Beneficial effect of curcumin in letrozole induced polycystic ovary syndrome
2016-10-18SushmaReddyNaziaBegumSumithMuthaVasudhaBakshi
P. Sushma Reddy, Nazia Begum, Sumith Mutha, Vasudha Bakshi
1Department of Pharmacology, School of Pharmacy, Anurag Group of Institutions, 500088, Hyderabad, India
2School of Pharmacy, Anurag Group of Institutions, 500088, Hyderabad, India
Beneficial effect of curcumin in letrozole induced polycystic ovary syndrome
P. Sushma Reddy1, Nazia Begum1, Sumith Mutha1, Vasudha Bakshi2*
1Department of Pharmacology, School of Pharmacy, Anurag Group of Institutions, 500088, Hyderabad, India
2School of Pharmacy, Anurag Group of Institutions, 500088, Hyderabad, India
ARTICLE INFO
Article history:
PCOS
Letrozole
Curcumin
Cysts
ABSTRACT
Objective: To investigated beneficial effect of Curcumin (a phenolic curcuminoid derivative from Curcuma longa) in Letrozole induced PCOS in female wistar rats. Methods: Letrozole (1mg/kg ) was administered per orally (p.o) for a period of 21days for the induction of PCOS, followed by dose of Curcumin (100 mg/kg and 200 mg/kg, p.o.) for 15 days using 0.5% w/v CMC as vehicle. Results: The administration of Letrozole led to abnormalcy in serum sex steroid profile, lipid profile, glucose and glycosylated haemoglobin levels and depletion in antioxidant activity. Curcumin was able to successfully exert its protective effect by restoring all the parameters to normal and disappearance of cysts in ovaries. Conclusion: Curcumin showed beneficial effects in Letrozole induced PCOS in female wistar rats.Its effect was comparable to that of Clomiphene citrate, most widely used treatment for ovulation induction in PCOS condition.
Document heading doi: 10.1016/j.apjr.2016.01.006
1. Introduction
Polycystic Ovary Syndrome (PCOS) is a common heterogenous endocrinological and metabolic disorder in women of reproductive age leading to infertility/subfertility. Women (5%-10%) of reproductive age are affected by PCOS [1, 2]. Clinical manifestations of PCOS include infrequent or absent menses, abdominal obesity, acanthosis nigricans and signs of androgen excess (hyperandrogenism) which include acne or seborrhea and insulin resistance [3-7]. Long term consequences include increased risk of endometrial cancer, type 2 diabetes mellitus, dyslipidemia, hypertension and cardiovascular disorders [8-9]. The etiology of PCOS is not clearly understood, but lipid imbalance, oxidative stress, insulin resistance and genetics are some of the contributing factors.
Various experimental models for PCOS have been developed in rats like administration of Estradiol Valerate, DHEA and prepubertal androgen excess [10]. Even though these models induce PCOS, none of them are fully convincing and identify with the conditions of human PCOS completely.
Letrozole, a non-steroidal aromatase inhibitor produces a PCOS model which in numerous ways depicts human PCOS [11]. It blocks conversion of testosterone and androstenedione to estradiol and estrone respectively and simulates PCOS like condition [12] by causing hormonal imbalance, circulating hyperandrogenism and intra ovarian androgen excess leading to appearance of polycystic ovary. Follicular atresia and abnormal follicular development is observed due to induced elevation of androgen levels inside the ovary [17]. Letrozole induction was reported to cause hyperglycemic condition which may contribute to insulin resistance, hyperlipidemia leading to metabolic syndrome [14, 15].Currently, many therapies are in use to manage PCOS condition and to induce ovulation. But these therapies have been reported to cause severe side effects ranging from arthritis, joint or muscle pain [16] and psychological disturbances [17]. Therefore, now-a-days focus is being laid on medicines from natural sources which show minimal or no side effects.
Curcumin is a water-insoluble, low molecular weight, polyphenolic curcuminoid derivative found in rhizomes of Indian spice, Curcuma longa (turmeric). Turmeric is extensively used as a food additive and coloring agent in Asian cuisine [18] and also in Indian herbal medicine. Curcumin constitutes to about 2%-8% of turmeric preparations.
Curcumin has been reported to possess a wide variety of biological effects like anti-inflammatory, anti-oxidant [19], hypoglycaemic [20] and antihyperlipidemic activities. Curcumin exhibits antiproliferative and apoptotic activities in several human cancer cell lines, like those derived from cancers of prostate, breast and ovary[21,22]. Recent study showed protective effect of Curcumin on Porcine Ovarian granulosa cells [23]. Apart from these, estrogenic effects of Curcumin were observed on breast cancer cell lines [24]. In this study we hypothesised that Curcumin may be beneficial in management of PCOS induced by Letrozole due to the reported activities.
