磁敏感加权成像在帕金森病脑核团铁含量的定量研究
2016-10-21唐守现左丽君张巍沈慧聪戴建平
唐守现,左丽君,张巍,沈慧聪,戴建平*
磁敏感加权成像在帕金森病脑核团铁含量的定量研究
唐守现1,左丽君2,张巍2,沈慧聪1,戴建平1*
目的 利用磁敏感加权成像测量和分析PD脑深部核团铁含量特点。材料与方法 研究对象为40例PD患者和36例正常志愿者,利用3.0 T磁共振扫描仪获取研究对象SWI图像,通过SPIN软件在获取的SWI相位图中测量脑深部灰质核团苍白球、壳核、黑质相位值,并将获取的研究对象核团相位值进行配对样本t检验,P<0.05为差异具有统计学意义。结果 PD组黑质相位值平均值最小(R,2034.84;L,2030.55)。PD组与对照组相比,右侧苍白球(P=0.018)、黑质相位值(P=0.008)和左侧苍白球(P=0.040)、黑质相位值(P=0.000)均具有显著性差异。PD组右侧苍白球与右侧黑质(P=0.000)、右侧壳核与右侧黑质(P=0.000)、左侧壳核与左侧黑质(P=0.000)相位值对比差异具有统计学意义。结论 PD病患者黑质铁含量最多,苍白球和黑质铁含量成对称性增多。磁敏感加权成像可为PD病诊断提供参考信息。
帕金森病;磁共振成像;铁
ACKNOWLEDGMENTS This work was part of the General Program of National Natural Science Foundation of China (No. 81271541).
唐守现, 左丽君, 张巍, 等. 磁敏感加权成像在帕金森病脑核团铁含量的定量研究. 磁共振成像, 2016, 7(9): 647-650.
1 材料与方法
1.1一般资料
选取2014年至2015年就诊于北京天坛医院的40例PD患者为研究对象,研究对象诊断均符合1992年英国PD脑库制定的原发性PD诊断标准。PD病诊断标准:(1)运动迟缓(随意运动、进行性语言和重复动作幅度变小);(2)至少符合下列表现之一,肌强直、4~6 Hz静止性震颤及姿势不稳(并非由视觉、前庭功能、小脑或本体感觉障碍引起)。PD诊断确立的支持诊断标准(确诊PD需满足以下3条或以上):单侧起病;存在静止性震颤;进行性病程;症状长期不对称,起病一侧症状明显;对左旋多巴反应良好(70%~100%);出现左旋多巴诱导的舞蹈症;对左旋多巴有反应持续5年或以上;临床病程10年或以上。
选取2011年至2015年在我院行头颅MRI及SWI检查年龄匹配正常志愿者36例。入选标准:(1)无阳性神经系统症状和体征;(2)无神经系统疾病史。
1.2扫描和处理
利用Siemens 3.0 T Trio扫描仪获取图像,8通道线圈,标准头部解剖位,行SWI序列横断扫描。三轴定位系统,经正中矢状面,平行于前后联合连线;扫描参数:TR 29 ms,TE 20 ms,FOV 230 mm× 230 mm,反转角15°,层厚5 mm,间隔2 mm;图像重建像素448×512。
利用SPIN软件分别测量相位图上感兴趣区(region of interest,ROI)黑质、苍白球、壳核相位值(图1,2),神经放射专业医师勾画ROI (ROI大小由核团决定),软件自动测量ROI相位值三次,并取其均值。
1.3统计学方法
采用SPSS 17.0软件进行统计分析。测得相位值采用均数±标准差表示,并进行配对样本t检验,P<0.05为差异具有统计学意义。
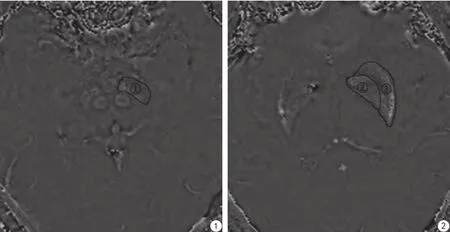
图1,2 显示测试ROI,标号①、②、③分别为左侧黑质,左侧苍白球、左侧壳Fig. 1,2 Marks ①, ②, ③ indicated left SN, left GP, left PUT.
