孔有效利用的活性炭基非对称电极膜/电容脱盐系统
2016-10-31JiyoungKimDongHyunPeckByungrokLeeSeongHoYoonDooHwanJung
Jiyoung Kim, Dong-Hyun Peck, Byungrok Lee, Seong-Ho Yoon, Doo-Hwan Jung
(1.Advanced Energy and Technology, University of Science and Technology (UST), Yuseong-gu, Daejeon,305-333, Republic of Korea;2.New and Renewable Energy Research Division, Korea Institute of Energy Research (KIER), Yuseong-gu, Daejeon,305-343, Republic of Korea;3.Institute for Materials Chemistry and Engineering, Kyushu University, Kasuga, Fukuoka,816-8580, Japan)
孔有效利用的活性炭基非对称电极膜/电容脱盐系统
Jiyoung Kim1,2,Dong-Hyun Peck1,2,Byungrok Lee2,Seong-Ho Yoon3,Doo-Hwan Jung1,2
(1.AdvancedEnergyandTechnology,UniversityofScienceandTechnology(UST),Yuseong-gu,Daejeon,305-333,RepublicofKorea;2.NewandRenewableEnergyResearchDivision,KoreaInstituteofEnergyResearch(KIER),Yuseong-gu,Daejeon,305-343,RepublicofKorea;3.InstituteforMaterialsChemistryandEngineering,KyushuUniversity,Kasuga,Fukuoka,816-8580,Japan)
采用活性炭纤维为原料制备出膜/电容脱盐系统,提纯含氯化钠的水。OG系列活性炭纤维作为电极的活性材料,其比表面积和孔分布不同而呈现不同的活化程度。将这些材料用于膜/电容脱盐系统,评价了他们对钠离子或氯离子的脱盐性能。膜/电容实验在不同操作电位窗口、含盐溶液的进料速率和浓度下进行。OG系列活性炭纤维对每种离子的脱盐效率和电吸附量来评价膜/电容性能。结果表明,BET比表面积是确保高性能的必要因素。另外,炭材料最上端的浅孔有助于活性炭纤维比表面积的充分利用。OG7A样品的孔结构适合于钠离子吸附,OG10A和 OG15A适于大量孔吸附氯离子。因此,非对称电极排列施加于吸附离子的尺寸,应考虑炭材料比表面积和孔面积的有效利用,以得到高性能的膜/电容脱盐系统。
电容去离子化; 活性炭纤维; 孔尺寸; 氯离子和钠离子; 非对称电极
1 Introduction
A desalination process that can remove some amount of salt or minerals from water has been drawn an increasing attention due to the global water crisis[1]. Most currently used methods such as thermal evaporation, reverse osmosis and electro-dialysis must overcome the high cost of facility and maintenance[2,3]. Recently, capacitive deionization (CDI), an electrochemical water purification method by reversible adsorption and desorption of ions onto the surface of electrical charged electrode under an external power source, has attracted a great deal of attention because it is an alternative low-energy-consumption and eco-friendly technology[4-8].
In recent years, the studies have been focused on the electrode active material to improve desalination performance. Carbons with a variety of forms and porosity have been chosen as electrode active materials owing to their excellent characteristics, such as large surface area, good electrical conductivity and chemical stability[9-11]. Many studies have been carried out to improve CDI performance using various forms of carbon materials including carbon aerogels[12-14], activated carbon fibers[15,16], activated carbon fiber cloth[17], carbon nanofiber networks[18], mesoporous carbons[19,20], graphene[21-23], carbon nanofibers[24], carbon nanotubes[24]and carbon-based composites[25]. However, basic investigations on achieving a high ion removal capacity and rapid kinetics of CDI systems with a low cost have not yet been sufficiently conducted. Because ions are held through the electrical double layers on the internal surface of carbon pores, revealing the relationship between the internal structure of carbons and the size of ions is essentially important to achieve a high desalination performance. Nevertheless, a few experiments and theoretical predictions are reported about the relations between pore structure and desalination performance[26-28].
Activated carbon fibers (ACFs), which have a large surface area and good conductivity, have been used in various fields such as catalyst supports[29], energy storage materials and adsorbents[30]. There have been numerous efforts to elucidate the pore structure of ACFs to extend their application fields. Among the conventional ACFs, the OG-series pitch-based ACFs from Osaka Gas Co. Ltd., Japan, have high surface areas and tunable pore structures depending on their degree of activation. The structural analysis and practical use of OG-series ACFs as chemical adsorbents have been already demonstrated in many papers[31-34].
The present study investigated the relationship between the pore structure of ACFs and desalination performance for sodium chloride aqueous solution using CDI. A powder activated carbon (Maxsorb-III) with an extremely high surface area was used as a counter and reference electrode materials, and four types of OG-series ACFs with predominant open pores were employed as working electrodes to study their pore structure effects on the desalination performance of sodium chloride solutions by CDI.
2 Experimental
2.1Materials
Four types of pitch-based ACFs (OG-series: OG7A, OG10A, OG15A and OG20A with an increased the degree of activation) were obtained from Osaka Gas Co., Ltd. (Japan). The extremely high surface area activated carbon for the counter/reference electrode material (Maxsorb-III) was provided by Kansai Coke and Chemicals Co., Ltd. (Japan). Ketjen Black was selected as the conductive additive to reduce electrode resistance. Carbons were sufficiently pulverized by a milling machine (FRITSCH, Germany; with 0.2 mm sieve rings) and ball-milled in ethanol for 24 h before the all experiments. Poly (vinylidene fluoride) (PVdF, Mw ~534 000) and N, N-Dimethylacetamide (DMAc, 99.8%) were purchased from Sigma-Aldrich and sodium chloride (NaCl, 99.5%) from Junsei Chemical Co. (Japan).
