害
1.1黄曲霉毒素
黄曲霉毒素(aflatoxin,AFT)是一类二氢呋喃氧杂萘邻酮的衍生物,主要由黄曲霉(Aspergillusflavus)和寄生曲霉(A.parasiticus)二次代谢产生,常见于霉变的玉米、花生、棉籽及某些干果中。该类毒素具有致癌[6]、致畸、致突变、基因毒性[7]及免疫抑制[8]等作用。黄曲霉毒素B1(AFB1,图1A)是其最主要、毒性最强的组分[1],国际癌症研究机构(International Agency for Research on Cancer,IARC)已将其列为人类致癌物[9]。AFB1在动物体内可代谢为黄曲霉毒素M1(AFM1,图1A)[1,10],常见于牛奶中,对人类危害巨大。欧盟规定坚果及玉米中总AFT的最大残留限量分别为4.00、10.0 μg·kg-1,谷类加工食品及婴儿食品和玉米中AFB1为100 ng·kg-1、5.00 μg·kg-1,而牛奶和婴儿奶粉中AFM1限量分别为50.0、25.0 ng·kg-1[11-12]。中国规定谷类加工食品及婴儿食品中AFB1限量为5.00 μg·kg-1,牛奶及奶粉中AFM1限量为500 ng·kg-1[13]。
1.2赭曲霉毒素
赭曲霉毒素(ochratoxin,OT)是由曲霉菌和青霉菌产生的一类重要的霉菌毒素,其中毒性最大、分布最广与人类健康关系最为密切的是赭曲霉毒素A(ochratoxin A,OTA,图1B)。该毒素具有肾毒性、肝毒性、免疫毒性、基因毒性和致突变作用[14-16]。动物摄入OTA后,易在组织内蓄积残留,对人类健康造成威胁。研究发现,丹麦曾经发生的由霉菌所引起的肾病以及巴尔干地方性肾病都与OTA有密切关系[17]。欧盟已制定OTA的最大残留限量标准,谷物及其制品、葡萄酒和婴儿食品中分别为5.00、2.00 μg·kg-1和500 ng·kg-1[12]。中国规定谷物及其制品和豆类中OTA限量为5.00 μg·kg-1[13]。
1.3玉米赤霉烯酮
玉米赤霉烯酮(zearalenone,ZEN,图1C)又称为F-2毒素,是黄色镰刀菌(Fusariumculmorum)和禾谷镰刀菌(F.graminearum)等镰刀菌属真菌的次级代谢产物。该毒素具有雌激素作用[18],主要作用于雌性动物生殖系统,引起繁殖机能异常甚至死亡,具有基因毒性和致癌性[18-19],免疫毒性[20]以及生殖毒性[21]。ZEN经动物摄入后,可代谢为玉米赤霉醇(zearalanol,ZAL)和玉米赤霉烯醇(zearalenol,ZEL)[22]。其中,α-ZAL曾广泛用作家畜促生长剂[23],因其能引起乳腺癌,扰乱人体内分泌系统[24],被欧盟禁用于动物源食品,中国也禁止将其用于食品动物促生长[25]。欧盟规定未加工谷物(玉米除外)、面包以及婴儿食品中ZEN的最大残留限量分别为100、50.0、20.0 μg·kg-1[26]。中国则规定小麦及玉米中ZEN最大残留限量为60.0 μg·kg-1[13]。
1.4T-2毒素
T-2毒素(图1D)是一种单端孢霉烯族化合物(trichothecenes,TS),由拟分枝孢镰刀菌(Fusariumsporotrichioides)和梨孢镰刀菌(Fusarlumpoae)等镰刀菌属真菌代谢产生。T-2毒素能够抑制DNA和蛋白质的合成[27-28],影响基因表达[29],具有生长抑制[30]和免疫抑制作用[10,31]。该毒素能导致蛋鸡产蛋量下降,增加裂壳蛋和空腔病变发生率,还能引起猪的轻度肾病、甲状腺缩小、胃黏膜增生及血液中白蛋白增加或其他血液指标变化[32]。有研究发现,T-2毒素可能与人类大骨节病所表现出的典型症状有关[33]。欧盟建议未加工燕麦、燕麦产品及婴儿食品中T-2毒素的残留限量分别为500、200、50.0 μg·kg-1[34]。
1.5脱氧雪腐镰刀菌烯醇
脱氧雪腐镰刀菌烯醇(deoxynivalenol,DON,图1E)又称呕吐毒素(vomitoxin,VT),是一种单端孢霉烯族毒素,主要由禾谷镰刀菌和粉红镰刀菌(Fusarlumroseum)代谢产生。人畜摄入过量被DON污染的食物后可引起中毒,表现出厌食和呕吐等症[35]。DON能抑制DNA和蛋白质的合成[36],具有生长抑制[37],免疫抑制[38],心脏毒性、致畸性[39]及神经毒性[40],对动物和人类危害巨大。欧盟规定未加工谷物、小麦和燕麦及婴儿食品中,DON的最大残留限量分别为1.25、1.75 mg·kg-1和200 μg·kg-1[26]。中国也对DON的最大残留限量作了明确规定,玉米、小麦和大麦中DON最大残留限量为1.00 mg·kg-1。
1.6伏马菌素
