Effect of growth regulators on rapid micropropagation and antioxidant acitivity of Canscora decussata (Roxb.) Roem. & Schult.-A threatened medicinal plant
2016-10-18LoganathanKousalyaNarmathaBai
Loganathan Kousalya, V. Narmatha Bai
Plant Tissue Culture Laboratory, Department of Botany, Bharathiar University, Coimbatore-641046, India
Effect of growth regulators on rapid micropropagation and antioxidant acitivity of Canscora decussata (Roxb.) Roem. & Schult.-A threatened medicinal plant
Loganathan Kousalya*, V. Narmatha Bai
Plant Tissue Culture Laboratory, Department of Botany, Bharathiar University, Coimbatore-641046, India
ARTICLE INFO
Article history:
Canscora decussata
Multiple shoots
Root induction
Callus induction
Antioxidant activity
ABSTRACT
Objective: An efficient in vitro plant regeneration protocol for Canscora decussata(Gentianaceae), (C. decussata) a threatened medicinal herb used in Ayurvedic system of medicine was developed. Seed germination was achieved on MS growth regulator free medium. Methods: The nodal explants were excised from the in vitro raised seedlings and inoculated on MS medium supplemented with various plant growth regulators such as BAP, KIN, TDZ and Zeatin individually and in combinations with or without GA3. BAP (2 mg/L) was proved to be effective for multiple shoot induction (30.20±6.53) among the cytokinin tested individually. Addition of NAA (1 mg/L) to cytokinin containing medium resulted in callus, with KIN (3 mg/L) produced highest percentage of callus (82%) per explant. Results: Among the various combination of cytokinin tested, BAP (0.5 mg/L) in combination with KIN (2 mg/L) induced highest number of multiple shoots (72.10±1.05 shoot per explant). Addition of 1 mg/L of GA3to the above medium induced highest number of shoots (100.80±3.20) with an average shoot length of (6.98±0.66 cm). Rooting was optimized in half-strength MS medium supplemented with IBA at 1.0 mg/l. The plantlets were successfully transferred to hardening medium containing vermiculite with 83% survival rate. Among the antioxidant activity of methanol extract of wild-grown plants and in vitro regenerants tested, half-MS medium supplemented with NAA (0.5 mg/L) derived callus has promising activity for total phenolics, DPPH, ABTS, FRAP and phosphomolymbdenum assays. Total flavonoid content was found to be high in callus derived from MS medium supplemented with KIN (2 mg/L) in combination with NAA (1 mg/L). Conclusion: Our present study suggest that in vitro derived callus of C. decussata represent a promising alternative source to meet the pharmaceutical demands for commercial formulations and the protocol could effectively be applied for the conservation of C. decussata.
Document heading doi: 10.1016/j.apjr.2016.01.014
1. Introduction
Shankhpushpi is a drug of Ayurvedic ‘Medhya Rasayana’category which is used to boost memory and intellect. Canscora decussata (C. decussate). (Gentianaceae) is one of the plants used as‘Shankhpushpi’. The entire plant, as well as its fresh juice is used in medicine. It is used in the popular medicine for the treatment of insanity, epilepsy and nervous debility. It has proven its therapeutic potential in acetylcholinesterase inhibition, CNS stimulation, hypertension, convulsions, tuberculosis, immunomodulation, inflammation, hepatoprotection, spermatogenesis and postmenopausal osteoporosis[1]. It is reported to contain several types of xanthones, triterpenoids, loliolide, sterols and flavanoids[2]. Studies of this plant showed hepatoprotective, antidepressant, antianxiety, antistress and antimycobacterium tuberculosis activity[3]. The presence of mangiferin in C. decussata can thus be correlated to the cognitive and memory enhancing activity of C. decussata[4].
Indiscriminate practice of over harvesting makes the species to become increasingly vulnerable at a point that threatens its survival[5]. Besides, these nature-harvested plants are unlikely to meet the quality standards for botanical drugs[6]. As C. decussata plants are short life-cycled and seasonal in nature, ex situ multiplication ofthis plant using in vitro techniques seems to be a viable approach for coexistence of germplasm conservation biomass utilization[7]. As the domestication of the plant using conventional techniques has not yet been successfully employed, so the present studies aim to develop a protocol for the rapid propagation of this commercially important medicinal plant.
The present research work is based on to develop an efficient and rapid propagation protocol for C. decussata for large-scale production of uniform raw materials for future pharmaceutical compound extraction and to analyze the antioxidant activity of the multiple shoot and callus from some selected PGR concentration and also compared with in vivo plants (wild- grown plants). Effective plant growth regulators in development of plants with a greater antioxidant activity were determined by 2, 2-Diphenyl-1-picrylhydrazyl (DPPH), 2, 2-Azinobis (3-ethylbenzothiozoline-6-sulfonic acid) diammonium salt (ABTS), FRAP and phosphomolybdenum assays. This present study also focused on correlation of total flavonoid and phenol content for antioxidant activity of both the in vitro regenerants and wild plants.
2. Materials and methods
2.1. Source of plant material
The plant materials of C. decussate were collected from Kerala, India. An authentic sample was identified by BSI (Botanical Survey of India), Southern Circle, Coimbatore, India, and a voucher specimen has been deposited in the herbarium of BSI Coimbatore (Accession No: 1893).
2.2. Experimental procedure
Seeds collected from healthy plants were washed with running tap water followed by 5% (v/v) Teepol (detergent) treatment for 5–10 min and then treated with fungicide (1% Bavistin) for 20 min. The treated explants were washed with double distilled water. Subsequently the explants were disinfected with 0.1% mercuric chloride for 3–5 min and finally they were rinsed with sterile double distilled water under aseptic condition. MS medium[8] supplemented with 3% (w/v) sucrose was used in the experiments. The pH of the medium was adjusted to 5.7 before adding 0.8% (w/v) agar (Hi Media). Media (15 mL) were poured into (25伊150) mm culture tubes (Borosil, Mumbai) and autoclaved at 121 ℃ and 1.06 kg/cm2pressure for 20 min. The cultures were incubated at (25 ± 2) ℃ under a 16 hrs photoperiod of 50–60 l mol/m2/s flux density provided by cool white fluorescent tubes.
2.2.1. Multiple shoot induction
Experiments were carried out on shoot induction and proliferation of C. decussata. The pretreated seeds were inoculated on MS medium without any growth regulator. The in vitro seedling derived nodal segments were cultured on various cytokinins, such as BAP, Kin, Zeatin and Thidiazuron (TDZ) (0.5-3.0 mg/L). The total number and length of shoots were calculated after 5 weeks of culture. In order to increase shoot multiplication and shoot elongation in in vitro derived nodal explants, different concentrations of Gibberellic acid (GA3) in combination with BAP, KIN, Zeatin and TDZ (0.5-3.0 mg/L) was tested. For callus induction and multiple shoot proliferation, NAA (1.0 mg/L) was combined with cytokinin at different concentrations. The maximum number of shoots and shoot length were calculated after 5 weeks of culture.