2.2Structural and electrochemical characteristics of ACFs
The porous structure of pulverized ACFs was evaluated by nitrogen adsorption at 77 K using a BELSORP-mini adsorption apparatus. The specific surface areas were calculated by the BET (Braunauer-Emmett-Teller) equation, and their pore size distributions were derived from adsorption isotherms using an NLDFT method[35]. Electrochemical capacitances of ACFs were investigated by cyclic voltammetry using a conventional three-electrode electrochemical cell configuration in a 0.5 M NaCl aqueous solution. A Pt wire and a silver/silver-chloride electrode (SSCE) were used as the counter and reference electrodes, respectively. The dispersed solution of activated carbons and binder substances was dropped onto the glassy carbon as a working electrode, after which the electrode was immersed in electrolyte and outgassed under vacuum for 10 min. Cyclic voltammogram for the assessment of carbon capacitance was performed with a potential sweep from -0.4 to 0.6 V at a scan rate from 5 to 100 mV·s-1in 5 cycles. The average capacitance of samples was calculated from the 5thsweep from -0.2 to 0.4 V. The capacitances were also evaluated by the same procedure in an electrolyte (0.1 M HClO4) and with a sweep range from -0.2 to 0.8 V.
2.3Capacitive deionization testing
An activated carbon paste was prepared by a mixture of 88 wt% of activated carbon, 5 wt% of Ketjen Black as conductive filler and 7 wt% of PVDF as a binder. The carbon paste was coated on the graphite foil as a current collector by a bar-coating machine with a bar height of 400 μm. The carbon electrode was dried at 80 ℃ in a vacuum oven for 2 h and cut into an effective area of 10 × 10 cm2. As shown in Fig. 1, the CDI unit-cells for the ion adsorption experiments were fabricated with a pair of carbon electrodes with a nylon spacer to enable streaming of the salty solution. Within the carbon electrode and spacer, ion-selective membranes (NEOSEPTA AMX/CMX membranes from ASTOM Corporation, Japan) were used to prevent adsorption on the opposite site during the desorption period to enhance the desalination performance[36]. An OG-series ACF electrode with a cation-permeable membrane was coupled with a negative electronic source to evaluate the adsorption performance of sodium ion in relation to the pore structure of ACFs. The counter electrode was made of an extremely high surface area activated carbon, Maxsorb-III. The opposite arrangement was used to evaluate the adsorption performance of chloride ion. The CDI experiments were conducted in a continuous flow mode. The salty water was fed from a reservoir to the CDI cell. The conductivity meter was placed at the outlet of the cell to measure the concentration of the effluent solution, purified or concentrated water. The effect of salt concentration and feeding rate of salty water on the adsorption performance was also tested. For the salt concentration tests, the 250 or 1 000 ppm NaCl aqueous solution was chosen with a flow rate of 10 mL/min. For the feeding rate tests, the solution concentration of 250 ppm was used under various flow rates of 10, 30 and 50 mL/min. In all tests, the cell voltage between both electrodes was applied at 1.0 or 1.5 V for 3 min. Afterward, the ions on both electrodes were desorbed by an electrical short for 3 min. These charge and discharge processes were repeated for three times. Each CDI performance test was performed three times and the third cycle of the experiments was used to obtain an average result.
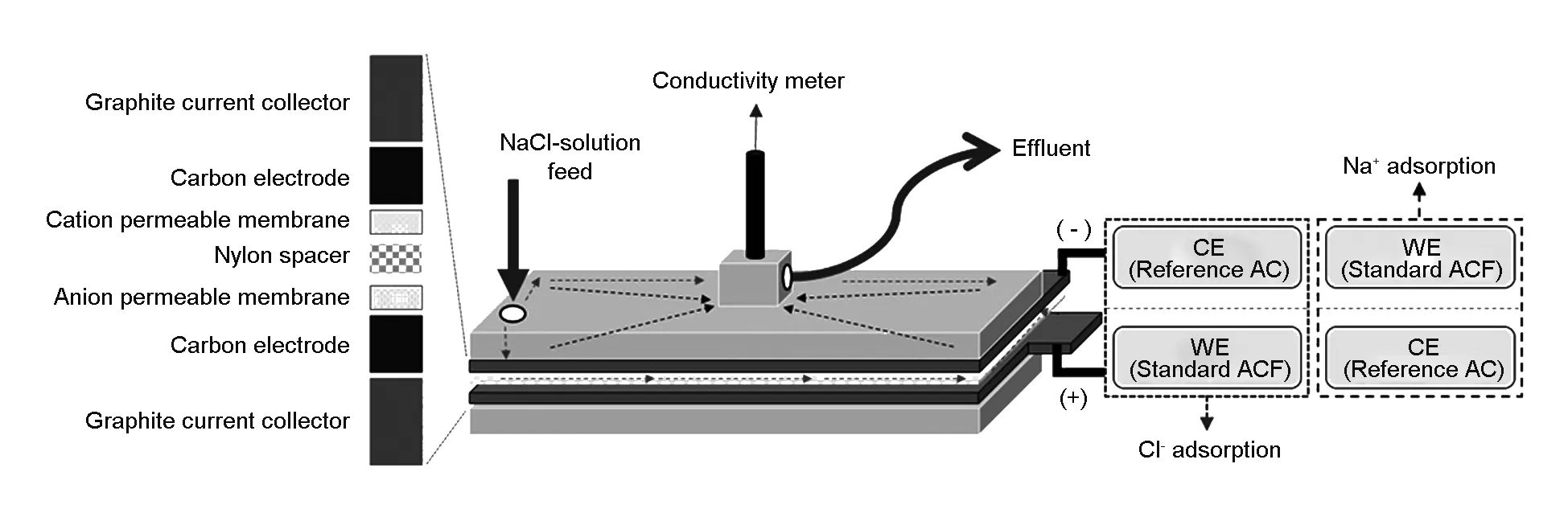
Fig. 1 Schematic illustration of the capacitive deionization experiment.