伏马菌素(fumonisin,FB,图1F)是一类多氢醇和丙三羧酸的双酯化合物,主要由串珠镰刀菌(FusariummoniliformeSheld)和多育镰刀菌(Fusariumproliferatum)代谢产生。FB与鞘脂类代谢过程中的神经鞘氨醇(sphingosine,So,图1G)和二氢神经鞘氨醇(sphinganine,Sa,图1H)的结构极为相似,是Sa N-酰基转移酶(又称神经酰胺合成酶)的有效抑制剂,能干扰鞘脂类代谢,引发疾病[41]。FB能引起马脑软化症和猪肺水肿[42-43],具有肝毒性[44],而且与人类食管癌[45]和肝癌[46]有关,IARC将其列为人类可能的致癌物[47]。目前已发现的FB有15种以上,主要包括FA1、FA2、FB1、FB2、FB3、FB4和FC1,其中,FB1是最主要也是毒性最强的组分[48]。欧盟对某些食品中FB1和FB2总量的最大残留限量作出规定,未加工玉米及婴儿食品中分别为4.00 mg·kg-1和200 μg·kg-1[26]。
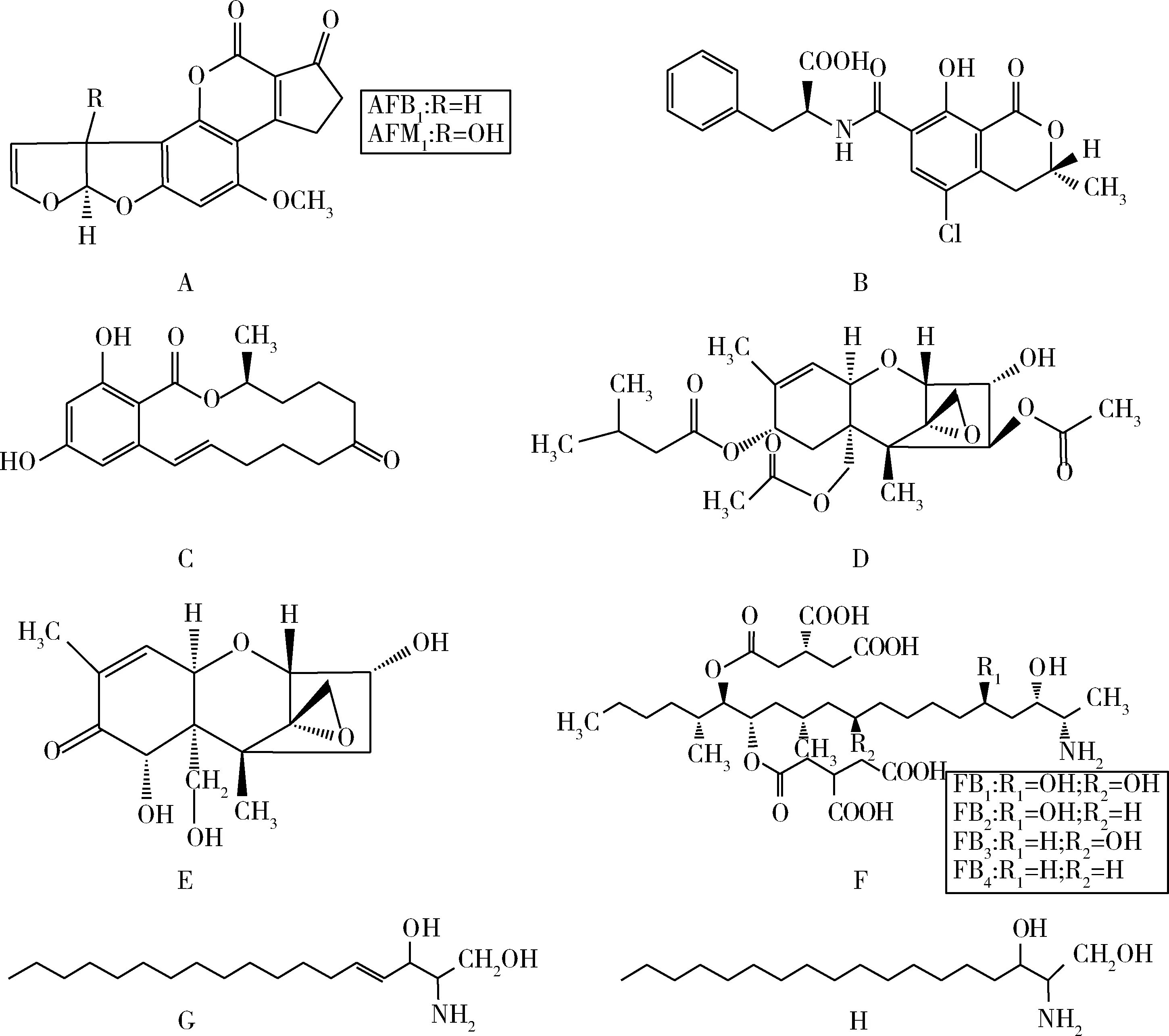
A.黄曲霉毒素;B.赭曲霉毒素;C.玉米赤霉烯酮;D.T-2毒素;E.脱氧雪腐镰刀菌烯醇;F.伏马菌素;G.神经鞘氨醇;H.二氢神经鞘氨醇A.Aflatoxin;B.Ochratoxins;C.Zearalenone;D.T-2 toxin;E.Deoxynivalenol;F.Fumonisin;G.Sphingosine;H.Sphinganine图1 几种主要霉菌毒素、神经鞘氨醇及二氢神经鞘氨醇的结构Fig.1 Structures of several main mycotoxins,sphingosine and sphinganine
2 霉菌毒素快速检测技术
2.1生物传感器法
生物传感器(biosensor)是一种对生物物质具有选择性和可逆性响应并能将其浓度转换为电信号进行检测的仪器(图2A)。它主要由两个元件组成:一个是生物学识别元件;另一个是与数据采集、处理系统相连接的信号转换元件[49]。目前常用的生物识别元件主要有辅因子、酶、抗体、微生物、细胞器、组织和高等生物细胞等[50]。由于酶特有的灵敏性和特异性,应用最为广泛[51],但其纯化过程费时,且昂贵[52]。随着抗体技术的应用,以抗体作为识别元件的生物传感器(又称免疫传感器)逐渐兴起。生物传感器法具有选择性好、响应快、灵敏度高、易于操作、高通量及适合现场检测等优点[53]。此外,生物传感器法的样品前处理简单易行,适合大批量样品的多残留快速分析检测[54]。V.P.Nancy等[55]将多层碳纳米管改良的玻璃碳电极与连续流动装置相结合,设计成免疫传感器,用于检测玉米中ZEN,检测过程仅需15.0 min,而且其检测限和灵敏度都优于酶联免疫吸附法(enzyme-linked immunosorbent assay,ELISA)。M.Mirasoli等[56]结合竞争的侧流免疫测定法,建立了一种化学发光生物传感器,对玉米中FB1和FB2进行检测,LOD达到 2.50 μg·L-1,灵敏度较高,回收率为90.0%~115%,包括样品前处理在内总耗时仅25.0 min。M.Masikini等[48]设计了一种基于多层碳纳米管结合玻璃碳表面碲化钯量子点的阻抗免疫传感器,用于检测玉米中FB1、FB2和FB3含量,LOD分别为14.0、11.0、11.0 μg·kg-1,满足检测需要。Z.Martina等[57]以AFB1和FB1偶联BSA建立竞争性侧流免疫测定法,结合便携式电荷耦合元件,构建了一种多元化学发光生物传感器,对玉米粉中AFB1及B族伏马菌素进行检测,LOD分别低于6.00和1.50 μg·kg-1,回收率为80.0%~115%,变异系数小于20.0%,检测过程仅需30.0 min。
2.2酶联免疫吸附法
酶联免疫吸附法是利用酶(如HRP)标记抗原或抗体,与样品中相应的抗体或抗原按不同的步骤与固相载体表面的抗原或抗体发生反应[58],如图2B。以洗涤的方法使固相载体上形成的抗原抗体复合物与其他物质分开,最后结合在固相载体上的酶量与样品中待测物的量成一定比例,然后利用复合物上标记的酶催化底物显色,根据其颜色深浅对待测样品中抗原或抗体的量进行判断,是一种将酶的高效性与抗原抗体反应的特异性相结合的微量分析技术[59]。ELISA方法具有低成本、快速、操作简单、便捷等优点,尤其适合现场检测[60]。蔡齐超[61]采用肟化法在AFM1的分子结构上引入羧基,偶联蛋白质后免疫小鼠,制备单克隆抗体并建立ELISA方法,IC50为3.68 ng·mL-1,奶粉中的添加回收率在89.0%~93.0%,变异系数均小于15.0%,准确度较高,但难以满足多组分检测需求。S.C.Pei等[62]以半抗原ZEN-CMO偶联蛋白获得单克隆抗体,建立了一种高通量筛选ELISA,IC50为1.79 ng·g-1,LOD达100 ng·kg-1,添加回收率为80.0%~128%,与β-ZEL、α-ZAL和β-ZAL的交叉反应率分别为24.1%、189%和43.9%,满足多组分检测需求。Y.Li等[63]合成了半抗原3-HS-T-2并与蛋白进行偶联,制备单克隆抗体并建立了稻米中T-2毒素ELISA方法,LOD达5.80 μg·kg-1,回收率为72.0%~109%,但与其他毒素交叉反应率较低。H.M.Lee等[64]以DON-CDI作半抗原合成免疫原,经小鼠免疫获得单克隆抗体,建立ELISA方法用于检测猪饲料中DON,IC50为23.4 ng·mL-1,仅能用于DON检测。S.Ling等[65]采用戊二醛法合成免疫原,免疫小鼠并制备单克隆抗体,建立了一种FB1ELISA 方法,IC50为32.0 ng·mL-1,LOD达1.00 ng·mL-1,平均回收率为 93.8% ± 6.90%,但与其他霉菌毒素交叉反应差。