2.2.2. Root induction
For root induction, excised shoots were transferred to half strength MS medium supplemented with three auxins including Indole-3-acetic acid (IAA), Indole-3-butyric acid (IBA), 毩-Naphthaleneacetic acid (NAA) at different concentrations (0.5-3.0 mg/L). Root number and length of roots were recorded after 3 weeks of culture. Healthy plantlets with well-developed roots were potted on paper cups containing vermiculite (100%). Subsequently the plantlets were transferred into greenhouse condition. Explants inoculated onto growth regulator free MS medium were served as controls for all the above mentioned experiments.
2.3. Antioxidant studies
2.3.1. Extraction method
The air-dried powdered of in vitro regenerants plants and callus derived from the various treatments of PGR and wild plants was used for extracted by maceration method with methanol (48 h) and the extracts were filtered. The extracts were concentrated by rotary vacuum evaporator and then air-dried. The extracts obtained were used directly for the estimation of total phenolic content and also for the assessment of antioxidant potential through various biochemical assays.
2.3.2. Determination of total phenol & flavonoid content
The total phenol content was determined according to the method described by[9] & total flavonoid contents estimated as per described by[10].
2.3.3. In vitro antioxidant activity
The radical scavenging activity of the C. decussata methanol extract of wild- grown plants and in vitro propagated plants and callus was evaluated using DPPH.[11], ABTS.+cation radical [12], ferric reducing antioxidant power (FRAP) activity[13] and phosphomolybdenum method [14].
2.4. Statistical analysis
All the experiments were conducted with a minimum of 5 replicates per treatment. The experiments were repeated thrice.The significance of differences among means was carried out using Duncan’s multiple range test (DMRT) at P<0.05 (SPSS 20.0 version). The results are expressed as a means ± SD of three experiments. All experiments of antioxidant studies were repeated at least three times. Results were reported as mean±SD. The antioxidant activities of all the extracts were tested by one-way analysis of variance (ANOVA). Correlation analysis was performed using Pearson correlation (two-tailed) test.
3. Results
3.1. In vitro seed germination
The plant specimen with flowering stage was showed in Figure 1. A protocol for the axillary multiplication of C. decussata was established in present study. There are no reports on studies relating to germination of seed and micropropagation of C. decussata. Full-strength MS medium was used for seed germination to arise aseptic seedlings of C. decussata (Figure 2A & B). The pretreated seeds showed 36.2% of seed germination in the present study. The nodal explants derived from aseptically raised seedlings were used for culture initiation (Figure 2C). The germination of seeds were recorded and percentage of seed germination was calculated by the formula:
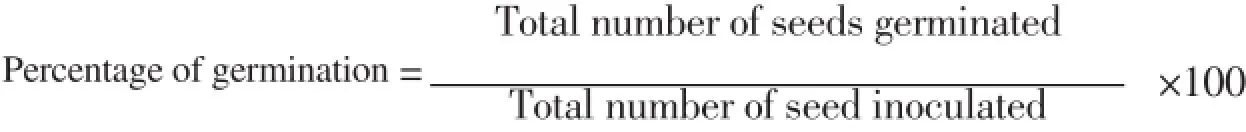
3.1.1. Effect of cytokinin on multiple shoot induction
Preliminary experiments were conducted to study the effect of various concentrations of cytokinins such as BAP, 6-Furfurylaminopurine (KIN), TDZ, and Zeatin (0.5-3.0 mg/L) with MS basal as control medium on shoot bud induction from in vitro nodal explants. Each of BAP, KIN, TDZ and Zeatin separately showed a significant variation in terms of number of shoot bud induced per explant (Table 1). A maximum number of (30.20±6.53) shoots per explants were induced on MS medium containing BAP (2 mg/L) (Figure 2D). In comparison to the response of nodal explants on media supplemented with cytokinins, no shoot buds were formed on MS basal media.
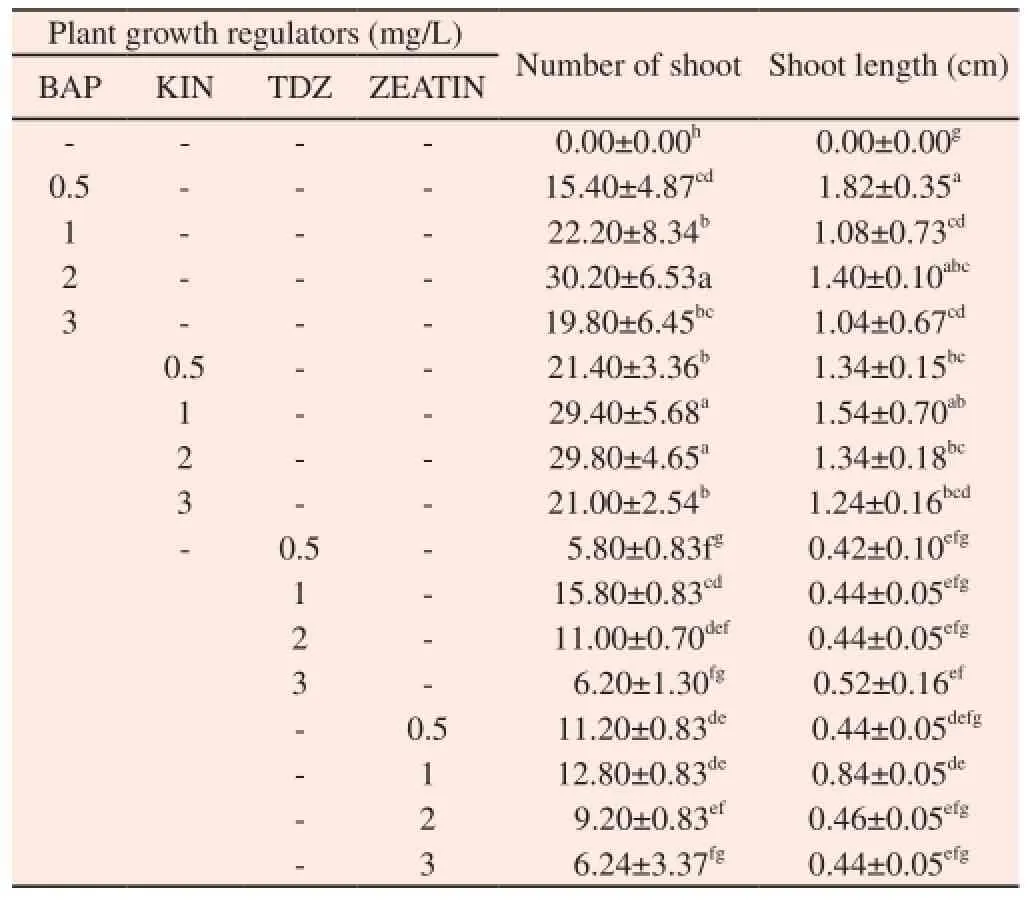
Table 1 Multiple shoots induction from in vitro nodal explants of C. decussata
3.1.2. Effect of combination of cytokinin on multiple shoot induction
In order to increase the shoot multiplication, BAP (0.5-3.0 mg/L) in combination with KIN, TDZ and Zeatin (0.5-3.0 mg/L) were tested. An increase in the number of the shoots (72.10± 1.05) was observed when the in vitro nodal explants were cultured on MS medium supplemented with BAP (0.5 mg/L) and KIN (2.0 mg/L) (Table 2) (Figure 2E). GA3was combined with well resulted combinations of cytokinins in order to increase the multiple shoot induction as well as shoot elongation. Among these concentrations combined with GA3(0.5-3.0 mg/L), combination of BAP (0.5 mg/L) and KIN (2.0 mg/L) along with GA3(1 mg/L) induced maximum number of (100.80±3.20) shoots per explants with an average shoot length of (6.98±0.66) cm per shoots (Table 3) (Figure 2F & G).