2. Materials and methods
2.1. Experimental animals
Virgin, cyclic, adult female Wistar Albino rats (160-200 g) were employed for the study. Animals were acquired from Animal house of Teena Labs, Kukatpally, Hyderabad and housed in our institution’s animal house and allowed to acclimatise for two weeks. During the study all animals were caged in standard polypropylene cages and maintained in controlled environment of (22±3)˚C temperature, (55 ± 5)% humidity and a 12 hour light/dark cycle. They were fed with standard diet and water provided ad libitum. The study was duly approved by Institutions Animal Ethics Committee for the use of animals and care of the animals was carried out as per the guidelines of committee for the purpose of control and supervision of experiments on animals (CPCSEA) with protocol number. I/ IAEC/LCP/016/2013/WR-30.
2.2. Drugs and reagents
Curcumin was acquired from Sigma Chemiclas Co., St. Louis, MO, USA. Letrozole was obtained from Natco Pharma Limited, Hyderabad. Clomiphene Citrate (Fertyl-Super) tablets were procured from Ar-Ex Laboratories Private Limited, Goregaon(E), Mumbai. All other chemicals used were of analytical grade. The Glucose, Cholesterol, Triglycerides, HDL and Glycosylate Hemoglobin kits were obtained from ERBA Diagnostic, USA.
2.3. PCOS induction
All the experimental animals except control group, were orally administered with Letrozole at a dose of 1mg/kg dissolved in 0.5% Carboxy Methyl Cellulose (CMC) once daily for 21 days [12]. Control group received vehicle only (0.5% CMC). Vaginal Smears were collected daily and evaluated microscopically using Giemsa stain to confirm the induction of PCOS.
2.4. Study design
The study consisted of 30 female albino Wistar rats equally divided into five groups designated as group 1 (served as control group), group 2 (served as PCOS induced group), group 3 (served as standard group), groups 4 and 5 served as treatment groups. Following letrozole administration, standard group was administered with Clomiphene Citrate at a dose of 1 mg/kg in 0.5% CMC per oral and treatment groups 4 and 5 were administered Curcumin with the dose of 100 mg/kg (Low Dose) and 200 mg/kg (High Dose) body weight respectively in 0.5% CMC per oral for 15 days i.e., from day 22 to day 36.
After 21 days, PCOS control group and after 36 days, animals from other groups were fasted overnight and anaesthetised with diethyl ether. Blood was collected by retino orbital puncture and serum was separated by centrifugation and was used for estimation of hormones, glucose, glycosylated haemoglobin and lipid parameters. The animals were then sacrificed, ovaries and uterus excised, cleaned of fat and weighed. After excision, ovaries were freed from blood and cleaned with ice cold saline and homogenised using 10% ice cold potassium chloride for antioxidant and TBARS evaluation.
2.5. Measurement of invasive blood pressure and heart rate
At the end of the study, four animals from each group were anesthetized with Ketamine. Arterial blood pressure (BP) was recorded from carotid artery. A polyethylene catheter (PE-50[1mm O.D.] for rats was attached to a pressure transducer (both were filled with heparinised saline), and inserted into the carotid artery and tied in place. Pressure fluctuations in the artery were transmitted along the catheter tubing to the transducer’s diaphragm, which moved in response. The diaphragm movements were converted into a varying electrical signal that was amplified through a bridge amplifier and recorded by a Power lab system (AD instruments, Australia).
2.6. Biochemical estimations
2.6.1. Hormonal assay
Hormones were assayed by Competitive Chemiluminescent Immunoassay using automated instrument ADVIA Centaur, SiemensHealthcare Diagnostics Inc., USA. The testosterone was estimated using ADVIA Centaur TSTO kit, estrogen using ADVIA Centaur E2-6 kit, and progesterone using ADVIA Centaur PRGE kit.
2.6.2. Measurement of fasting blood glucose (FBG)
FBG was measured by Trinder’s method using a commercial diagnostic kit from ERBA Diagnostics, USA.
2.6.3. Measurement of glycosylated hemoglobin (HbA1c) levels
HbA1c was assayed by cation-exchange method using a diagnostic kit from ERBA Diagnostics, USA.
2.6.4. Assessment of lipid profile
Lipid profile [total-cholesterol (TC), triglycerides (TG), and HDL-cholesterol (HDL-C)] were estimated by using enzymatic kits procured from ERBA Diagnostics, USA. LDL-cholesterol (LDL-C) was calculated by using Friedewald’s equation.