2.3免疫层析法
免疫层析法(Immunochromatography)具有快速、简便、成本低等优点,已被广泛应用于临床、食品、农业及环境等领域[66]。其原理是将抗体固定于硝化纤维素膜,当一端浸入样品后,由于毛细管作用,样品将向前移动,当移动至固定有抗体的区域时,样品中相应抗原即与抗体发生特异性结合,根据颜色变化,实现对样品的检测分析[67],如图2C。杨扬[68]采用活性酯法将OTA与BSA偶联合成人工抗原,免疫小鼠制备单克隆抗体,并建立了一种OTA胶体金免疫层析法,LOD达到5.00 ng·mL-1,与其他毒素交叉反应率低。骆敏儿等[69]以人工抗原ZEN-CMO-OVA免疫小鼠,制备单克隆抗体并建立胶体金免疫层析法,对ZEN进行检测,LOD为100 ng·mL-1,与β-ZEL和α-ZAL的交叉反应率仅为12.8%和1.30%,难以满足多组分检测需要。Y.Sun等[70]合成了半抗原ZEN-1,4-丁二醇缩水甘油醚,偶联BSA免疫小鼠以获得单克隆抗体,并建立了一种胶体金免疫层析法,用于检测玉米中ZEN含量,LOD为20.0 μg·kg-1,与玉米赤霉酮的交叉反应率为53.1%,回收率达91.3%~97.1%。Z.Wang等[71]用荧光微球标记FB1单克隆抗体,以提高方法的灵敏度,建立了一种免疫层析法,用于玉米中FB1检测,IC50为1.32 ng·mL-1,LOD达120 ng·L-1,与FB2和FB3的交叉反应率分别为1.50%和67.3%,回收率为91.4%~118%,满足多组分检测需要。
2.4荧光偏振免疫分析法
荧光偏振免疫分析法(fluorescence polarization immunoassay,FPIA)是一种均相、定量、竞争性免疫分析技术,其原理是基于荧光标记抗原(Tracer)在结合特异性抗体前后其荧光转速及荧光偏振值(P值,1 P=1 000 mP)发生变化,用竞争性方法直接测定溶液中待测物含量[72]。FPIA的基本原理如图2D所示。Tracer体积较小,在体系当中的布朗运动速度较快,产生的P值较小,一般为30~50 mP[73]。当样品中不含待测物时,Tracer与抗体发生特异性结合,形成较大的抗原抗体复合物,运动速度变慢,P值升高,一般为150~300 mP[74]。若样本中待测物浓度较高,待测物将与Tracer竞争有限的抗体结合位点,从而抑制Tracer与抗体的结合,多数Tracer将以游离的小分子形式存在于样品中,P值便会降低,一般达到30~60 mP即认为达到完全抑制[75]。P值的大小与样品中待测物浓度呈反比,通过测定待测物标准品后制作标准曲线,从标准曲线上可以精确地分析样品中待测物的相应含量[76]。FPIA典型的标准曲线如图3所示[77]。

A.生物传感器;B.酶联免疫吸附法;C.免疫层析法;D.荧光偏振免疫分析法A.Biosensor;B.Enzyme-linked immunosorbent assay;C.Immunochromatography;D.Fluorescence polarization immunoassay图2 几种基于抗体的免疫学快速检测方法原理Fig.2 The principle of several antibody-based immunological rapid detection methods

图3 荧光偏振免疫分析法的典型标准曲线Fig.3 The typical standard curve of fluorescence polarization immunoassay
FPIA操作过程简单,仅仅是将特异性抗体、Tracer和样品加入到反应体系中混匀,经过几分钟甚至是几秒钟的孵育便可测定荧光偏振光强度,从而快速获得待测物浓度,适于大批量样品分析[78]。即FPIA检测时间仅取决于加样过程和测定过程的时间,明显缩短了检测时间[75]。S.Y.Jie等[79]采用AFB1单克隆抗体,以荧光标记AFB1作为Tracer,建立了一种黄曲霉毒素类FPIA,对于AFB1的IC50和LOD分别是23.3、13.1 ng·mL-1,与AFB2、AFG1、AFG2、AFM1、AFM2的交叉反应率分别达到65.7%、143%、23.5%、111%、2.00%,检测96个样品总耗时少于5.00 min,适用于黄曲霉毒素类检测。C.Li等[80]通过设计3种Tracer,与7种单克隆抗体组合筛选,建立了一种FPIA方法,用于检测玉米中FB1和FB2,LOD分别为157、291 μg·kg-1,回收率为84.7%~93.6%,变异系数小于9.90%,是一种快速、高通量检测方法。C.Li等[81]分别设计了3种Tracer,经TLC纯化后与相应抗体组合,建立多路复用FPIA,对玉米中DON、T-2及FB1进行检测,LOD分别为 242、17.8和332 μg·kg-1,回收率达76.5%~106%,变异系数小于21.7%。