3.1.3. Effect of combination of cytokinin & NAA on multiple shoot induction
The effect of NAA in combination with cytokinin on multiple shoot induction and callus induction was studied. In the present study, combination of cytokinin with NAA produced lower number of shoots due to callus formation and proliferation at the base of shoot clumps. Of various combinations of NAA tested, KIN (3.0 mg/L) +NAA (1.0 mg/L) which produced 82% showed good callusing followed by TDZ (3.0 mg/L) + NAA (1.0 mg/L) (71%) (Table 4). The callus observed in NAA supplemented with cytokinin was fragile turned into compact, green and regenerative in nature with few adventitious shoot buds in the same medium. But the regenerative potential was found to be very low (Figure 2I).

Figure 1. C. decussata habit & its herbarium.

Figure 2. Effect of cytokinin and auxin on multiple shoot induction and callus induction of C. decussata from in vitro nodal explants.
3.1.4. Effect of auxin on root induction
Among the various auxins tested, IBA proved to be the most effective for root induction (15.80±0.83 root per explant) (Figure 2J). Although NAA and IAA also responded for root induction but number of rooting is poor and roots were thin and delicate (Table 5). However, half strength MS medium containing NAA at 0.5 mg/L showed the highest callus induction (Figure 2H). The healthy plantlets developed on MS + IBA (1.0 mg/L) were removed from the culture tubes and washed thoroughly in sterile distilled water. Then plantlets were treated with Bavistin (1%) for 5 min and it was washed thoroughly with sterile distilled water and transferred to vermiculite (Figure 2L). The plantlets survived 83% without any phenotype changes.
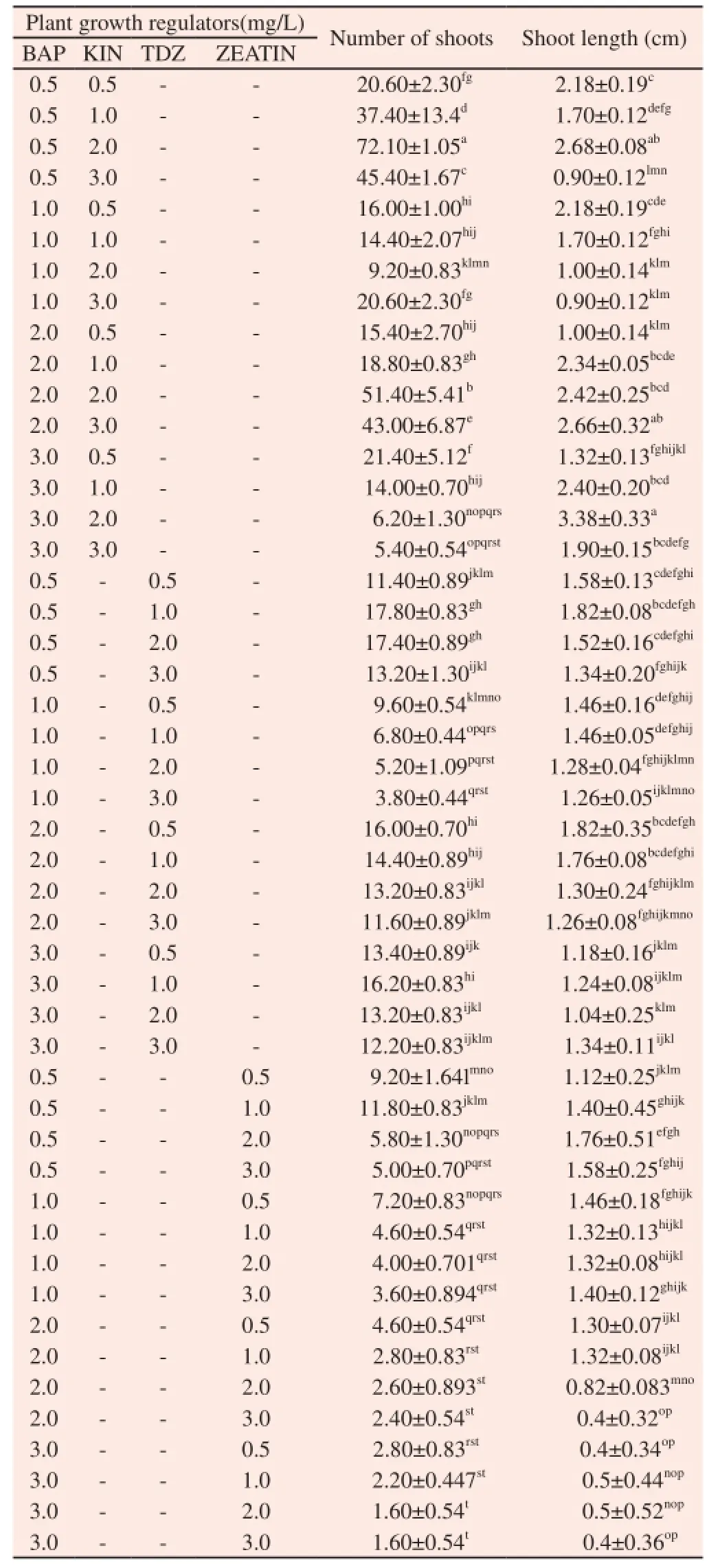
Table 2 Effect of combination of cytokinin on multiple shoot induction on C. deccusata.