Salt removal efficiency (%) was calculated from the concentrations of effluent versus influent solution[37]. Normalized electrosorption capacity, the amount of adsorbed sodium chloride divided by the specific surface area of ACF, ug-NaCl/m2-activated carbon, was compared to evaluate the relationship between the adsorption performance and pore structure for each ion.
3 Results and discussion
3.1Pore structure of OG-series ACFs
Fig. 2 shows the nitrogen adsorption/desorption isotherms and pore size distributions of pulverized OG-series ACFs calculated by the NLDFT method. The porous properties of the ACFs are listed in Table 1. The surface areas and pore size ACFs increased with the degree of activation. No pronounced difference in surface area was found between OG15 and OG20. However, the proportions of meso pores of OG20 were higher than those of OG15. The peak pore radii of ACFs increased from 0.69 to 1.52 nm with the degree of activation. The results of the surface area and pore properties were in close agreement with those found in the earlier experimental study[33]. The BET surface area of Maxsorb-III was over 3 000 m2/g, nearly twice the value of OG-20. Although the pore size of Maxsorb-III was similar to that of the OG-series ACFs, the large surface area and pore volume were most likely due to the activation method.
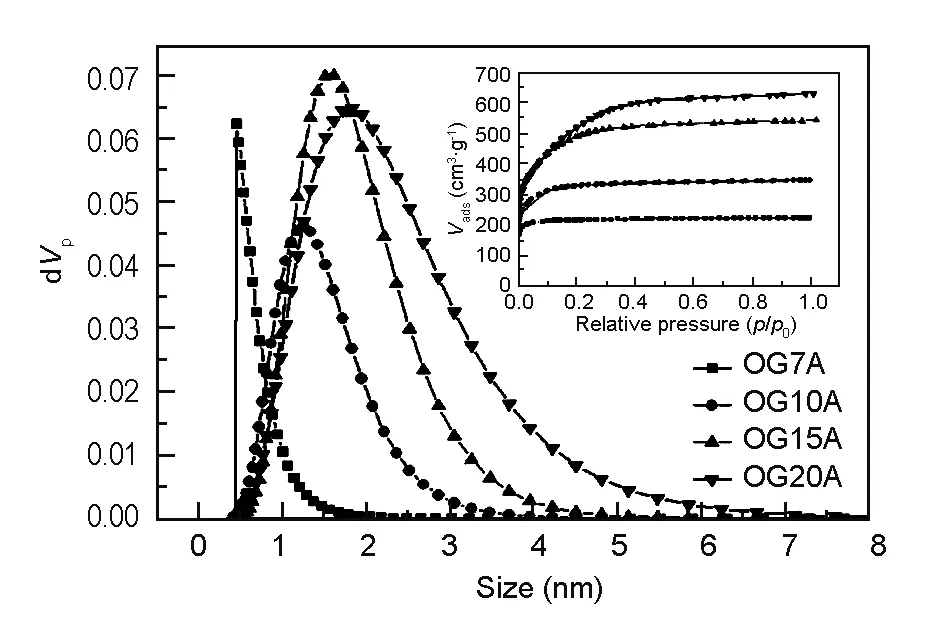
Fig. 2 The pore size distribution calculated
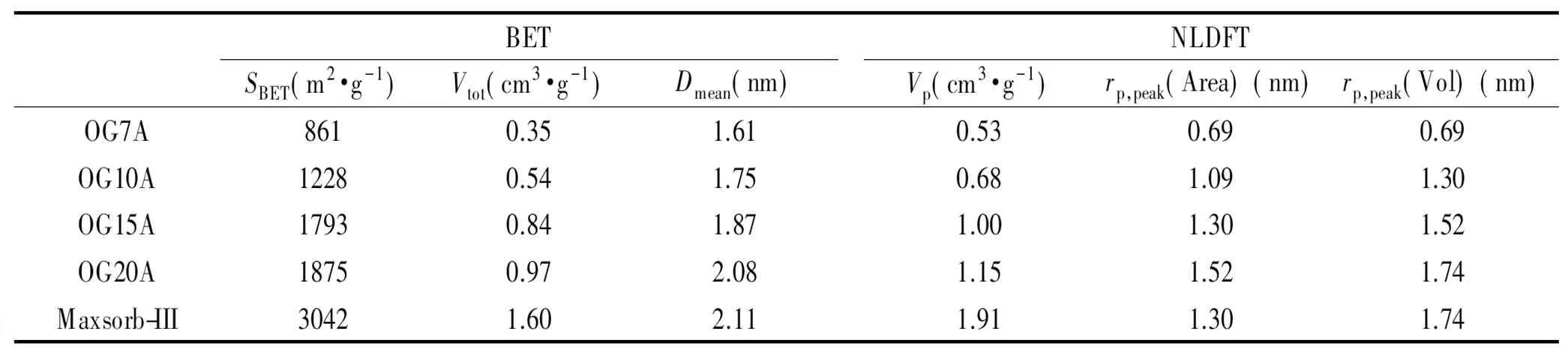
BETSBET(m2·g-1)Vtot(cm3·g-1)Dmean(nm)NLDFTVp(cm3·g-1)rp,peak(Area)(nm)rp,peak(Vol)(nm)OG7A8610.351.610.530.690.69OG10A12280.541.750.681.091.30OG15A17930.841.871.001.301.52OG20A18750.972.081.151.521.74Maxsorb-III30421.602.111.911.301.74
3.2Electrochemical characteristics
The electrochemical double layer capacitances of ACFs were measured via the cyclic voltammetry in 0.5 M NaCl and 0.1 M HClO4electrolyte. The CV profile of ACFs presents an expected rectangular and ideal capacitive behavior at different scan rates (Fig. S1 and S2-Supplementary data). The specific capacitances calculated from the CV profiles at different scan rates are indicated in Fig. 3.