2 结果
PD患者男22例 (55%),女18例(45%),年龄40~78岁,平均61.8岁,病程0.5~26.0年,平均5年;临床均表现静止性震颤和肌肉强直。
PD组测得黑质相位值平均值最小,对照组黑质相位值左右侧差异具有显著性。在PD组与对照组之间右侧苍白球、黑质相位值和左侧苍白球、黑质相位值差异均具有显著性,壳核相位值差异无统计学意义(表1)。PD组右侧苍白球和右侧黑质、右侧壳核和右侧黑质、左侧壳核和左侧黑质相位值配对对比差异具有显著性,对照组左侧苍白球和左侧黑质相位值配对对比差异具有显著性(表2)。
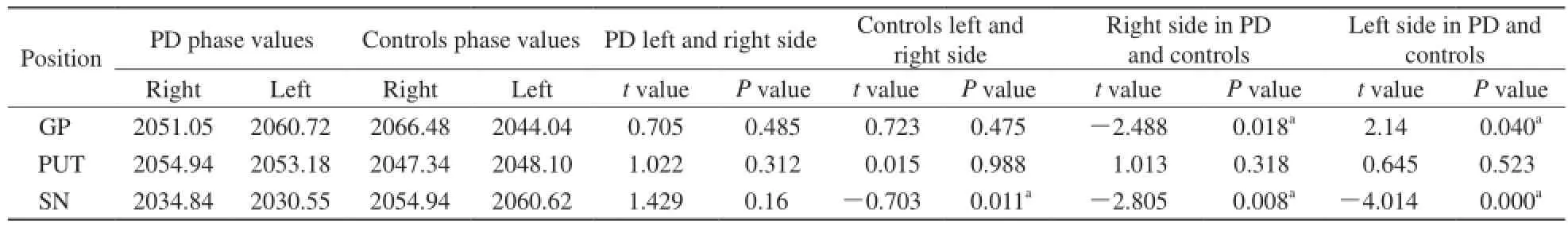
表1 PD组与对照组核团平均相位值Tab. 1 Phase values in nucleus of the PD subjects and controls
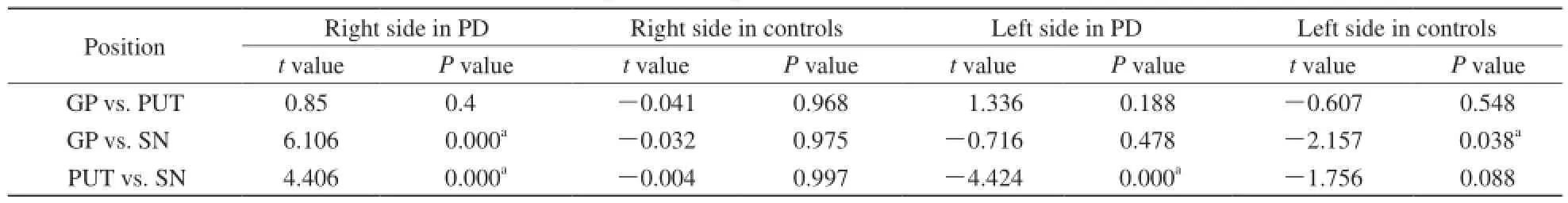
表2 不同核团之间相位值比较Tab. 2 Comparison of phase values in different nucleus
3 讨论
研究结果表明PD患者黑质铁含量明显增加。PD组测得黑质相位值平均值较对照组小,而相位值和铁含量呈现负相关,因此PD患者同侧黑质铁含量较对照组铁含量高。这一结果与目前研究报道PD患者黑质铁含量异常增加相一致,有研究报告表明PD患者黑质多巴胺能神经元变性与铁含量异常增加密切相关[6-7,12]。以往研究报告黑质为PD病变部位及黑质铁含量异常沉积[13-14]。而利用SWI序列测量PD患者黑质含量并与对照组对比的研究报道结果存在差异。通过1.5 T磁共振扫描仪,Gupta等[15]发现PD患者黑质铁含量与正常对照组相比改变不明显,相反,Jin等[16]得出黑质铁含量增加的结论,Wang等[17]利用3.0 T扫描仪和Lotfipour等[18]利用7 T扫描仪同样发现黑质铁含量增加。
研究结果表明PD患者苍白球铁含量异常增加,壳核铁含量无明显异常。PD患者同侧苍白球相位值均较对照组明显降低,壳核相位值与对照组相比差异无统计学意义。苍白球铁含量较对照组增高,这与以往研究PD患者病变部位累及苍白球、苍白球铁含量异常结论一致[13-14]。可见通过对苍白球、壳核相位值测试可发现是否有相应部分铁含量异常沉积。这也进一步说明SWI图像对于PD病的诊断可提供参考信息[19], PD患者苍白球铁含量相对增加,壳核铁含量不见增加,而有研究报道多系统萎缩伴PD症状患者苍白球和壳核相位值均可见明显增加[15]。
PD患者病变核团铁含量增加具有对称性特点。我们测量结果表明同一核团左右侧对比分析显示仅对照组黑质左右相位值差异具有显著性。PD患者黑质、苍白球左右侧对称性铁含量差异无统计学意义,同时均较对照组含量有所增加,这与PD患者病变核团对称性特点相一致。对照组黑质左右侧相位值有差异,左侧黑质和苍白球相位值有差异,这与铁在人脑内分布有差异性及随龄增加性特点相符[1-2,20]。
研究不足之处:一是偶然性的苍白球生理性钙化可能导致感兴趣相位值测量存在差异。二是研究对象病程和治疗期限并没有进行考虑。三是测量部位核团数量相对较少。
总之,PD患者出现临床症状时,双侧黑质、苍白球铁含量异常。黑质铁含量最高,苍白球和黑质铁含量成对称性增高。证明了SWI可发现PD患者灰质核团铁含量异常增加,可为PD病诊断提供重要的参考信息。
[References]
[1] Hagemeier J, Geurts JJ, Zivadinov R, et al. Brain iron accumulation in aging and neurodegenerative disorders. Expert Rev Neutother, 2012, 12(12): 1467-1480.