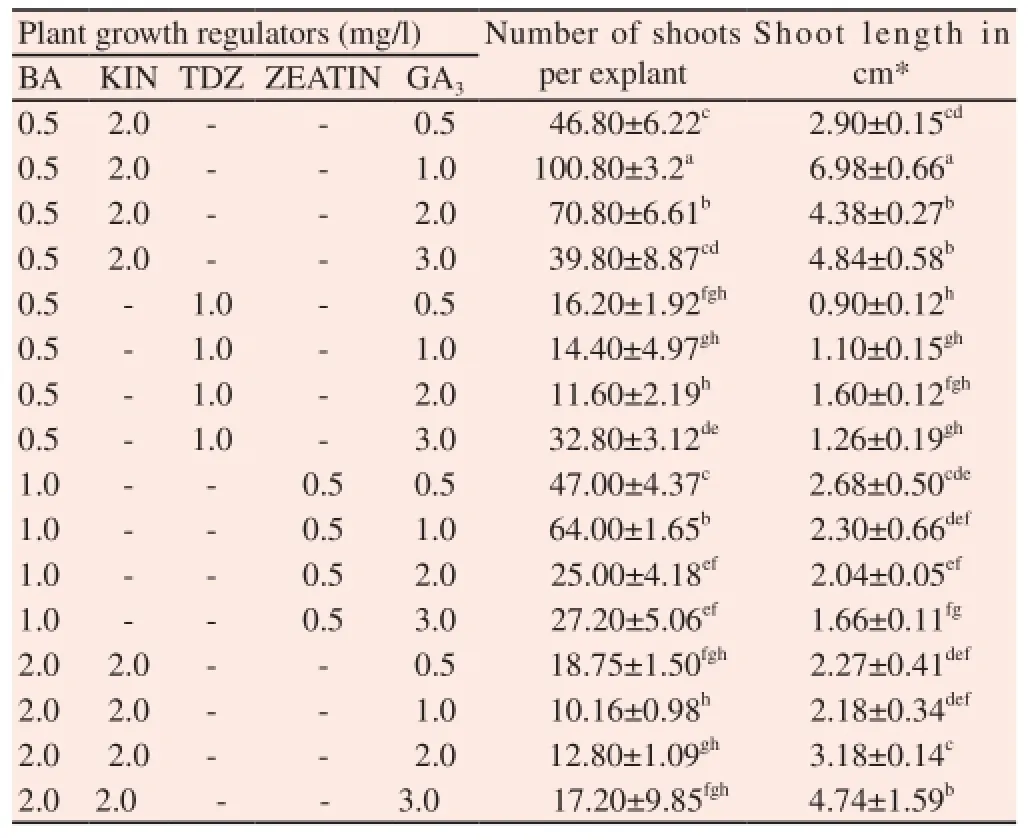
Table 3 Effect of GA3in combination with cytokinins on multiple shoot induction of C. deccusata.
3.2. Antioxidant activity
Plant cell and tissue cultures hold great promise for controlled production of numerous useful secondary metabolites. In vitro cultured cells, organs and regenerated plants synthesize, accumulate and sometimes show many classes of secondary metabolites have been studied in various plant species. The literature further reveals that the regenerating callus have wide use in both basic research and industrial applications. To study the antioxidant activity, we selected those in vitro plants and callus which produced the best yield when treated with various plant growth regulators and compared with in vivo plant (nature grown). The selected concentration of tissue culturally grown plants was listed in the Table 6 used to analyzed for total phenolics content and antioxidant activity by DPPH, ABTS, FRAP and phosphomolybdenum assays.
3.2.1. Determination of total phenol content
The results obtained from the assay were expressed as means standard deviation of triplicate analyses and are presented in Table 6. Highest phenol content (577.77±15.18 mg GAE/g DW) was observed in the methanol extract of callus obtained from half MS containing 0.5 mg/L NAA of C. decussata which is also higher than that of methanol extract of in vivo plants. A good correlation of total phenol content with total flavonoid content (r2= 0.761), ABTS (r2= 0.922), Phosphomolybdenum (r2= 0.934) and FRAP assay (r2= 0.812) was achieved for tested samples. A negative correlation was achieved between total phenol and DPPH scavenging assay which clearly implies that increase in the phenol content which lowers the DPPH radicals (r2= -0.866) (P<0.05) (Table 7).
3.2.2. Determination of total flavonoid content
Highest flavonoid contents (179.16±10.92 mg Rutin equivalents /g DW) was observed in the methanol extracts of in vitro derived callus obtained from MS containing KIN (2.0 mg/L) + NAA (1.0 mg/L) which is comparatively higher than in vivo plant (wild plant). There is a correlation between total flavonoid content with total phenol content (r2= 0.761), ABTS (r2= 0.777), Phosphomolybdenum (r2= 0.802) and FRAP assay (r2= 0.543) for tested samples (r2= 0.761) (Table 7). The contribution of total flavonoid with DPPH assay was confirmed by their negative correlation because the flavonoid which tends to inhibits the DPPH radicals (r2= -0.695).
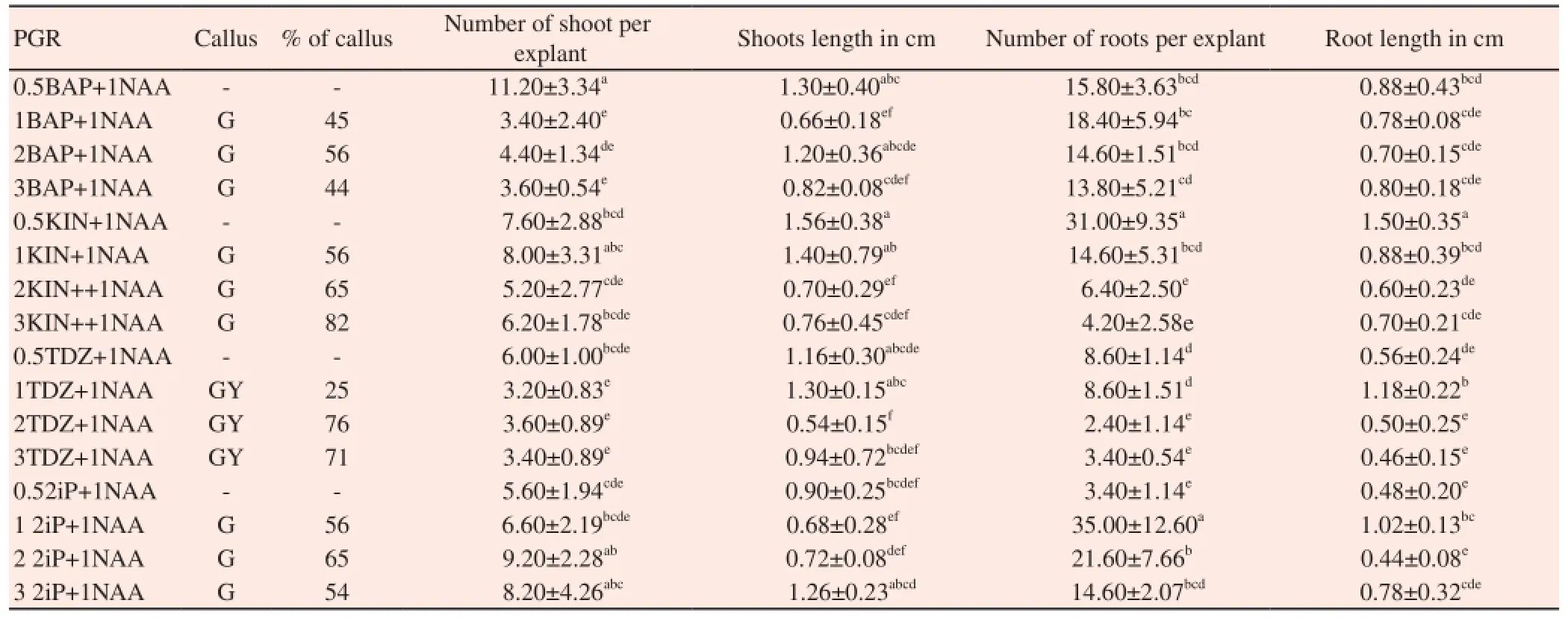
Table 4 Effect of NAA in combination with cytokinins in callus and multiple shoot induction of C. deccusata.