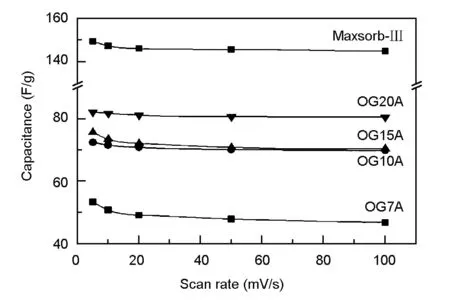
Fig. 3 The specific capacitances calculated from
As the scan rate increased, the capacitance slightly decreased. The above finding indicates that the CV measurement was accurately conducted. The capacitance of ACFs increased with specific surface area, but not exactly proportional. It was confirmed that the Maxsorb-III as a counter electrode material had much a higher capacitance than the OG-series ACFs. Table 2 lists the specific capacitance (F/g) and normalized capacitance (F/m2) divided by BET surface area at a scan rate of 10 mV/s in 0.5 M NaCl and 0.1 M HClO4. For both electrolytes, the capacitances of all carbons increased with the specific surface area even though not exactly proportional. However, OG7A had the highest normalized capacitance efficiency. These results indicate that despite the increase in specific capacitance with the specific surface area related to the degree of activation, the deep pores connected to the mesopore by severe activation are not effectively used. In addition, the capacitance in the 0.1 M HClO4electrolyte was two times higher than those in 0.5 M NaCl. The different capacitance between the two electrolytes imply that the amount of adsorbed ion is also related to the types of ions[38].
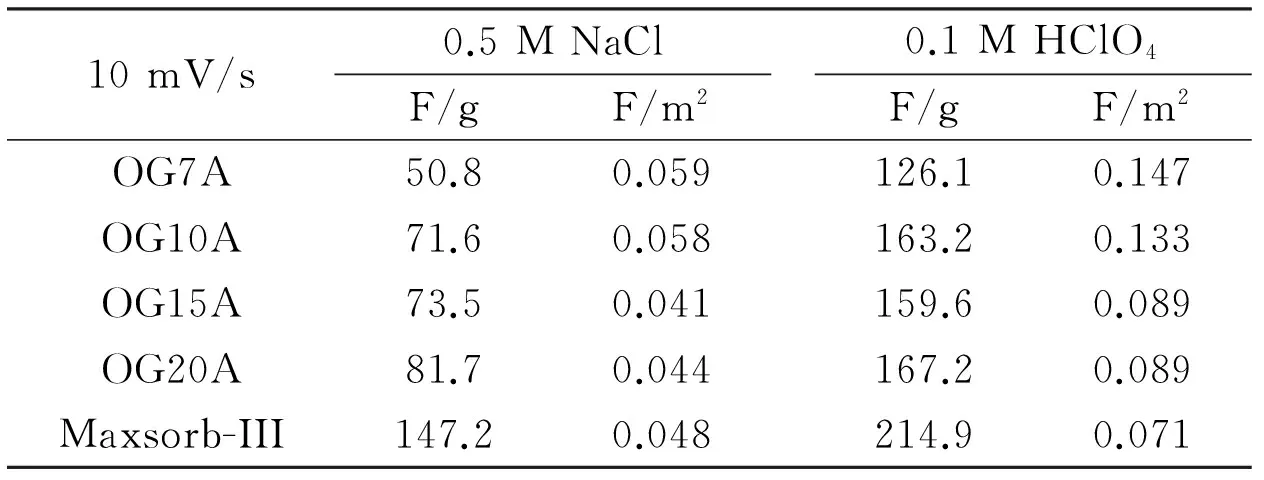
Table 2 The specific and surface area normalized capacitances of OG-series ACFs and Maxsorb-III. Capacitances were obtained in different electrolyte: 0.5 M NaCl or 0.1 M HClO4.
3.3Capacitive deionization performance
The carbon electrodes for the CDI tests were prepared from four types of ACFs and Maxsorb-III. The thickness of graphite foil as a current collector was 210 μm, and the carbon mixture layer was well casted on it with a thickness of approximately 200 μm (Fig. S3). The availability of Maxsorb-III as a counter and reference electrode material was already confirmed by a surface area analysis and cyclic voltammetry experiment. In the CDI experiment, a Maxsorb-III was verified to have the best desalination performance for both sodium and chloride. Fig. S4 and S5 show the adsorption and desorption curves of sodium and chloride ions. In all experiments, Maxsorb-III exhibited the lowest concentration during the adsorption period among the OG-series ACFs. Therefore, using the Maxsorb-III as a counter and reference electrode is reasonable.