2.6.5. Antioxidant assay
2.6.5.1. Superoxide dismutase
Superoxide dismutase activity was determined by the pyrogallol oxidation method. This is an indirect method that is based on the ability of the enzyme to inhibit the auto-oxidation of pyrogallol. Superoxide dismutase activity was determined by monitoring the rate of oxidation of pyrogallol by superoxide radicals. The reaction is initiated by adding pyrogallol and the change in optical density was recorded at 420 nm [25].
2.6.5.2. Catalase
Catalase activity was determined in 50 ml of sample mixed with 50 ml of substrate for 60s, then 100 ml of 32.4 mM ammonium molybdate solution was added and absorbance change was measured at 405 nm. One unit of the enzyme was defined as µmoles of H2O2 degraded/min/mg of protein [25].
2.6.5.3. Reduced glutathione (GSH)
Glutathione content was estimated according to a previously reported method (Moren et al., 2012). 0.25ml of 10% ovarian homogenate was added to equal volume of ice cold 5% Trichloroacetic acid (TCA). The precipitate was removed by centrifugation at 4000 rpm for 10 minutes. To 1 ml aliquot of supernatant, 0.25ml of 0.2M phosphate buffer, pH 8.0 and 0.5ml of 5, 5’-Dithio-bis 2-nitrobenzoic acid (DTNB) was added and mixed well. The absorbance was read at 412 nm using spectrophotometer. The values were expressed in units/mg protein.
2.6.6. Assay of thiobarbituric acid reacting species (TBARS) content
The method used to estimate the rate of Lipid peroxidation (LPO) was as mentioned below. Homogenate (0.25 mL) was pipetted into 15×100 mm test tubes and incubated at 37°曟 in a metabolic shaker for 1 hr. An equal volume of homogenate was pipetted into a centrifuge tube, placed at 0°曟 and marked at 0 h incubation. After 1 hr of incubation, 0.5 ml of 5% (w/v) chilled trichloroacetic acid (TCA), followed by 0.5 mL of 0.67% TBA (w/v) was added to each test tube and centrifuge tube, and centrifuged at 1 000 × g for 15 min. Thereafter, the supernatant was transferred to other test tubes and was placed in a boiling water bath for 10 min. The absorbance of pink color produced was measured at 535 nm in a spectrophotometer (Shimadzu-1601, Japan). The TBARS content was calculated by using a molar extinction coefficient of 1.56伊105M-1cm-1and expressed as n mol of TBARS formed min-1mg-1of protein [25].
2.7. Ovarian histomorphology
Excised ovaries were fixed in 10% Neutral Buffered Formalin. They were subjected to tissue processing by dehydration through an ascending ethanol series, clearing in xylene and embedding completely in paraffin wax into blocks. The blocks were then serially sectioned at 5µm thickness using microtome and were mounted on poly-lysine coated slides, deparaffinised using xylene, rehydrated and stained with haematoxylin and eosin, dehydrated, cleared and mounted on DPX under glass cover slips. The slides were then observed under light microscope connected to a camera to capture images.
2.8. Statistical analysis
The data was statistically analysed using one-way ANOVA followed by Newman-Keuls multiple comparison tests and expressed as mean ± standard error of mean. P<0.05 was considered to be statistically significant. The statistical analysis was carried out with Graph pad prism 5.0 software.
3. Results
3.1. Arterial blood pressure and heart rate
There was no significant difference among the control group and PCOS induced group in terms of arterial BP and Heart Rate.
3.2. Organ weights
In terms of ovarian weights, there were no significant changes among the groups. However, Letrozole treatment advanced to a significant decrease in uterine weight (P<0.01) as compared to control group. Repeated administration of Low dose (P<0.05) and High dose (P<0.01) of curcumin significantly inhibited the decrease in uterine weight (Figure 1).

Figure 1. Effect of various treatments on uterine weights.
3.3. Serum hormonal profile
The serum levels of Testosterone were remarkably increased in PCOS induced group (P<0.001) while those of Progesterone and Estradiol decreased significantly (P<0.01 and P<0.05 respectively) in comparision to the control group. A significant fall (P<0.001) in testosterone levels was observed in standard, low dose and high dose groups. Progesterone levels were also increased significantly (P<0.01) in all the treatment groups i.e., in groups 3, 4 and 5. Only standard group and High dose group showed significant increase (P<0.05) in estradiol levels when compared to PCOS induced group (Table 1).