2.5霉菌毒素混合污染免疫检测技术
从霉菌毒素污染特点来看,传统的单组分检测技术已难以满足检测需求,快速、简便、高灵敏度、高通量的多组分检测技术将是今后霉菌毒素检测技术发展的趋势。李鑫[82]基于竞争性免疫层析反应原理,研制出具有三条检测线的免疫层析试纸条,可同步检测AFB1、OTA和ZEN,可视检测限分别为0.250、0.500、1.00 ng·mL-1;首次建立了一种能同步检测三种霉菌毒素的聚合物刷微阵列免疫芯片,对AFB1、OTA和ZEN的LOD分别为4.00、4.00、3.00 pg·mL-1,花生样品的添加回收率达85.9%~109.2%。另外,基于混合抗体的免疫检测技术,即在固相上包被混合或具有合适亲和力的抗原,采用混合抗体对样品中多种待测物进行定性和半定量检测,已成功应用于小分子药物的多残留检测。A.Strasser等[83]以葡萄糖氧化酶作载体蛋白,采用混合抗体酶免疫检测法对牛奶中链霉素、磺胺嘧啶、磺胺甲嘧啶和氯唑西林进行检测,LOD分别为14.0、20.0、13.0、1.70 ng·mL-1,低于最大残留限量标准,能够满足检测需要。谢焕龙等[84]基于孔雀石绿和隐孔雀石绿的高特异性单克隆抗体,采用混合抗体模式建立了可同时检测两种物质的ELISA方法,IC50为3.27 ng·mL-1,LOD达0.240 ng·mL-1,线性范围为0.620~17.2 ng·mL-1。将该技术用于霉菌毒素多组分检测是一种很好的发展方向。
3 影响霉菌毒素抗体质量的因素
抗体是指抗原刺激机体后,产生免疫反应,由机体浆细胞合成并分泌的能与抗原特异性结合的免疫球蛋白。抗原结构、动物免疫方法及免疫剂量均能对免疫反应造成影响。多克隆抗体是将抗原注射入实验动物体内,免疫细胞会不同程度受到抗原刺激,产生不同类型的抗体,然后采集动物血清而获得。单克隆抗体则需要提取针对特定抗原表位的B淋巴细胞,在体外与骨髓瘤细胞融合,然后对杂交瘤细胞进行培养,筛选出所需细胞群,通过体外或体内培养,从培养液或动物腹水中获取抗体。
霉菌毒素为小分子化合物,不具备免疫原性,需要依靠分子中某些活性基团(如-COOH、-NH2)与相应载体连接构建人工抗原。因此,选择适当的半抗原是制备理想抗体的关键。Y.Sun等[70]以ZEN-1,4-丁二醇缩水甘油醚作半抗原,偶联BSA后免疫小鼠,所制备单克隆抗体IC50为1.12 ng·mL-1,与ZAL交叉反应率为53.1%,其他同类化合物小于4.00%;而李沐洁等[85]合成半抗原ZEN-CMO,以活泼酯法与BSA偶联作免疫原,所得抗体IC50达22.9 pg·mL-1,与α-ZEL交叉反应率为95.0%,其他同类化合物也优于前者。半抗原设计中应尽量保留待测物的特征基团,还应具有一定的复杂性,如含苯环、杂环和支链或在待测物上连接二肽基团,可增强半抗原的特异性和免疫活性[78]。为突出待测物分子内特征基团,半抗原设计时常在特征结构和载之间引入一定长度的连接臂,目前认为以3~6 个碳原子的连接臂最佳[86]。连接臂太短,半抗原有可能被载体的空间位阻掩盖而不利于其充分暴露,过长又会造成半抗原分子构型折叠[87],不利于产生特异性抗体。但Y.Sheng等[88]在制备FB1单克隆抗体过程中,设计合成了FB1-GA-BSA(5个C原子连接臂)、FB1-GA-OVA(5个C原子连接臂)、FB1-KLH(无连接臂)3种免疫原,其中以FB1-KLH免疫小鼠所得抗体效果最好,IC50为2.20 ng·mL-1,与FB2的交叉反应率154%。
载体的选择在抗体生产过程中同样至关重要,要综合考虑分子量、活性基团、溶解度及来源、价格等因素。目前用于人工抗原合成的载体主要有蛋白质类,包括牛血清蛋白(BSA)、卵清蛋白(OVA)、钥孔血蓝蛋白(KLH)、人血清蛋白(HSA)、兔血清白蛋白(RSA)等;多肽聚合物,如多聚赖氨酸(PLL)等;大分子聚合物,主要有羧甲基纤维素、聚乙烯吡咯烷酮等[89]。最常用的为蛋白类载体。其中,BSA具有物化性质稳定、不易变性、价廉易得、结合为点较多等优点,最为常用;KLH分子上结合位点多,效果也好,但较昂贵;OVA与半抗原合成的免疫原,免疫后在动物体内易脱落,免疫效果不确实。此外,半抗原与载体蛋白的偶联比等因素,同样影响抗体生产[90]。
4 总结与展望
霉菌毒素是造成食品安全问题的重要因素,建立快速、准确测定饲料及畜禽产品中霉菌毒素的方法显得至关重要。基于抗体的快速检测方法简单易行,具有检测速度快、特异性强等优点,在饲料及畜禽产品中霉菌毒素快速检测领域应用前景广阔。由于霉菌毒素污染大多是几种不同毒素同时存在,而目前市场上所应用的快速检测技术多数难以满足同时检测多种霉菌毒素的要求。因此,未来霉菌毒素快速检测技术将有两个重要研究方向:一是制备可识别多种霉菌毒素的抗体;二是建立混合污染免疫检测技术。
[1]RICHARD J L.Some major mycotoxins and their mycotoxicoses--an overview[J].IntJFoodMicrobiol,2007,119(1-2):3-10.