Fig. 4 shows the salt removal efficiency of each ion when the feed solution with different concentrations of 250 ppm or 1 000 ppm NaCl was pumped into the cell at a fixed rate of 10 mL/min. The external power voltage was applied at 1.0 V or 1.5 V. The average values and the margin of errors from testing in triplicate are presented. The salt removal efficiency appeared to have a tendency to increase with surface area of ACFs. This tendency is more obvious at lower concentration than at high concentration. OG20A had the highest salt removal efficiency of 91% at 250 ppm and 50% at 1 000 ppm under under 1.5 V. Fig. 5 indicates the normalized electrosorption capacity to investigate the effectiveness of pore structure of ACFs for each sodium/chloride ion. For sodium ion adsorption, OG7A had the most effective use of surface area. For chloride ion adsorption, OG10A and OG15A had the most effective use of surface area. Although OG20A presented the highest salt removal efficiency due to its highest surface area, the availability of entire carbon surface was not the highest.
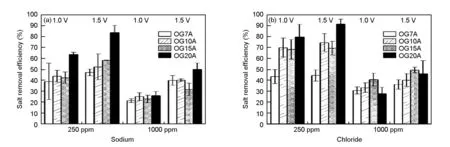
Fig. 4 The salt removal efficiency of each individual ion in concentration CDI tests. The concentration of salty solution is 250 ppm or 1 000 ppm, and the feed rate is fixed at 10 mL/min.
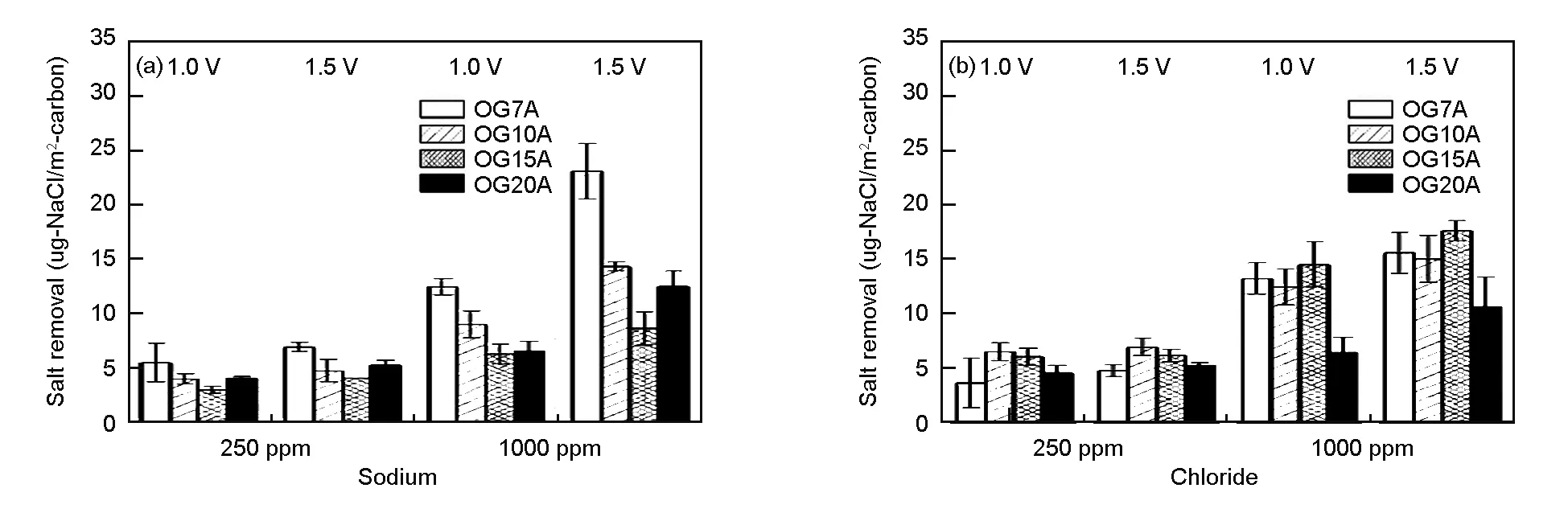
Fig. 5 Normalized electrosorption capacities of OG-series ACFs in the concentration test.
Fig. 6 shows the salt removal efficiency of sodium and chloride for OG-series ACFs at different feed rates of NaCl solution. The salt removal efficiency decreased with the flow rate, due to the accumulation of salt amount. The relationship between the adsorption capacity of ions and the surface area of OG-series ACFs was similar to that of the concentration experiments. A higher performance was obtained from the ACF with a higher surface area. The normalized electrosorption capacity in relation to the feed rate is shown in Fig. 7, which agree well with that of the concentration tests. OG7A was best for adsorbing sodium ions while OG10A and OG15A were more effective for chloride ion adsorption based on electrosorption capacity.