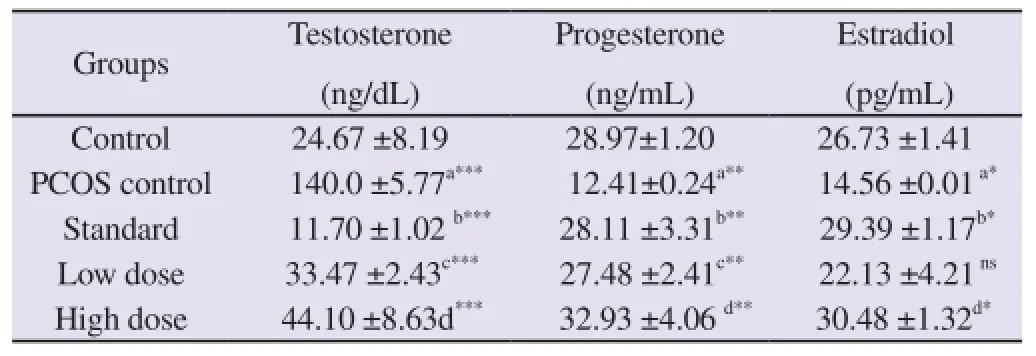
Table 1 Effect of various treatments on serum sex steroids.
3.4. Curcumin reduces FBG and HbA1c levels in serum
Figure 2 and 3 interpret the effect of Curcumin on serum glucose and HbA1c levels respectively. PCOS induced group showed significant increase in glucose level (P<0.001) and HbA1c level (P<0.01) in comparision to control group. All the treatment groups exhibited significant decrease in levels of both the parameters (P<0.001) when compared to PCOS induced group.
3.5. Curcumin normalised serum lipid profile
Letrozole treatment caused significant changes in serum lipid as compared to control. TG’s, TC and LDL were greatly increased as P<0.001, P<0.001 and P<0.01 respectively while HDL levels were notably decreased (P<0.001) in PCOS induced group. Table 2 portrays effect of Curcumin on lipid profile. Clomiphene treatment significantly decreased TG (P<0.001), TC (P<0.001) and LDL (P<0.01) levels when compared to PCOS induced group. Low dose of Curcumin decreased the levels of TG (P<0.001), TC (P<0.01) and LDL (P<0.01) significantly but did not affect HDL levels. High dose of Curcumin significantly reduced TG, TC and LDL levels (P<0.001). It also increased HDL levels significantly (P<0.001) in comparision to PCOS induced group.
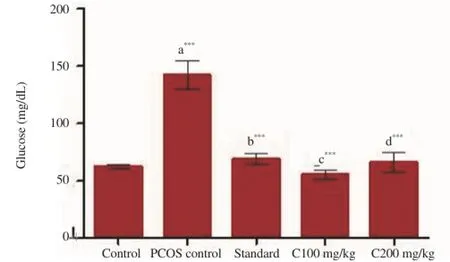
Figure 2. Effect of various treatments on serum fasting blood glucose levels.
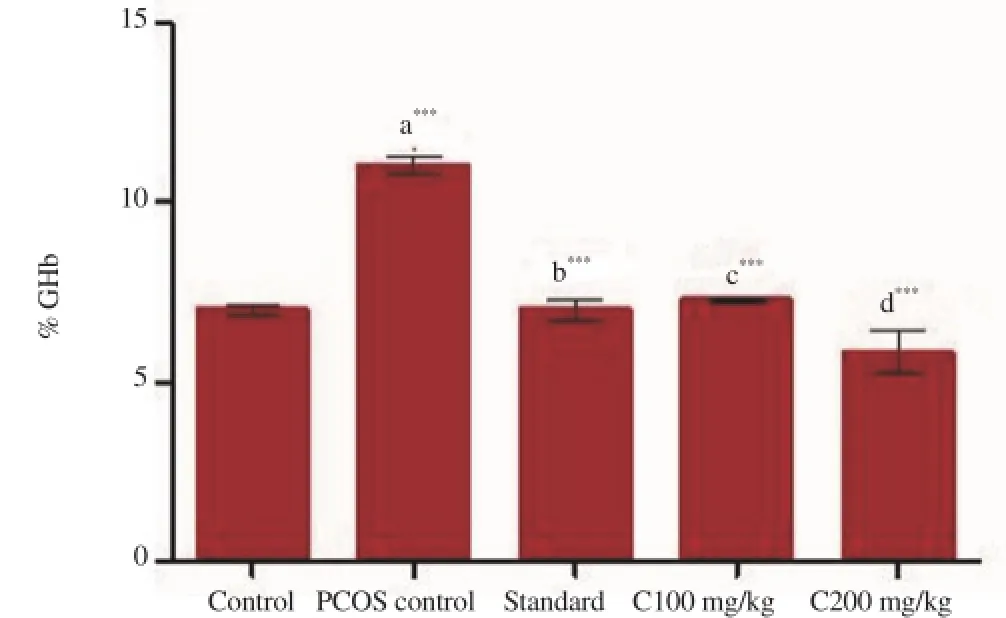
Figure 3. Effect of various treatments on serum glycosylated hemoglobing levels.