[2]AKANDE K E,ABUBAKAR M M,ADEGBOLA T A,et al.Nutritional and health implications of mycotoxins in animal feeds:a review[J].PakistanJNutr,2006,5(5):398-403.
[3]SULYOK M,BERTHILLER F,KRSKA R,et al.Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize[J].RapidCommunMassSpectrom,2006,20(18):2649-2659.
[4]CAST.Mycotoxins:risks in plant,animal and human Systems[S].TaskForceReportNo139,Ames,Iowa (USA),January,2003.
[5]KINSELLA B,O’MAHONY J,MALONE E,et al.Current trends in sample preparation for growth promoter and veterinary drug residue analysis[J].JChromatogrA,2009,1216(46):7977-8015.
[6]WANG Y,WENJUAN T,WANG C C,et al.Aflatoxin B1augments the synthesis of corticotropin releasing hormone in JEG-3 placental cells[J].ChemBiolInteract,2015,237:73-79.
[7]ZHANG J,ZHENG N,LIU J,et al.Aflatoxin B1and aflatoxin M1induced cytotoxicity and DNA damage in differentiated and undifferentiated Caco-2 cells[J].FoodChemToxicol,2015,83:54-60.
[8]LANYASUNYA T P,WAMAE L W,MUSA H H,et al.The risk of mycotoxins contamination of dairy feed and milk on smallholder dairy farms in Kenya[J].PakistanJNutr,2005,4(3):162-169.
[9]LIU B H,YU F Y,CHAN M H,et al.The effects of mycotoxins,fumonisin B1and aflatoxin B1,on primary swine alveolar macrophages[J].ToxicolApplPharmacol,2002,180(3):197-204.
[10]KANORA A,MAES D.The role of mycotoxins in pig reproduction:a review[J].VetMed,2009,54(12):565-576
[11]EC.Commission regulation (EU) No.165/2010 amending regulation (EC) No.1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins.[S].Off J Eur Union,2010,L 50:8-12.
[12]EC.Commission regulation (EU) No.1881/2006 of 19 december 2006 setting maximum levels for certain contaminants in foodstuffs[S].Off J Eur Union 2006,L 364:5-24.
[13]食品安全国家标准.GB 2761-2011 食品中真菌毒素限量[S].2011.
GB.GB 2761-2011 Standard of Mycotoxins in Food[S].2011.(in Chinese)
[14]SOLERI R,DEMEY H,TRIA S A,et al.Peptide conjugated chitosan foam as a novel approach for capture-purification and rapid detection of hapten——example of ochratoxin A[J].BiosensBioelectron,2015,67:634-641.
[15]MANTLE P G,FAUCET-MARQUIS V,MANDERVILLE R A,et al.Structures of covalent adducts between DNA and ochratoxin a:a new factor in debate about genotoxicity and human risk assessment[J].ChemResToxicol,2010,23(1):89-98.
[16]CARIDDI L N,SABINI M C,ESCOBAR F M,et al.Polyphenols as possible bioprotectors against cytotoxicity and DNA damage induced by ochratoxin A[J].EnvironToxicolPharmacol,2015,39(3):1008-1018.
[17]KROGH P.Role of ochratoxin in disease causation[J].FoodChemToxicol,1992,30(3):213-224.
[18]WANG Y K,ZOU Q,SUN J H,et al.Screening of single-stranded DNA (ssDNA) aptamers against a zearalenone monoclonal antibody and development of a ssDNA-based enzyme-linked oligonucleotide assay for determination of zearalenone in corn[J].JAgrFoodChem,2015,63(1):136-141.
[19]OUANES Z,ABID S,AYED I,et al.Induction of micronuclei by zearalenone in Vero monkey kidney cells and in bone marrow cells of mice:protective effect of vitamin E[J].MutatRes,2003,538(1-2):63-70.
[20]VLATA Z,PORICHIS F,TZANAKAKIS G,et al.A study of zearalenone cytotoxicity on human peripheral blood mononuclear cells[J].ToxicolLett,2006,165(3):274-281.
[21]COLLINS T F,SPRANDO R L,BLACK T N,et al.Effects of zearalenone on in utero development in rats[J].FoodChemToxicol,2006,44(9):1455-1465.
[22]DONG M,HE X J,TULAYAKUL P,et al.The toxic effects and fate of intravenously administered zearalenone in goats[J].Toxicon,2010,55(2-3):523-530.