3.4Correlations between pore structure and CDI performance
The aim of this study is to examine the correlation between the pore structure of OG-series ACFs and the desalination performance of NaCl aqueous solution to design high performance carbon materials for CDI. First, as is well known, a high specific surface area is necessary to obtain a high salt removal capacity. Although the performance is not exactly proportional to the surface area of the electrode material, the salt removal efficiency generally increases with surface area. From our results, OG20A, with the highest surface area among the ACFs, represents the highest salt removal efficiency in all the CDI experiments. Next, shallow pores that expose to the salt solution directly favor the best use of all ACF surfaces. Shiratori et al[33]have reported the pore structure of the OG-series ACFs based on the concept of a microdomain structure. They found that the ACFs activated under mild conditions such as OG7A had shallow slit-shape micropores. However, the highly activated microdomain in OG20A forms a mesoporous region from an extension of intermicrodomain and generates novel micropores in the core part of ACFs as deep pores. From all the cyclic voltammetry and CDI cell experiments, OG7A with shallow pores presents the highest normalized electrosorption capacity than that of OG20 ACF. OG20A needs supercharged dragging force and long time to use the whole surface of inner pores because of a long and complex pathway to reach the inner pores. In the real CDI system, a switch from adsorption to desorption at a short time interval is essential to achieve the effective use of CDI device. Therefore, a quick operation and rapid ion moving kinetics under a low external electrical field needs abundant shallow pores on the surfaces of carbon particles. Finally, the size of adsorbed ions is definitely considered to affect the effective use of the surface and pore area of carbons for the CDI electrode. The pore size dependency on the types of adsorption ion can be inferred from the different capacitance values related to the different electrolyte ions in cyclic voltammetry, such as that in Table 2. Additionally, Fig. 5 and Fig. 7 show that the maximum electrosorption capacity of sodium ion is found for OG7A and that of chloride ion is found for OG10A and OG15A. Smith et al[39]simulated the size of hydrated sodium ions to be 0.66 nm and that of chloride to be 0.72 nm. Additionally, Kalluri et al[40]reported that the effect of pore size on the capacitance of nanoporous carbon was evaluated by electrochemical experiments and molecular dynamics simulations. The results of this study suggested that the proper pore size of carbon for the adsorption of hydrate sodium and chloride ions are 0.65 and 0.79 nm, respectively. These reports agree well with the results of the present study that the carbon for chloride ion removal needs the larger sized pores than for sodium ion removal. Thus, OG7A with a peak pore radius of 0.69 nm is suitable for sodium ion adsorption, and OG10A and OG15A with a median pore size of 1.09 and 1.52 nm, respectively have pores large enough for chloride ion adsorption. The experimental results in this study show that enhanced performance is expected by an asymmetric electrode configuration, in which pore size of each electrode materials should have pore size that match or is larger than the corresponding radius of hydrated sodium ions. Moreover, both high surface area and shallow pores of electrode materials favor a high ion removal efficiency whether the materials are used as cathode or anode.
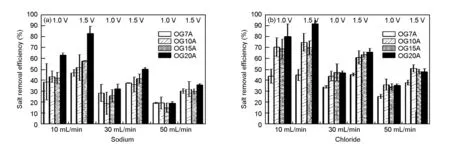
Fig. 6 The salt removal efficiency of OG-series ACFs in the feed rate test.
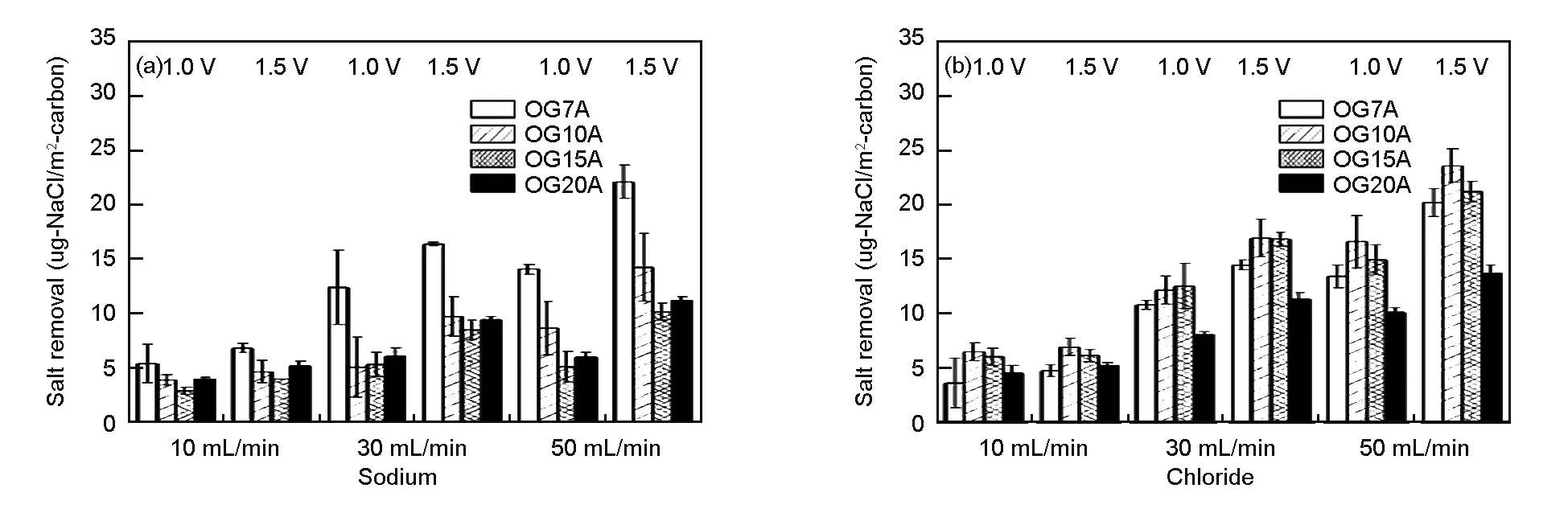
Fig. 7 Normalized electrosorption capacities of OG-series ACFs in feed rate tests.