Table 2 Effect of various treatments on serum lipid profile.
3.6. Effect of curcumin on antioxidant activity and lipid peroxidation (TBARS)
Table 3 illustrates effect of Curcumin on antioxidant activity and lipid peroxidation. PCOS induced group showed conspicuous depletion in antioxidant enzyme activity- SOD (P<0.05), GSH (P<0.01), Catalase (P<0.01) and increase in lipid peroxidation (P<0.01). Standard group could restore only Catalase (P<0.05) and
TBARS (P<0.01) activity close to those in the control group. Low dose (100mg/kg) showed its after effect by increasing the activity of SOD (P<0.05), Catalase (P<0.05) and TBARS (P<0.01). High dose (200 mg/kg) potentially augmented the antioxidant enzyme activity significantly (P<0.01) and reduced TBARS level (P<0.001) when compared to control group [25].
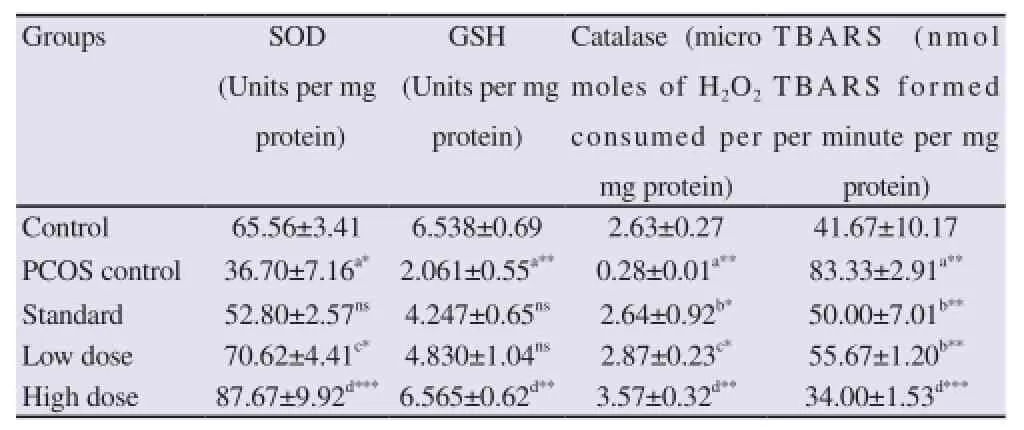
Table 3 Effect of various treatments on antioxidation and lipid peroxidation.
3.7. Histomorphological changes
Sections of ovaries from control group animals showed healthy follicles with oocyte at different stages of development (Figure 4). Letrozole treated rats exhibited numerous subcapsular cysts, with a very thin or no granulosa layer (Figure 5). Corpora lutea were completely absent indicating anovulation. Few follicles were observed at their early stages of development. In addition, they were accompanied with atretic follicles containing fluid filled antrum and higher incidence of pyknotic granulosa cells. Clomiphene citrate treatment led to disappearance of cysts and appearance of healthy follicles and corpora lutea. Sections from low dose of Curcumin (100 mg/kg) group exhibited follicles larger in size and few corpora lutea (Figure 6). Cysts were absent and normal sized healthy follicles at different developmental stages with oocytes were found in section from high dose (200 mg/kg) group (Figure 7). Also with the high dose many corpora lutea and antral follicles with clearly differentiated oocyte, granulosa cell layer, corona radiate, cumulus oophorus and thecal cells were observed.
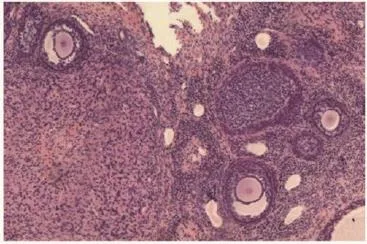
Figure 4. Section of ovary from control animal showing follicles (H&E 伊10).
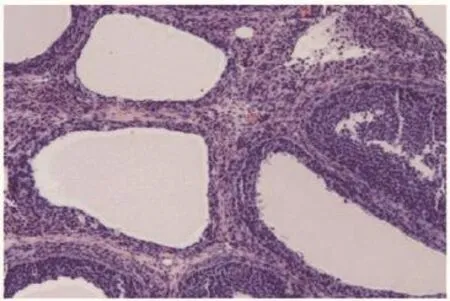
Figure 5. Section of ovary from PCOS induced animal showing multiple cysts (H&E 伊10).