[23]VALENZUELA-GRIJALVA N V,GONZLEZ-RIOS H,ISLAVA T Y,et al.Changes in intramuscular fat,fatty acid profile and cholesterol content induced by zeranol implantation strategy in hair lambs[J].JSciFoodAgric,2012,92(7):1362-1367.
[24]MATRASZEK-ZUCHOWSKA I,WOZNIAK B,ZMUDZKI J.Determination of zeranol,taleranol,zearalanone,α-zearalenol,β-zearalenol and zearalenone in urine by LC-MS/MS[J].FoodAdditContamA,2013,30(6):987-994.
[25]刘媛,刘贤进,余向阳,等.玉米赤霉醇酶标抗原的制备及其直接和间接竞争ELISA检测方法的建立与比较[J].分析科学学报,2008,24(2):141-144.
LIU Y,LIU X J,YU X Y,et al.Synthesis of enzyme labeled antigens and establishment of direct competitive ELISA and indirect competitive ELISA methods for zeranol[J].JournlofAnalyticlScience,2008,24(2):141-144.(in Chinese)
[26]EC.Commission regulation (EU) No.1126/2007 of 28 september 2007 amending regulation (EC) No.1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards fusarium toxins in maize and maize products[S].OffJEurUnion,2007,L 255:14-17.
[28]KALANTARI H,MOOSAVI M.Review on T-2 toxin[J].JundishapurJNatPharmPro,2010,5(1):26-38
[29]YANG J Y,ZHANG Y F,MENG X P,et al.T-2 toxin inhibits gene expression and activity of key steroidogenesis enzymes in mouse Leydig cells[J].ToxicolInVitro,2015,29(5):1166-1171.
[30]LI Y,WANG Z,BEIER R C,et al.T-2 toxin,a trichothecene mycotoxin:review of toxicity,metabolism,and analytical methods[J].JAgricFoodChem,2011,59(8):3441-3453.
[31]WAN Q,WU G,HE Q,et al.The toxicity of acute exposure to T-2 toxin evaluated by the metabonomics technique[J].MolBiosyst,2015,11(3):882-891.
[32]JECFA.Safety evaluation of certain mycotoxins in food:fifty-sixth report of the Joint FAO/WHO Expert Committee on food additives[R].WorldHealthOrganTechRepSer906.Geneva, Switzerland,2002,1-62.
[33]LI Y,WANG Z,BEIER R C,et al.T-2 toxin,a trichothecene mycotoxin:review of toxicity,metabolism,and analytical methods[J].JAgrFoodChem,2011,59(8):3441-3453.
[34]EDWARDS S G,BARRIER-GUILLOT B,CLASEN P E,et al.Emerging issues of HT-2 and T-2 toxins in European cereal production[J].WorldMycotoxinJ,2009,2(2):173-179.
[35]NAEF A,SENATORE M,DéFAGO G.A microsatellite based method for quantification of fungi in decomposing plant material elucidates the role of fusarium graminearum DON production in the saprophytic competition with trichoderma atroviride in maize tissue microcosms[J].FemsMicrobiolEcol,2006,55(2):211-220.
[36]JI F,LI H,XU J,et al.Enzyme-linked immunosorbent-assay for deoxynivalenol (DON)[J].Toxins(Basel),2011,3(8):968-978.
[37]PESTKA J J.Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol[J].WorldMycotoxinJ,2010,3(3):323-347.
[38]SOBROVA P,ADAM V,VASATKOVA A,et al.Deoxynivalenol and its toxicity[J].InterdiscipToxicol,2010,3(3):94-99.
[39]LI Y,LIU G,FU X,et al.High-sensitive chemiluminescent ELISA method investigation for the determination of deoxynivalenol in rice[J].FoodAnalMethod,2015,8(3):656-660.
[40]ROTTER B A,PRELUSKY D B,PESTKA J J.Toxicology of deoxynivalenol (vomitoxin)[J].JToxicolEnvironHealth,1996,48(1):1-34.
[41]MERRILL A H J,SULLARDS M C,WANG E,et al.Sphingolipid metabolism:roles in signal transduction and disruption by fumonisins[J].EnvironHealthPerspect,2001,109(Suppl 2):283-289.
[42]SEGVIC M,PEPELJNJAK S.Fumonisins and their effects on animal health-a brief review[J].VetArhiv,2001,71(5):299-323.
[43]WANG J H,ZHANG J B,LI H P,et al.Molecular identification,mycotoxin production and comparative pathogenicity of fusarium temperatumIsolated from maize in China[J].JPhytopathol,2014,162(3):147-157.
[44]GELDERBLOM W C,ABEL S,SMUTS C M,et al.Fumonisin induced hepatocarcinogene-sis:mechanisms related to cancer initiation and promotion[J].EnvironHealthPerspect,2001,109(Suppl 2):291-300.
[45]SUN G,WANG S,HU X,et al.Fumonisin B1contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China[J].FoodAdditContam,2007,24(2):181-185.
[46]UENO Y,IIJIMA K,WANG S D,et al.Fumonisins as a possible contributory risk factor for primary liver cancer:a 3-year study of corn harvested in Haimen,China,by HPLC and ELISA[J].FoodChemToxicol,1997,35(12):1143-1150.
[47]IARC.Traditional herbal medicines,some mycotoxins,napthalene,and styrene[R].IARCMonographsontheEvaluationofCarcinogenicRiskstoHumans,2002,82-171.
[48]MASIKINI M,MAILU S N,TSEGAYE A,et al.A fumonisins immunosensor based on polyanilino-carbon nanotubes doped with palladium telluride quantum dots[J].Sensors(Basel),2015,15(1):529-546.
[49]PATEL P D.(Bio)sensors for measurement of analytes implicated in food safety:a review[J].TracTrendAnalChem,2002,21(2):96-115.
[50]LEI Y,CHEN W,MULCHANDANI A.Microbial biosensors[J].AnalChimActa,2006,568(1-2):200-210.
[51]D’SOUZA S F.Microbial biosensors[J].BiosensBioelectron,2001,16(6):337-353.
[52]SU L,JIA W,HOU C,et al.Microbial biosensors:a review[J].BiosensBioelectron,2011,26(5):1788-1799.