[2] Mao L, Dai JP, Sun B. Susceptibility-weighted imaging:a study of cerebral iron content in healthy people. Chin J Med Imaging Technol, 2009, 25(6): 996-998.毛磊, 戴建平, 孙波. 磁敏感加权成像观察健康人群脑内铁含量.中国医学影像技术, 2009, 25(6): 996-998.
[3] Zhang W, Sun SG, Jiang YH, et al. Determination of brain iron content in patients with Parkinson's disease using magnetic susceptibility imaging. Neurosci Bull, 2009, 25(6): 353-360.
[4] Liu TY, Bai Y, Ma XY, et al. The value of "swallow tail" appearance of nigrosome on ESWAN at 3.0 T MR in the diagnosis of Parkinson's disease. Chin J Magn Reson Imaging, 2016, 7 (46): 265-269.刘太元, 白岩, 马潇越, 等. 3.0 T MR非高分辨ESWAN上黑质“燕尾征”在帕金森病诊断中的价值. 磁共振成像, 2016, 7(46): 265-269.
[5] Fang XW, Hu T, Wu YH, et al. Metabolic changes of striatum and substantia nigra in patients with early Parkinson's disease demonstrated by 1H-MRS. Chin J Magn Reson Imaging, 2015, 6(7): 481-485.方学文, 胡涛, 武玉华, 等. 1H-MRS检测早期帕金森病纹状体、黑质的功能代谢. 磁共振成像, 2015, 6(7): 481-485.
[6] Gao JH, Yan ZF, Liu Z, et al. Investigation on the neuroinfammatory mechanism of iron-induced selective dopaminergic neurodegeneration. Chin J Neurol, 2011, 44(7): 493-499.高俊华, 闫兆芬, 刘卓, 等. 铁选择性损伤多巴胺能神经元的神经免疫炎症机制. 中华神经科杂志, 2011, 44(7): 493-499.
[7] Peng J, Peng L, Stevenson FF, et al. Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson's disease accelerate age-related neurodegeneration. J Neurosci, 2007, 27(26): 6914-6922.
[8] Langkammer C, Krebs N, Goessler W, et al. Quantitative MR imaging of brain iron a postmortem validation study. Radiology, 2010, 257(2): 455-461.
[9] Schafer A, Forstmann BU, Neumann J, et al. Direct visualization of the subthalamic nucleus and its iron distribution using highresolution susceptibility mapping. Hum Brain Mapp, 2012, 33(12): 2831-2842.
[10] Huang YW, Jeng JS, Tsai CF, et al. Transcranial imaging of substantia nigra hyperechogenicity in a Taiwanese cohort of Parkinson's disease. Mov Disord, 2007, 22(4): 550-555.
[11] Haacke EM, Xu Y, Cheng YC, et al. Susceptibility weighted imaging (SWI). Magn Reson Med, 2004, 52(3): 612-618.
[12] Oakley AE, Collingwood JF, Dobson J, et al. Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology, 2007, 68(21): 1820-1825.
[13] Cosottini M, Frosini D, Pesaresi I, et al. MR imaging of the substantia nigra at 7 T enables diagnosis of Parkinson disease. Radiology, 2014, 271(3): 831-838
[14] Rossi M, Ruottinen H, Soimakallio S, et al. Clinical MRI for iron detection in Parkinson's disease. Clin Imaging, 2013, 37(4): 631-636.
[15] Gupta D, Saini J, Kesavadas C, et al. Utility of susceptibilityweighted MRI in differentiating Parkinson's disease and atypical parkinsonism. Neuroradiology, 2010, 52(12): 1087-1094.