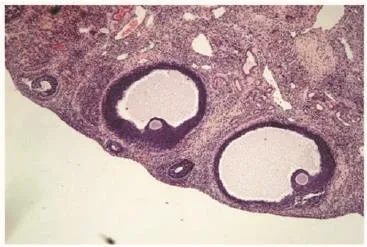
Figure 6. Section of ovary from curcumin treated (100 mg/kg) animal showing large follicles (H&E 伊5).
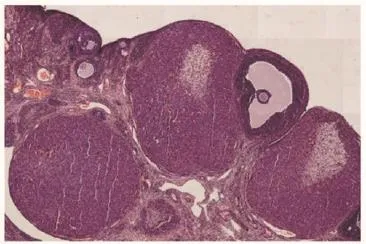
Figure 7. Section of ovary from curcumin treated (200 mg/kg) animal showing follicles at different developmental stages and corpora lutea (H & E伊10).
4. Discussion
In the present study, Letrozole- aromatase inhibitor, was used to induce Polycystic Ovary Syndrome in female Wistar rats. Previous reports suggest that Letrozole induced PCOS condition depicts human PCOS in many ways [12]. The working of this model was confirmed by regular examination of vaginal smears and presence of persistent vaginal cornification. As evidenced, there was a marked increase in testosterone levels when compared to control animalsindicating the hyperandrogenism status in PCOS condition.
Curcumin was able to normalize serum Testosterone levels similar to that of Clomiphene citrate. Serum levels of Progesterone and Estradiol were decreased in PCOS induced group. These results are in accordance with the earlier studies [12, 26-28]. Decreased progesterone levels are also indicative of anovulation[10] and Curcumin successfully restore its level to normal. Decreased estradiol concentration due to inhibition of aromatase in PCOS induced group was significantly increased by repetitive administration of high dose (200 mg/kg) of Curcumin, confirming its earlier reported phytoestrogenic activity [24]. Even though, there were no significant changes in ovarian weights, uterine weights were reduced due to Letrozole treatment [12, 29]. Curcumin treatment significantly increased the uterine weights which matched to those in control animals.
PCOS is also a metabolic disorder associated with type 2 diabetes mellitus [30] and manifests as hyperglycemia in initial stages that leads to insulin resistance gradually. In our study PCOS induced animals showed marked increase in fasting blood glucose and glycosylated haemoglobin levels. This is in congriuty with the earlier findings that reported induction of hyperglycemia in Letrozole induced PCOS rats[15]. Hence, our study also examined serum glucose levels as well as glycosylated haemoglobin levels because in hyperglycemic condition excess of glucose present in blood reacts with haemoglobin to form glycosylated haemoglobin[31]. Oral administration of Curcumin significantly prevented the rise in levels of fasting blood glucose and HbA1c, which indicates beneficial effect of Curcumin in preventing insulin resistance and diabetic complication.
One of the consequences of PCOS is dyslipidemia. Imbalances in lipid profile are attributed to hyperandrogenemia[32, 33]. Present study exhibited similar results in lipid profile. PCOS induced group showed notable increase in TC, TG’s, LDL and decrease in HDL levels. Curcumin displayed its antihyperlipidemic action by considerably decreasing serum TC, TG’s, LDL while increasing HDL levels.
Many studies reported oxidative stress as one of the pathological factor for PCOS [34, 35]. Increased oxidant levels may alter the stereo diagnosis in ovaries contributing to increased androgen production and polycystic ovaries [34]. In the present study, it was observed that the PCOS animals exhibited elevated oxidative stress markers and reduced endogenous antioxidants in ovary. SOD, Catalase and GSH activity were significantly diminished in the PCOS group and concominant treatment with Curcumin restored their activities. This is in unison with the earlier reported antioxidant activity of Curcumin [19].
Lipid Peroxidation is generally used as one of the marker for oxidative tissue damage, as it induces free radical damage to the components of cell membrane which leads to cell necrosis and inflammation [36]. TBARS is formed as a by-product of lipid peroxidation. In our study, TBARS formation significantly increased in Polycystic Ovaries. Treatment with Curcumin regularized its level.
Women with PCOS are prone to higher risk of hypertension as a long term consequence [37]. To find out whether Letrozole induction of PCOS causes any changes in blood pressure and heart rate, in our study, we measured these parameters. The results of our study projected that there were no significant differences in these parameters among the control and PCOS induced groups.