[53]TOLDRA F,REIG M.Methods for rapid detection of chemical and veterinary drug residues in animal foods[J].TrendsFoodSciTech,2006,17(9):482-489.
[54]REDER-CHRIST K,BENDAS G.Biosensor applications in the field of antibiotic research-a review of recent developments[J].Sensors(Basel),2011,11(12):9450-9466.
[55]NANCY V P,FRANCO A B,ELOY S.Zearalenone determination in corn silage samples using an immunosensor in a continuous-flow/stopped-flow systems[J].BiochemEngJ,2010,51(1-2):7-13.
[56]MIRASOLI M,BURAGINA A,DOLCI L S,et al.Chemiluminescence-based biosensor for fumonisins quantitative detection in maize samples[J].BiosensBioelectron,2012,32(1):283-287.
[57]MARTINA Z,FABIO D N,LAURA A,et al.A multiplex chemiluminescent biosensor for type B-fumonisins and aflatoxin B1quantitative detection in maize flour[J].Analyst,2015,140(1):358-365.
[58]MU H,WANG B,XU Z,et al.Stereospecific recognition and quantitative structure-activity relationship between antibodies and enantiomers:ofloxacin as a model hapten[J].Analyst,2015,140(4):1037-1045.
[59]XU Z,ZHENG L,YIN Y,et al.A sensitive competitive enzyme immunoassay for detection of erythrosine in foodstuffs[J].FoodControl,2015,47:472-477.
[60]ZHANG B,DU D,MENG M,et al.Determination of amaranth in beverage by indirect competitive enzyme-linked immunosorbent assay (ELISA) based on anti-amaranth monoclonal antibody[J].FoodAnalMethod,2013,7(7):1498-1505.
[61]蔡齐超.黄曲霉毒素M1免疫学快速检测方法的研究[D].郑州:河南科技大学,2014.
CAI Q C.Study of aflatoxin M1immunological rapid detection method[D].Zhengzhou:Henan University of Science and Technology,2014.(in Chinese)
[62]PEI S C,LEE W J,ZHANG G P,et al.Development of anti-zearalenone monoclonal antibody and detection of zearalenone in corn products from China by ELISA[J].FoodControl,2013,31(1):65-70.
[63]LI Y,LUO X,YANG S,et al.High specific monoclonal antibody production and development of an ELISA method for monitoring T-2 toxin in rice[J].JAgricFoodChem,2014,62(7):1492-1497.
[64]LEE H M,SONG S O,CHA S H,et al.Development of a monoclonal antibody against deoxynivalenol for magnetic nanoparticle-based extraction and an enzyme-linked immunosorbent assay[J].JVetSci,2013,14(2):143-150.
[65]LING S,PANG J,YU J,et al.Preparation and identification of monoclonal antibody against fumonisin B1and development of detection by Ic-ELISA[J].Toxicon,2014,80(3):64-72.
[66]GIROTTI S,EREMIN S,MONTOYA A,et al.Development of a chemiluminescent ELISA and a colloidal gold-based LFIA for TNT detection[J].AnalBioanalChem,2009,396(2):687-695.
[67]NESTERENKO I S,NOKEL M A,EREMIN S A.Immunochemical methods for the detection of sulfanylamide drugs[J].JAnalChem,2009,64(5):435-444.
[68]杨扬.赭曲霉毒素A的单克隆抗体制备及胶体金检测方法研究[D].北京:北京农学院,2012.
YANG Y.Preparation of monoclonal antibodies of ochratoxin A and research on colloidal gold detecting technology[D].Beijing:Beijing Agriculture College,2012.(in Chinese)
[69]骆敏儿,唐勇,向军俭,等.玉米赤霉烯酮单克隆抗体的制备及胶体金免疫层析法的建立[J].细胞与分析免疫学杂志,2013,29(7):729-733.
LUO M E,TANG Y,XIANG J J,et al.Preparation of anti-zearalenone monoclonal antibody and preliminary establishment of colloidal gold immunochromatographic assay for zearalenone[J].XiBaoYuFenZiMianYiXueZaZhi,2013,29(7):729-733.(in Chinese)
[70]SUN Y,HU X,ZHANG Y,et al.Development of an immunochromatographic strip test for the rapid detection of zearalenone in corn[J].JAgricFoodChem,2014,62(46):11116-11121.
[71]WANG Z,LI H,LI C,et al.Development and application of a quantitative fluorescence-based immunochromatographic assay for fumonisin B1in maize[J].JAgricFoodChem,2014,62(27):6294-6298.
[72]OBERLEITNER L,EREMIN S A,LEHMANN A,et al.Fluorescence polarization immunoassays for carbamazepine-comparison of tracers and formats[J].AnalMethods,2015,7(14):5854-5861.
[73]米铁军.动物性食品中喹诺酮类药物残留的荧光偏振免疫分析研究[D].北京:中国农业大学,2013.
MI T J.Fluorescence polarization immunoassays for determination of quinolones residue in animal-origin foods[D].Beijing:China Agricultural University,2013.(in Chinese)
[74]王战辉.动物性产品中磺胺类和喹诺酮类等兽药残留的免疫分析检测技术研究[D].北京:中国农业大学,2007.
WANG Z H.Immunoassay techniques for determination of sulfonamides and fluoroquinolones residue in animal products[D].Beijing:China Agricultural University,2007.(in Chinese)
[75]王战辉,张素霞,沈建忠,等.荧光偏振免疫分析在农药和兽药残留检测中的研究进展[J].光谱学与光谱分析,2007,27(11):2299-2306.
WANG Z H,ZHANG S X,SHEN J Z,et al.Development of fluorescence polarization immunoassay for determination of pesticides and veterinary drugs[J].SpectroscopyandSpectralAnalysis,2007,27(11):2299-2306.(in Chinese)
[76]袁利鹏,杨金易,徐振林,等.荧光偏振免疫分析法检测沙丁胺醇[J].食品科学,2013,34(16):139-142.