[16] Jin L, Wang J, Zhao L, et al. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson's disease. Brain, 2011, 134(1): 50-58.
[17] Wang Y, Butros SR, Shuai X, et al. Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. AJNR Am J Neuroradiol, 2012, 33(2): 266-273.
[18] Lotfipour AK, Wharton S, Schwarz ST, et al. High resolution magnetic susceptibility mapping of the substantia nigra in Parkinson's disease. J Magn Reson Imaging, 2012, 35(1): 48-55.
[19] Meijer FJ, van Rumund A, Fasen BA, et al. Susceptibility-Weighted Imaging Improves the Diagnostic Accuracy of 3T Brain MRI in the Work-Up of Parkinsonism. AJNR Am J Neuroradiol, 2015, 36(3): 454-460.
[20] Xu XJ, Wang QD, Zhang MM. Age, gender, and hemispheric differences in iron deposition in the human brain: an in vivo MRI study. Neuroimage, 2008, 40(1): 35-42.
A quantitative study about iron content of the cerebral nucleus in patients with Parkinson's disease
TANG Shou-xian1, ZUO Li-jun2, ZHANG Wei2, SHEN Hui-cong1, DAI Jian-ping1*
1Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100050, China
2Department of Geriatric, Beijing Tiantan Hospital, Capital Medical University, Beijing 100050, China
*Correspondence to: Dai JP, E-mail: daijianping_2008@126.com
Objective: Aiming to measure and analyse the iron content in cerebral nucleus of the Parkinson's disease (PD) subjects. Materials and Methods: Forty PD subjects and 36 volunteers were recruited in the cross-sectional study. Susceptibility weighted images (SWI) was acquired for all participants. Phase values of globus pallidum (GP), putamen (PUT), substantia nigra (SN) were measured by SPIN software. Paired-sample t test was used to compare the phase values of nucleus, P<0.05 was considered to be statistically signifcant. Results: Phase values in SN of PD subjects were least (right, 2034.84. left, 2030.55). Phase values of bilateral GP (R, P=0.018. L, P=0.040) and bilateral SN (R, P=0.008. L, P=0.000) showed signifcantly statistical differences between PD subjects and volunteers. There were significantly statistical differences in phase values between right GP and SN (P=0.000), right PUT and SN (P=0.000), left PUT and SN (P=0.000) in PD subjects. Conclusions: SN showed highest iron content in patients with PD, iron content increased symmetrically in GP and SN of patients with PD. SWI provided information for the diagnosis of PD.
Parkinson disease; Magnetic resonance imaging; Iron
1 June 2016, Accepted 10 Aug 2016
国家自然科学基金面上项目(编号:81271541)
1. 首都医科大学附属北京天坛医院放射科,北京 100050
2. 首都医科大学附属北京天坛医院老年病科,北京 100050
戴建平,E-mail:daijianping_2008@ 126.com
2016-06-01
接受日期:2016-08-10
R445.2;R742
A
10.12015/issn.1674-8034.2016.09.002
铁在人脑中分布呈随龄增加性[1-2],并具有部位差异性,按含量从高至低依次为苍白球(globus pallidum,GP)、壳核(putamen,PUT)、黑质(substantia nigra,SN)、尾状核(caudate nucleus)、大脑白质(white matter)及小脑(cerebellum)[3]。其中苍白球、壳核及黑质是脑内多巴胺能神经元最富集部位, 纹状体和黑质也是PD病变主要部位之一[4-5]。而PD病患者出现临床症状时黑质多巴胺能神经元变性丢失约70%。同时实验证明铁离子可以诱发氧化应激机制损伤神经元,同时催化多巴胺氧化为毒性6-羟基-DA(MPTP)和异喹啉样物质,诱导多巴胺能神经元变性死亡。研究发现多种内外源性物质与铁结合作用通过神经免疫炎症机制进行性损伤多巴胺能神经元[6-7]。因此铁含量异常可能促使PD深部核团神经元变性。目前脑内铁沉积测量方法主要有R2及R2*值测量[8]、磁敏感加权成像相位值测量[9]、经颅超声成像测量[10]。磁敏感加权成像(susceptibility weighted imaging,SWI)是以T2*序列为基础并进行改进的磁共振扫描成像技术,包括相位值图像和磁矩值图像。相位值图像中相位值反映不同组织间磁敏感性细微差异,通过校正测得的相位值大小与脑铁沉积呈负相关[11],进而可以间接测量反映脑内核团铁含量。文章通过利用SWI技术测量PD患者黑质、苍白球和壳核核团相位值进而分析铁含量特点。