4 Conclusions
The relationship between the pore structure of ACFs and the sort of ions to be desalinated have been investigated to improve the CDI performance of the ACF electrodes. OG-series ACFs, whose pore size distributions were fully analyzed, were used to evaluate their deionization performance for sodium and chloride ions. The cyclic voltammetry and CDI unit cell performance test indicated that the specific capacitance and salt removal efficiency increased with surface area of active materials. However, a pore utilization ratio on the carbon surface was different, which depend on the size match between pore size of ACFs and radius of hydrated ions. Pore depth is another factor that affect the pore utilization ratio. Shallower pore favors a better use of carbon surface. Therefore, a best performed CDI should have an asymmetric electrode configuration with pore size of each electrode material match with the size of hydrated ions in addition to high surface area and shallow pores of electrode materials. This was verified by experimental results in this investigation.
Acknowledgements
This work was conducted under the framework of the Research and Development Program (B4-2443-03) of the Korea Institute of Energy Research (KIER).
[1]Elimelech M, Phillip W A. The future of seawater desalination: Energy, technology, and the environment[J]. Science, 2011, 333: 712-717.
[2]Welgemoed T J, Schutte C F. Capacitive deionization technologyTM: An alternative desalination solution[C]. European Conference on Desalination and the Environment, ITALY, 2005,183: 327-340.
[3]Oda H, Nakagawa Y. Removal of ionic substances from dilute solution using activated carbon electrodes[J]. Carbon, 2003, 41: 1037-1047.
[4]Lee J B, Park K K, Eum H M, et al. Desalination of a thermal power plant wastewater by membrane capacitive deionization[J]. Desalination, 2006, 196: 125-134.
[5]Lee J H, Bae W S, Choi J H. Electrode reactions and adsorption/desorption performance related to the applied potential in a capacitive deionization process[J]. Desalination, 2010, 258: 159-163.
[6]Biesheuvel P M, Bazant M Z. Nonlinear dynamics of capacitive charging and desalination by porous electrodes[J]. Physical Review E, 2010, 81: 031502.
[7]Hou C H, Huang C Y, Hu C Y. Application of capacitive deionization technology to the removal of sodium chloride from aqueous solutions[J]. Int J Environ Sci Technol, 2013, 10: 753-760.
[8]Nugrahenny A U, Kim J, Kim S K, et al. Preparation and application of reduced graphene oxide as the conductive material for capacitive deionization[J]. Carbon Letters, 2014, 15: 38-44.
[9]Oren Y. Capacitive deionization (CDI) for desalination and water treatment-past, present and future (a review)[J]. Desalination, 2008, 228: 10-29.
[10]Anderson M A, Cudero A L, Palma J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete?[J]. Electrochimica Acta, 2010, 55: 3845-3856.
[11]Porada S, Zhao R, van der Wal A, et al. Biesheuvel, review on the science and technology of water desalination by capacitive deionization[J]. Progress in Materials Science, 2013, 58: 1388-1442.
[12]Farmer J C, Fix D V, Mack G V, et al. Capacitive deionization of NaCl and NaNO3solutions with carbon aerogel electrodes[J]. J Electrochem Soc, 1996, 143: 159-169.
[13]Jung H H, Hwang S W, Hyun S H, et al. Capacitive deionization characteristics of nanostructured carbon aerogel electrodes synthesized via ambient drying[J]. Desalination, 2007, 216: 377-385.
[14]Kohli D K, Singh R, Singh A, et al. Enhanced salt-dsorption capacity of ambient pressure dried carbon aerogel activated by CO2for capacitive deionization application[J]. Desalination and Water Treatment, 2014: 1-7.
[15]Avraham E, Yaniv B, Soffer A D. Aurbach, developing ion electroadsorption stereoselectivity, by pore size adjustment with chemical vapor deposition onto active carbon fiber electrodes. Case of Ca2+/Na+separation in water capacitive desalination[J]. J Phys Chem C, 2008, 112: 7385-7389.
[16]Huang Z H, Wang M, Wang L, et al. Relation between the charge efficiency of activated carbon fiber and its desalination performance[J]. Langmuir, 2012, 28: 5079-5084.
[17]Chou W L, Cheng L C, Hu J L, et al. Desalination by electrochemically enhanced adsorption using activated carbon fiber cloth electrodes[J]. Fresenius Environmental Bulletin, 2013, 22: 117-122.
[18]Chen Y, Yue M, Huang Z H, et al. Electrospun carbon nanofiber networks from phenolic resin for capacitive deionization[J]. Chemical Engineering Journal, 2014, 252: 30-37.
[19]Tsouris C, Mayes R, Kiggans J, et al. Mesoporous carbon for capacitive deionization of saline water[J]. Environ Sci Technol, 2011,45: 10243-10249.
[20]Wang G, Qian B, Dong Q, et al. Highly mesoporous activated carbon electrode for capacitive deionization[J]. Separation and Purification Technology, 2013, 103: 216-221.
[21]Li H, Lu T, Pan L, et al, Electrosorption behavior of graphene in NaCl solutions[J]. J Mater Chem, 2009, 19: 6773-6779.
[22]Wang H, Zhang D, Yan T, et al. Graphene prepared via a novel pyriding-theral strategy for capacitive deionization[J]. J Mater Chem, 2012, 22: 23745-23748.
[23]Jia B, Zou L. Graphene nanosheets reduced by a multi-step process as high-performance electrode material for capacitive deionization[J]. Carbon, 2012, 50: 2315-2321.
[24]Li H, Gao Y, Pan L, et al. Electrosorptive desalination by carbon nanotubes and nanofibers electrodes and ion-exchange membranes[J]. Water Research, 2008, 42: 4923-4928.