YUAN L P,YANG J Y,Xu Z L,et al.Flurescence polarization immunoassay for the detection of salbutamol[J].FoodScience,2013,34(16):139-142.(in Chinese)
[77]SMITH D S,EREMIN S A.Fluorescence polarization immunoassays and related methods for simple,high-throughput screening of small molecules[J].AnalBioanalChem,2008,391(5):1499-1507.
[78]薛钢,雷红涛,吴青,等.荧光偏振免疫分析技术研究进展[J].食品工业科技,2008,29(3):289-292.
XUE G,LEI H T,WU Q,et al.Research progress of fluorescence polarization immunoassay[J].ScienceandTechnologyofFoodIndustry,2008,29(03):289-292.(in Chinese)
[79]JIE S Y,SERGEI E,JUN M T,et al.The Development of a fluorescence polarization immunoassay for aflatoxin detection[J].BiomedEnvironSci,2014,27(2):126-129.
[80]LI C,MI T,CONTI G O,et al.Development of a screening fluorescence polarization immunoassay for the simultaneous detection of fumonisins B1and B2in maize[J].JAgricFoodChem,2015,63(20):4940-4946.
[81]LI C,WEN K,MI T,et al.A universal multi-wavelength fluorescence polarization immunoassay for multiplexed detection of mycotoxins in maize[J].BiosensBioelectron,2015,79:258-265.
[82]李鑫.基于免疫分析的农产品真菌毒素混合污染同步检测技术研究[D].武汉:中国农业科学院,2014.
LI X.Immunoanalysis based simultaneous detection of mycotoxins composite contamination in agro-products[D].Wuhan:Chinese Academy of Agricultural Sciences,2014.(in Chinese)
[83]STRASSER A,DIETRICH R,USLEBER E,et al.Immunochemical rapid test for multiresidue analysis of antimicrobial drugs in milk using monoclonal antibodies and hapten-glucose oxidase conjugates[J].AnalChimActa,2003,495(1-2):11-19.
[84]谢焕龙,王宇,徐振林,等.基于混合抗体的酶联免疫分析方法同时检测孔雀石绿和隐孔雀石绿[J].现代食品科技,2015,31(12):325-330.
XIE H L,WANG Y,XU Z L,et al.Simultaneous detection of malachite green and leucomalachite green based on hybrid antibody ELISA analysis method[J].ModernFoodScienceandTechnology,2015,31(12):325-330.(in Chinese)
[85]李沐洁,张明洲,奚茜,等.玉米赤霉烯酮单克隆抗体制备及免疫分析[J].中国食品学报,2013,13(1):145-152.
LI M J,ZHANG M Z,XI X,et al.Preparation of monoclonal antibody and detection of zearalenone by enzyme-linked immunosorbent assay[J].JournalofChineseInstituteofFoodScienceandTechnology,2013,13(1):145-152.(in Chinese)
[86]KIM Y J,CHO Y A,LEE H S,et al.Investigation of the effect of hapten heterology on immunoassay sensitivity and development of an enzyme-linked immunosorbent assay for the organophosphorus insecticide fenthion[J].AnalChimActa,2003,494(1-2):29-40.
[87]张泽英,袁宗辉.兽药人工抗原合成的研究进展[J].中国兽药杂志,2006,40(5):44-47.
ZHANG Z Y,YUAN Z H.Research progress on synthesis of artificial antigens for veterinary drugs[J].ChineseJournalofVeterinaryDrug,2006,40(5):44-47.(in Chinese)
[88]SHENG Y,JIANG W,DE SAEGER S,et al.Development of a sensitive enzyme-linked immunosorbent assay for the detection of fumonisin B1in maize[J].Toxicon,2012,60(7):1245-1250.
[89]徐加发,成义祥,沈萍萍.半抗原设计合成研究进展[J].江苏农业学报,2009,25(5):1178-1182.
XU J F,CHENG Y X,SHEN P P.Research progress on dsign and synthesis of hapten[J].JiangsuJournalofAgriculturalSciences,2009,25(5):1178-1182.(in Chinese)
[90]宋娟,王榕妹,王悦秋,等.半抗原的设计、修饰及人工抗原的制备[J].分析化学,2010,38(8):1211-1218.
SONG J,WANG R M,WANG Y Q,et al.Hapten design,modification and preparation of atificial antigens[J].ChineseJournalofAnalyticalChemistry,2010,38(8):1211-1218.(in Chinese)
(编辑白永平)
Research Progress on the Rapid Detection Technology of Mycotoxins in Feeds and Animal Products
DONG Guo-liang1,3,PENG Da-peng1,2,3*,HAN Xiao-ya1,3,LIU Zhen-li1,2,3,YUAN Zong-hui1,2,3
(1.NationalReferenceLaboratoryofVeterinaryDrugResidues(HZAU),TheKeyLaboratoryfortheDetectionofVeterinaryDrugResiduesofMinistryofAgriculture,Wuhan430070,China;2.LaboratoryofQuality&SafetyRiskAssessmentforLivestockandPoultryProducts(Wuhan)ofMinistryofAgriculture,Wuhan430070,China;3.CollegeofVeterinaryMedicine,HuazhongAgriculturalUniversity,Wuhan430070,China)
Mycotoxins are secondary metabolites of some fungi that distributed widely in nature,especially in feedstuffs and animal products,with great harm to animals and humans.This review introduces several mycotoxins that commonly presents in feedstuffs and animal products and their risks to animals and humans,summarizes the research progress on the rapid detection technology of mycotoxins,analyzes the main influence factors of antibody preparation against mycotoxins,and prospects the further development of the rapid detection technology of mycotoxins as well.
mycotoxin;rapid detection technology;antibody
10.11843/j.issn.0366-6964.2016.09.003
2016-05-10
青年教师科技创新专项(2662015QC030)
董国良(1990-),男,山东莒县人,硕士生,主要从事兽药残留与食品安全研究, E-mail:dgl@webmail.hzau.edu.cn
彭大鹏(1977-),男,副教授,硕导,主要从事兽药残留与食品安全研究,E-mail:pengdapeng@mail.hzau.edu.cn
S859.8
A
0366-6964(2016)09-1757-11