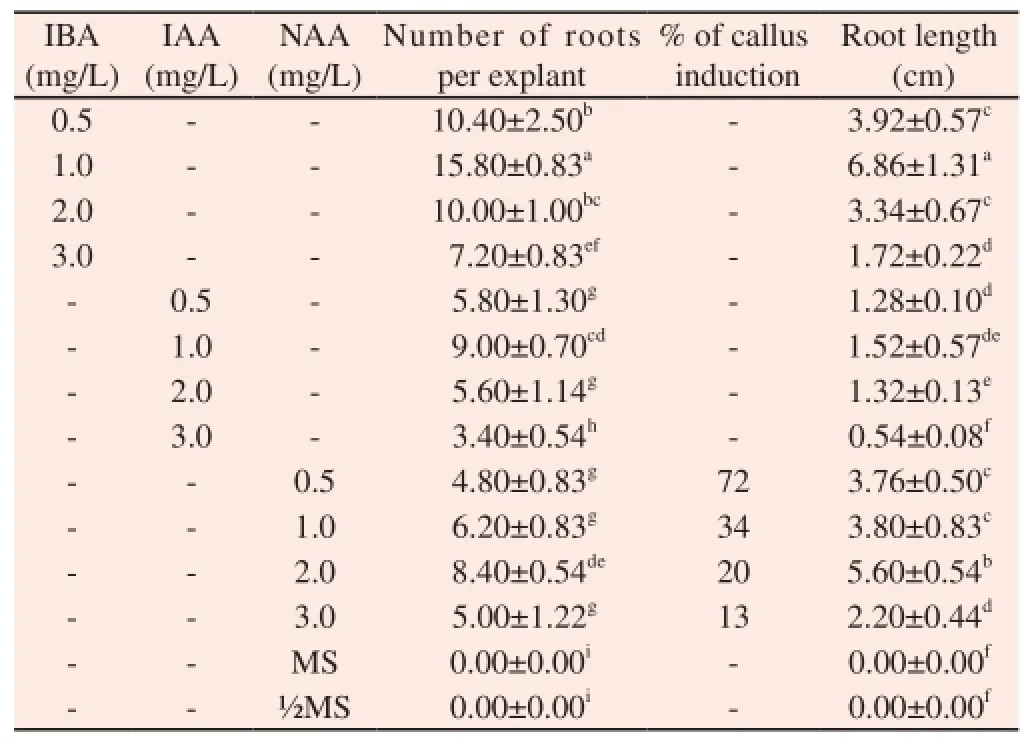
Table 5 Effect of auxins on root induction of C. deccusata.
3.2.3. DPPH scavenging assay
Among all the extract of in vitro derived plants and callus obtained from various PGR containing media and wild- grown plants (Table 6), highest DPPH radical scavenging activity, i.e. lowest IC50value, was observed in methanol extract of callus derived from half MS medium supplemented with 0.5 mg/L of NAA (IC50=20.88 µg/mL). This was followed by the methanol extracts of callus from 1.0 mg/L KIN+1 mg/L NAA (IC50=23.29 µg/mL). The IC50values of callus extract from 0.5 mg/L NAA was lower than all the extracts of in vitro and in vivo plant (wild- grown plant) extracts.
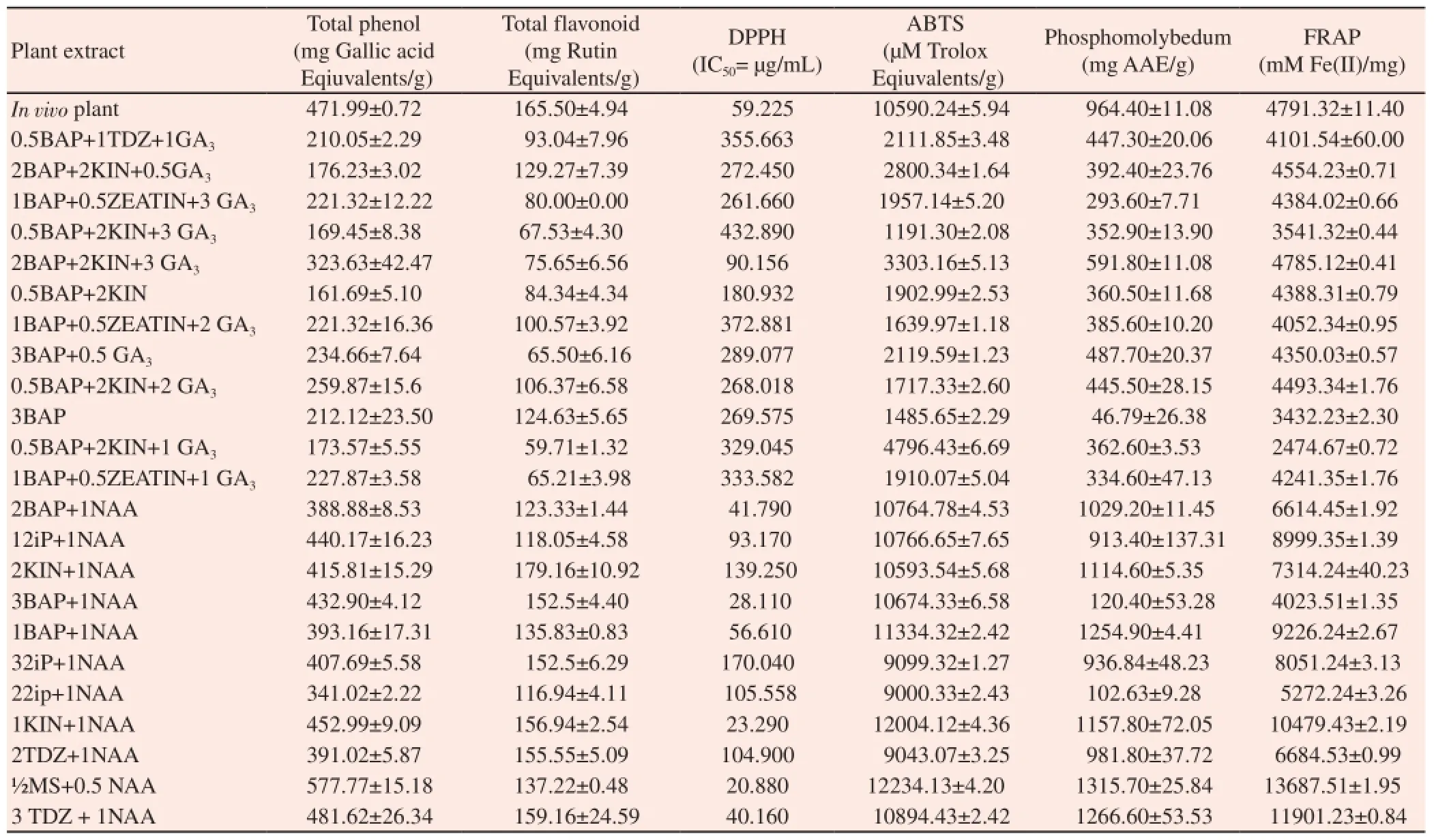
Table 6 Antioxidant activity of in vivo plant and in vitro derived plants of C. deccussata.