[25]Yang C M, Choi W H, Na B K, et al. Capacitive deionization of NaCl solution with carbon aerogel-silica gel composite electrodes[J]. Desalination, 2005, 174: 125-133.
[26]Porada S, Borchardt L, Bryjak M, et al. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization[J]. Energy Environ Sci, 2013, 6: 3700-3712.
[27]Biesheuvel P M, Zhao R, Porada S, et al. Theory of membrane capacitive deionzation including the effect of the electrode pore space[J]. Journal of Colloid and Interface Science, 2011, 360: 239-248.
[28]Han L, Karthikeyan K G, Anderson M A, et al. Exploring the impact of pore size distribution on the performance of carbon electrodes for capacitive deionization[J]. Journal of Colloid and Interface Science, 2014, 430: 93-99.
[29]Tien P D, Morisaka H, Satoh T, et al. Yamaguchi, efficient evolution of hydrogen from tetrahydronaphthalene upon palladium catalyst supported on activated carbon fiber[J]. Energy & Fuels, 2003, 17: 658-660 .
[30]J Miyawaki, T Shimohara, N Shirahama, et al. Removal of NOxfrom air through cooperation of the TiO2photocatalyst and urea on activated carbon fiber at room temperature[J]. Applied Catalysis B: Environmental, 2011, 110: 273-278.
[31]Kisamori S, Kuroda K, Kawano S, et al. Oxidative removal of SO2and recovery of H2SO4over poly(acrylonitrile)-based active carbon fiber[J]. Energy & Fuels, 1994, 8: 1337-1340.
[32]Lee S, Mitani I, Yoon S, et al. Mochida, Capacitance and H2SO4adsorption in the pores of activated carbon fibers[J]. Appl Phys A, 2006, 82: 647-652.
[33]Shiratori N, Lee KJ, Miyawaki J, et al. Pore structure analysis of activated carbon fiber by microdomain-based model[J]. Langmuir, 2009, 25: 7631-7637.
[34]Lee K J, Miyawaki J, Shiratori N, et al. Toward an effective adsorbent for polar pollutants: formaldehyde adsorption by activated carbon[J]. Journal of Hazardous Materials, 2013, 260: 82-88.
[35]El-Merraoui M, Aoshima M, Kaneko K. Micropore size distribution of activated carbon fiber using the density functional theory and other method[J]. Langmuir, 2000, 26: 4300-4304.
[36]Kim Y J, Choi J H. Enhanced desalination efficiency in capacitive deionization with an ion-selective membrane[J]. Separation and Purification Technology, 2010, 71: 70-75.
[37]Kim Y J, Choi J H. Improvement of desalination efficiency in capacitive deionization using a carbon electrode coated with an ion-exchange polymer[J]. Water Research, 2010, 44: 990-996.
[38]Simon P, Burke A. Nanostructured carbons: Double-layer capacitance and more, The electrochemical society interface[J]. 2008, 17: 38-43.
[39]Smith D E, Dang L X. Computer simulations of NaCl association in polarizable water[J]. J Chem Phys, 1994, 100: 3757-3766.
[40]Kalluri R K, Biener M M, Suss M E, et al. Unraveling the potential and pore-size dependent capacitance of slit-shaped graphitic carbon pores in aqueous electrolytes[J]. Phys Chem Chem Phys, 2013, 15: 2309-2320.
An asymmetrical activated carbon electrode configuration for increased pore utilization in a membrane-assisted capacitive deionization system
Jiyoung Kim1,2,Dong-Hyun Peck1,2,Byungrok Lee2,Seong-Ho Yoon3,Doo-Hwan Jung1,2
(1.AdvancedEnergyandTechnology,UniversityofScienceandTechnology(UST),Yuseong-gu,Daejeon,305-333,RepublicofKorea;2.NewandRenewableEnergyResearchDivision,KoreaInstituteofEnergyResearch(KIER),Yuseong-gu,Daejeon,305-343,RepublicofKorea;3.InstituteforMaterialsChemistryandEngineering,KyushuUniversity,Kasuga,Fukuoka,816-8580,Japan)
A membrane-assisted capacitive deionization (CDI) system was developed for the purification of water containing sodium chloride using activated carbon fibers (ACFs) as capacitor electrode materials. The ACFs have different degrees of activation with different surface areas and pore size distributions. Their desalination performance for sodium or chloride ions was investigated. Results indicate that the salt removal efficiency and surface area-normalized electrosorption capacity for each ion depend on the surface area, pore depth and the match between the pore sizes of the ACFs and the radius of each hydrated ion. A high surface area and shallow pores favor the salt removal efficiency and a high surface area-normalized electrosorption capacity. The ACF with a median pore size of 0.69 nm performs best for sodium ion removal and those with median pore sizes of 1.09 and 1.52 nm are best for chloride ion removal, which could be ascribed to the fact that the radius of a hydrated sodium ion (0.66 nm) is smaller than that of a hydrated chloride ion (0.72 nm). An asymmetric electrode material configuration is needed to optimize both the anion and cation adsorption in the membrane-assisted CDI system.
Capacitive deionization; Activated carbon fiber; Pore size; Sodium and chloride ions; Asymmetric electrode
Doo-Hwan Jung. E-mail: doohwan@kier.re.kr
date: 2016-06-28:Reviseddate: 2016-07-28
10.1016/S1872-5805(16)60020-3
1007-8827(2016)04-0378-08
TQ127.1+1
A
Doo-Hwan Jung. E-mail: doohwan@kier.re.kr
Supplementary data associated with this article can be found in the online version.
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