Curcumin treatment advanced to disappearance of cysts and decreased incidence of pyknotic granulosa cells. Varying number of corpora lutea were seen suggesting ovulation and normal estrous cyclicity. Follicles at different stages of development with oocytes and clear, visible granulosa cell layer were observed. Ovarian cortex appeared normal with many follicles.
In conclusion, curcumin showed many beneficial effects similar to Clomiphene citrate in treating PCOS condition and inducing ovulation. Curcumin restored the hormone and lipid profile, antioxidant and glycemic status as well as ovarian morphology in Letrozole induced PCOS animals. These effects may be ascribed to its multiple pharmacological activities like estrogenic, antihyperlipidmic, antioxidant and hypoglycemic effects which could be useful in managing PCOS condition and prevent ovarian cell dysfunction, ovulation and thereby improving fertility. Together broad spectrum biological effects of Curcumin make it a promising drug for treating clinical and pathological abnormalities in PCOS condition.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 2000; 85: 2434–8.
[2] Knochenhauer ES, Key TJ, Kashar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the Southeastern United States: a prospective study. J Clin Endocrinol Metab 1998; 83: 3078–3082.
[3] Joo yeon Lee, Chin-kun Baw, Sajal Gupta, Nabil Aziz, Ashok Agarwal. Role of oxidative stress in polycystic ovary syndrome. Curr Women’s Health Rev 2010; 6: 96-107.
[4] Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005; 352: 1223–1236.
[5] Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004; 89: 2745–2749.
[6] Pasquali R, Gambineri A. Polycystic ovary syndrome: a multifaceted disease from adolescence to adult age. Ann N Y Acad Sci 2006; 1092: 158–174.
[7] Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet 2007; 370: 685–697.
[8] Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, et al. Postmenopausal women with a history of irregular mensesand elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health—National Heart, Lung, and Blood institute sponsored women’s ischemia syndrome Evaluation. J Clin Endocrinol Metab 2008; 93: 1276–84.
[9] Birdsall MA, Farquhar CM, White HD. Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med 1997; 126: 32–5.
[10] Raj Kamal Srivastava and Amitabh Krishna. Pathophysiology of polycystic ovary syndrome (PCOS): lessons from animal studies. Proc India Natl Sci Acad 2006; B71: 191-205.
[11] Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 1998; 95: 6965–6970.
[12] Kafali H, Iriadam M, Ozardali I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res 2004; 35: 103-108.
[13] Catiele Garcia Gervasio, Marcelo Picinin Bernuci, Sa Rosa-c-Silva. The role of androgen hormones in early follicular development. ISRN Obstet Gynecol 2014; 2014:818010.
[14] Bhavna Desai N, Radha Maharjan H, Laxmipriya Nampoothiri P. Aloe barbadensis Mill. Formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Pharmacog Res 2012; 4(2): 109–115.
[15] Radha Maharjan H, Padamnabhi Nagar S, Laxmipriya Nampoothiri P. Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. J Ayurveda Integr Med 2010; 1(4): 273–279.
[16] Ahmed Badawy, Abubaker Elnashar. Treatment options for polycystic ovary syndrome. Int J Womens Health 2011; 3: 25-35.
[17] Choi SH, Shapiro H, Robinson GE, Irvine J, Neuman J, Rosen B, et al. Psychological side-effects of clomiphene citrate and human menopausal gonadotrophin. J Psychosom Obst. Gynecol 2005; 26: 93-100.
[18] Arun N, Nalini N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Food Hum Nutr 2002; 57: 41-52.
[19] Pari L, Murugan P. Effect of tetrahydrocurcumin on blood glucose, plasma insulin and hepatic key enzymes in streptozotocin induced diabetic rats. J Basic Clin Physiol Pharmacol 2005; 16(4): 257–274.
[20] Pari L, Murugan P. Antihyperlipidemic effect of curcumin and tetrahydro curcumin in experimental type 2 diabetic rats. Ren Fail 2007; 29(7): 881-889.
[21] Aggarwal BB, Banerjee S, Bharadwaj U, Sung B, Shishodia S, Sethi G. Curcumin induces the degradation of cyclin E expression through ubiquitin-dependent pathway and up-regulates cyclin dependent kinase inhibitors p21 and p27 in multiple human tumor cell lines. Biochem Pharmacol 2007; 73: 1024–1032.
[22] Watson JL, Greenshields A, Hill R, Hilchie A, Lee PW, Giacomantonio CA, et al. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinae activation and downregulation of Bcl-2 and survivin expression and Akt signalling. Mol Carcinog 2010; 49: 13–24.