3.2.4. ABTS assay
The ABTS radical scavenging activity of methanol callus extract obtained from in vitro from MS medium + 0.5 mg/L NAA has highest ABTS radical scavenging activity was observed (12234.13±43.20 µM TEAC/g DW) followed by methanol extract of in vitro callus from MS medium containing KIN at 1.0 mg/L + NAA at 1.0 mg/L (12004.12±2.81 µM TEAC/g DW) (Table 6). The ABTS assay of the C. deccusata extracts calculated as Trolox equivalents/g extracts (TEAC/g).
3.2.5. Phosphomolybdenum assay
Highest reducing power showed in methanol extract of in vitro callus from MS medium containing 0.5 mg/L NAA (1315.78 mg AAE/g) which was followed by MS medium containing TDZ (3.0 mg/L) + NAA(1.0 mg/L) (1266.66 mg AAE/g) (Table 6). We observed that the total phenolic content have more ability to reduce Mo+ion than the total flavonoid content of C. decussata. The phosphomolybdenum assay of the C. deccusata extracts calculated as ascorbic acid equivalents /g extracts(AAE/g).
3.2.6. FRAP assay
The ferric ion-reducing activities of C. decussata extracts is calculated as µmol Fe(II)/g extract. Among the various samples tested, methanol extract of callus derived from MS medium containing 0.5 mg/L NAA of C. decussata showed stronger Ferric reducing power (13687.51±1.95 µmol Fe(II)/g extract) which was consistent with the results obtained from the DPPH and ABTSassays (Table 6). The FRAP assay of the C. deccusata extracts calculated as Fe(II) equivalents /g extracts.
3.2.7. Correlation analysis
A good negative correlation was observed for all other assays such as ABTS (r2=-0.870), phosphomolybdenum assay (r2=-0.880) and FRAP assay (r2=-0.730) (Table 7). The inhibition of DPPH radicals tends to have the negative correlation with all the assays tested. Correlations among the ABTS, FRAP, and phosphomolybdenum assays were positively high and ranged between 0.74 and 0.96: the highest correlation was between ABTS and phosphomolybdenum (0.97) and the lowest correlation was between ABTS and FRAP (0.746) (P<0.05) (Table 7). From the correlation analysis, it is evident that the phenolics and flavonoids in the methanolic extract of callus derived from MS medium containing 0.5 mg/L NAA were responsible for highest antioxidant activity in all assays tested (Table 7). On the basis of the current findings, we conclude that MS medium supplemented with 0.5 mg/L NAA yields high total phenol content as well as higher antioxidant activity.
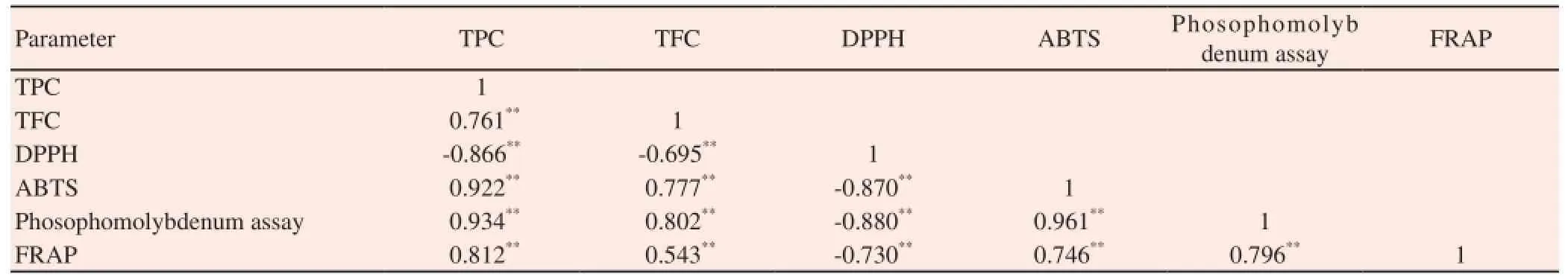
Table 7 Correlation between phenolics, flavonoids, and different antioxidant parameters of in vitro regenerated plants and wild-grown plants’ methanol extract of C. decussata.
4. Discussion
Recently, Gaikwad et al.[15] and Sethiya et al.[16] were studied in vitro propagation and pharmacological activities of C. decussata Schult. respectively. But, the critical examination on their figures which clearly shows it is only the species of Canscora diffusa (C. diffusa) resembles in vegetative forms as C. decussata and it might have been misidentified as C. decussata. From this result, we can conclude that our study is the first report on micropropagation and antioxidant activity of C. decussata. The objective of this study is to develop an effective in vitro regeneration protocol and to evaluate the antioxidant of both wild-grown and in vitro regenerated plants of C. decussata.
MS medium supplemented with BAP (2 mg/L) was effective for shoot multiplication from nodal segments of C. decussata. The effect of BAP on multiple shoot formation has also been studied in various medicinal plant species such as Ceropegia noorjahaniae (C. noorjahaniae) [17] Gymnema sylvestre (G. sylvestre) [18] and Stevia rebaudiana (S. rebaudiana) [19]. Any further increase in concentration more than optimum level of all cytokinins tested did not improve any parameters of shoot multiplication. In this research, application of cytokinin such as BAP in combination with KIN resulted in high-frequency shoot regeneration in C. decussata. The synergistic effect of BAP and KIN in promoting shoot multiplication has been reported earlier in Swertia chirata (S. chirata) [20,21], S. rebaudiana [22] and Achryrantes aspera (A. aspera) [23]. In vitro flowering was also observed when the culture was stored for longer period on the same medium (Figure 2K). Addition of GA3, not only increases shoot elongation but also increases the shoot multiplication. Similarly results were obtained in Gentiana triflora (G. triflora) [24], S. chirata[21], Enicostema axillare (E. axillare) [25] and G. sylvestre [26].
NAA play an important role in callus induction. NAA induced callus when combined with all cytokinin containing medium. Similar, synergistic effect of auxins with cytokinins in callus induction was reported by in Salvia officinalis (S. officinalis) [27], Salvadora oleoides (S. oleoides) [28] and Eustoma grandiflorium (E. grandiflorium) [29]. Exogenous application of cytokinin and auxin in a specific ratio may help to maintain the required ratio which favoured callus production. The replication and proliferation of callus was due to the essence of NAA, because this hormone belongs to auxins groups and these groups of hormones usually cause the cell elongation, tissue swelling, cellular division (callus formation), adventitious roots formation, prevention from adventitious and adverse branches and often embryogenesis in suspension cultures[30]. Superiority of NAA for callus induction has also been reported in different plant species, viz., in Erigeron breviscapus (E. breviscapus) [31] Rosmarinus officinalis (R. officinalis) [32] and E. grandiflorium[29].
There was a clear difference in rooting response of PGR-treated and untreated regenerated shoots of C. decussata. In the presence of auxin, regenerated shoots rooted earlier and had a much higher rooting rate than untreated shoots. IBA is a common auxin used for inducing rooting in several Gentianaceae plant species in Swertia chirata (S. chirata) [20] and G. austriaca [33]. Likewise, IBA hasbeen shown to be very effective in root induction as in various cases including Garcinia indica (G. indica) [34], Ceropegia noorjahaniae (G. noorjahaniae)[17] and Terminalia arjuna (T. arjuna) [35]. Similar results were achieved in Swertia corymbosa (S. corymbosa) [36] & E. grandiflorum [37]. The effectiveness of IBA in root formation may be due to its easier uptake/transport, constancy greater than other auxins, and successive gene activation.
Phenols are compounds that have the ability to destroy radicals because they contain hydroxyl groups. These important plant components give up hydrogen atoms from their hydroxyl groups to radicals and form stable phenoxyl radicals; hence, they play an important role in antioxidant activity. Therefore, determination of the quantity of phenolic compounds is very important in order to determine the antioxidant capacity of plant extracts [39-41]. Our results are in agreement with a previous report where a positive correlation between high total phenol content and total flavonoid content and antioxidant activities in Artemisia absinthium L. (A. absinthium) [42,43]. The antioxidant potential in various medicinal plants has been shown to be mainly due to phenolic compounds[44-47]. The results imply that both phenol and flavonoid content contributed in all the antioxidant assays tested.
The DPPH method is a preferred method because it is fast, easy and reliable and does not require a special reaction and device. DPPH is a stable, synthetic radical that does not disintegrate in water, methanol, or ethanol. The free radical scavenging activities of extracts depend on the ability of antioxidant compounds to lose hydrogen and the structural conformation of these components [48, 49]. The IC50values of callus extract from 0.5 mg/L NAA was lower than all the extracts of in vitro and in vivo plant (wild- grown plant) extracts. This shows that NAA played an important role for the antioxidant activity of C. decussata in in vitro cultures. The ABTS radical cation decolourization assay is another method commonly used to assess antioxidant activity. ABTS free radical on incubation with sodium persulfate forms ABTS cation, which is deep blue in colour and is highly reactive towards antioxidants. When mixed with an antioxidant, an electron is donated to the ABTS radicals which is converted to a non-radical form. Decrease in colour intensity indicates the reduction of the ABTS radical. ABTS assay is consistent with the results of DPPH where callus derived from MS medium + NAA at 0.5 mg/L shows highest activity.
The phosphomolybdenum assay is successfully used to quantify vitamin E in seed, and being simple and independent of other antioxidant assays commonly employed, it was decided to extend its application to plant extract [14]. We compare and evaluated for the capacity to reduce Mo(VI) to Mo(V), a green phosphate by the antioxidant compound present in the samples. This reduction ability was expressed in ascorbic acid equivalents (AAE). The FRAP assay mainly depends on the reducing capacity of Fe3+- Fe2+conversion and serves as a significant indicator of its potential antioxidant activity. The antioxidant activities have been attributed to various reactions, binding of transition metal ion catalysts, decomposition of peroxides, prevention of continuous proton abstraction and radical scavenging activity [50]. The FRAP is often used as an indicator of phenolic antioxidant activity. The antioxidant potential of sample was estimated by their abilities to reduce Fe(III)-TPTZ to Fe (II)-TPTZ [13]. Ferric reducing power (FRAP) of C. decussata was determined by using various samples in which methanol extract of callus derived from MS medium containing 0.5 mg/l NAA of C. decussata showed the strongest ferric reducing power (13687.51±1.95 µmol Fe(II)/ g extract) which was consistent with the results obtained from the DPPH, ABTS and phosphomolybdenum assays. From Pearson correlation coefficient test, we can confirm the total phenol and flavonoid content were responsible for the antioxidant activity of all the assays. By this, we can conclude that inhibition of DPPH radicals (lowest IC50value) tends to have the negative correlation with all the assays tested. There is no positive relationship among the DPPH assay and all other assays in the present study. Correlations among the DPPH, ABTS, FRAP, and phosphomolybdenum assays were highly positive i.e., ABTS = phosphomolybdenum assay (r2= 0.976, P <0.01), phosphomolybdenum assay = FRAP (r2= 0.796) (P <0.01) and ABTS = FRAP (r2= 0.746) 317 (P<0.01). From the correlation analysis, it is evident that the phenol and flavonoids were responsible for the antioxidant activity in all assays tested. There is a significant relationship occurs between all the assays due to the presence of phenolic compounds. There are studies in the literature that report a positive correlation between antioxidant activity and the quantity of phenolic compounds [49,50].
Callus culture is very useful to obtain commercially important secondary metabolites. The potential of in vitro plant culture systems for the production of an enormous variety of antioxidant compounds has been recognized. Addition of NAA had stimulatory effect on the level of flavonoids and total phenolics in the majority of the treatments. This may be due to the induction of callus in NAA added medium. Earlier studies have been undertaken on the investigations of total phenolic content in callus culture of various medicinal plants. Similarly, phenolics associated enhanced antioxidant activities over wild plants have been reported for the callus culture of Habenaria edgeworthii (H. edgeworthii) [51] and cell suspension and in vitro shoot cultures of Ruta graveolens (R. graveolens) [52].