[23] Atilla Kadasi, Alexander Sirotkin V, Nora Maruniakova, Adriana Kolesarova, Jozef Bulla, Roland Grossmann. The effect of curcumin on secretory activity, proliferation and apoptosis of the porcine ovarian granulose cells. JMBFS 2012; 2(1): 349-357.
[24] Bachmeier BE, Mirisola V, Romeo F, Generoso L, Esposito A, Dell’eva R, et al. Reference profile correlation reveals estrogen-like transcriptional activity of curcumin. Cell Physiol Biochem 2010; 26(3): 471-82.
[25] Md. Nur Alam, Nusrat Jahan Bristi, Md. Rafiquzzam. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceut J 2012; 21: 143-152.
[26] Baravalle C, Salvetti NR, Mira GA, Pezzone N, Ortega HH. Microscopic characterization of follicular structures in letrozole-induced polycystic ovarian syndrome in the rat. Arch Med Res 2006; 37: 830-839.
[27] Rezvanfar MA, Ahmadi A, Shojaei-Saadi HA, Baeeri M, Abdollahi M. Molecular mechanism of a novel selenium based complementary medicine which confers protection against hyperandrogenism induced polycystic ovary. Theriogenology 2012; 78: 620-631.
[28] Sasikala Sl, Shamila S. Unique rat model exhibiting biochemical fluctuations of letrozole induced polycystic ovary syndrome and subsequent treatment with allopathic and ayurvedic medicines. J Cell Tissue Res 2009; 9: 2013-2017.
[29] Mamata Jadhav, Sasikumar Menon, Sunita Shailajan. In vivo evaluation of Mimosa pudica linn. In the management of Polycystic ovary using rat model. Int J Appl Biol and Pharmaceut Technol 2013; 4(1): 285-292.
[30] Boudreaux MY, Talbott EO, Kip KE, Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8—year follow—up. Curr Diab Rep 2006; 6: 77-83.
[31] Lakhani K, Prelevic GM, Seifalian AM, Atiomo WU, Hardiman P. Polycystic ovary syndrome, diabetes and cardiovascular disease: risks and risk factors. J Obstet Gynaecol 2004; 24(6): 613-621.
{32] Von Eckardstein A. Androgens, cardiovascular risk factors and atherosclerosis. In: Nieschlag E, Behre HM. (eds.) Testosterone: Action, deficiency, substitution. 2nd ed. Berlin, Heidelberg, New York: Springer; 1998,p.229-258.
{33] Croston GE, Milan LB, Marschke KB, Reichman M, Briggs MR. Androgen receptor-mediated antagonism of estrogen-dependent low density lipoprotein receptor transcription in cultured hepatocytes. Endocrinology 1997; 138: 3779-3786.
[34] Sabuncu T, Vural H, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. J Clin Biochem 2001; 34(5): 407-413.
[35] Liu J, Zhang D. The role of oxidative stress in pathogenesis of polycystic ovary syndrome. Sichuan Da Xue Xue Bao Yi Xue Ban 2012; 43(2): 187-90.
[36] Antonio Ayala, Mario F. Munoz, Sandro Arguelles. Lipid peroxidation: production, metabolism and signalling mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev 2014; 2014: 360438.
[37] Cibula D, Cífkova R, Fanta M, Poledne R, Zivny J, Skibova J, et al. Increased risk of non-insulin dependent diabetes mellitus, arterial hypertension and coronary artery disease in perimenopausal women with a history of the polycystic ovary syndrome. Hum Reprod 2000; 15(4): 785-789.
5 December 2015
Vasudha Bakshi, Department of Pharmacology, Lalitha College of Pharmacy, Anurag Group of Institutions, Ghatkesar, Ranga Reddy Dist-500088, Hyderabad, Telangana, India.
E-mail: vasudhapharmacy@cvsr.ac.in
Received in revised form 2 January 2016 Accepted 8 January 2016
Available online 1 March 2016
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Effect of growth regulators on rapid micropropagation and antioxidant acitivity of Canscora decussata (Roxb.) Roem. & Schult.-A threatened medicinal plant
- A new nucleotide variant G1358A potentially change growth differentiation factor 9 profile that may affect the reproduction performance of Friesian Holstein cattle
- Pregnancy rate in Bulgarian White milk goats with natural and synchronized estrus after artificial insemination by frozen semen during breeding season
- Effect of heparin, caffeine and calcium ionophore A 23187 on in vitro induction of the acrosome reaction of fresh ram spermatozoa
- The characterisation and cryopreservation of Venda chicken semen
- Effects of intramuscular injections of vitamin E-selenium and a gonadotropin releasing hormone analogue (GnRHa) on reproductive performance and blood metabolites of post-molt male broiler breeders