In in vitro cultures, especially after the addition of NAA (0.5 mg/L) to the medium, those total phenolics and antioxidant activity was significantly high compared to field-grown plants. This may be due to the presence of NAA which induces high stress level which tends to accumulated more phenolics by producing callus in in vitro condition. Stress conditions during in vitro cultivation may have stimulated polyphenol production, and plant growth regulatorcytokinis and auxins might have been responsible. NAA regenerated callus proved to be better for the accumulation of secondary metabolite. Therefore, the protocol developed in the present study can be efficiently used for the large-scale production of secondary metabolites in pharmaceutical industries.
Although the previous study on C. decussata of Gaikawad et al. 2015 has made a misidentification of C. diffusa as C. decussata. By this, the present study is the first report on efficient rapid regeneration protocol for C. decussata. Due to over exploitation of natural populations and difficulty in the cultivation of C. decussata, it become threatened and going to be extinct in few years. The protocol standardized in the present study enabled high rate of mass multiplication and could be applied for pharmaceutical industries for isolation of selective bioactive compounds. The in vitro callus derived from half strength MS medium supplemented with NAA (0.5 mg/L) has a stronger antioxidant activity compared to field-grown plants and could be used for the extraction of bioactive compounds for large-scale production in the field of pharmacy and medicine without disturbing the natural habitat of this threatened plant sps. The in vitro produced bioactive compounds by callus culture are of medically huge interest is a viable alternative in comparison to traditional methods, being able to exceed the productivity of in situ plant. Further investigation on the phytochemistry to these calli is of great curiosity in order to elucidate which molecules are responsible for the higher antioxidant activity.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Sethiya NK, Nahata A, Mishra SH, Dixit VK. An update on Shankhpushpi, a cognition-boosting ayurvedic medicine. J Chin Integr Med 2009; 7(11):1001-1022.
[2] Sethiya NK, Nahata A, Dixit VK. Simultaneous spectrofluorimetric determination of scopoletin and mangiferin in a methanolic extract of Canscora decussata Schult. Asian J Trad Med 2008; 3(6): 224-229.
[3] Madan B, Ghosh B. Canscora decussata promotes adhesion of neutrophils to human umbilical vein endothelial cells. J Ethnopharmacol 2002; 79(2):229-235.
[4] Sethiya NK, Nahata A, Dixit VK, Mishra SH. Cognition boosting effect of Canscora decussata (a South Indian Shankhpushpi). Eur J Integr Med 2012; 4: 113–121.
[5] Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, et al. Plants and human health in the twenty-first century. Trends Biotechnol 2002; 20: 522–531.
[6] Carmona F, Pereira AMS. Herbal medicines: old and new concepts, truths and misunderstandings. Rev Bras Farmacogn Braz J Pharmacogn 2013; 23: 379–385.
[7] Canter PH, Thomas H, Ernst E. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol 2012; 23: 180–185.
[8] Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 1962; 15: 473–497.
[9] Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem 2003; 51: 2144-2155.
[10] Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64: 555–559.
[11] Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958; 29: 1199-1200.
[12] Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. J Free Radic Biol and Med 1999; 26: 1231–1237.
[13] Pulido R, Bravo L, Saura-Calixto F. Antioxidant of dietary polyphenols as determined by a modified ferric reducing antioxidant power assay. J Agric Food Chem 2000; 46: 3396-3402.
[14] Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 1999; 269: 337-341.
[15] Gaikwad NK, Moon UR, Bhadoria PS, Mitra A. In vitro propagation of Canscora decussata Schult. and comparative assessment of anticholinesterase and antioxidant capacities of wild-harnessed and in vitrogrown plant extracts. Plant Cell Tiss Organ Cult 2015; doi:10.1007/ s11240-015-0770-y.
[16] Sethiya NK, Mishra S. Simultaneous HPTLC analysis of ursolic acid, betulinic acid, stigmasterol and lupeol for the identification of four medicinal plants commonly available in the Indian Market as Shankhpushpi. J Chromatog Sci 2014; 1–8. doi:10.1093/chromsci/ bmu111.
[17] Chavan JJ, Nalawade AS, Gaikwad NB, Gurav RV, Dixit GB, Yadav SR. An efficient in vitro regeneration of Ceropegia noorjahaniae: an endemic and critically endangered medicinal herb of the Western Ghats. Physiol Mol Biol Plants 2014; 20(3):405–410.
[18] Komalavalli N, Rao MV. In vitro micropropagation of Gymnema sylvestre–A multipurpose medicinal plant. Plant Cell, Tissue & Organ Culture 2000; 61: 97-105.
[19] Thiyagarajan M, Venkatachalam P. Large scale in vitro propagation of Stevia rebaudiana (bert) for commercial application: Pharmaceutically important and antidiabetic medicinal herb. Industrial Crops and Products 2012; 37:111–117.
[20] Chaudhuri RK, Pal A, Jha TB. Production of genetically uniform plants from nodal explants of Swertia chirata Buch. Ham. ex Wall-an endangered medicinal herb. In vitro Cell Dev Biol Plant 2007; 43: 467-472.
[21] Balaraju K, Agastian P, Ignacimuthu S. Micropropagation of Swertia chirata Buch.-Hams. ex Wall: a critically endangered medicinal herb. Acta Physiol Plant 2009; DOI: 10.1007/s11738-008-0257-0.
[22] Sridhar TM, Aswath CR. Influence of additives on enhanced in vitro shoot multiplication of Stevia rebaudiana (Bert.) —An important antidiabetic medicinal plant. Am J Plant Sci 2014; 5: 192-199.
[23] Sen MK, Nasrin S, Rahman S, Mostofa Jamal AH. In vitro callus induction and plantlet regeneration of Achyranthes aspera L., a high value medicinal plant. Asian Pac J of Trop Biomed 2014; 4: 40–46.
[24] Zhang Z, Leung DWMS. Factors influencing the growth of micropropagated shoots and in vitro flowering of Gentian. J Plant Growth Regul 2000; 36: 245-251.
[25] Kousalya L, Narmatha Bai V. High frequency in vitro plantlet regeneration and antioxidant activity of Enicostema axillare (Lam.) Raynal ssp. littoralis (Blume) Raynal: an important medicinal plant. Asian Pac J Reprod 2014; 3(3): 241-248.
[26] Thiyagarajan M, Venkatachalam P. A reproducible and high frequency plant regeneration from axillary node explants of Gymnema sylvestre (Gurmur) - An important antidiabetic endangered medicinal plant. Indus Crops Prod 2013; 50: 517-527.
[27] Kintzios S, Nikolaou A, Skoula M. Somatic embryogenesis and in-vitro rosmarinic acid accumulation in Salvia officinalis and S. fruticosa leaf callus cultures. Plant Cell Rep 1999; 18: 462-466.
[28] Phulwaria M, Ram K, Gahlot P, Shekhawat NS. Micropropagation of Salvadora persica- a tree of arid horticulture and forestry. New Forests 2011;42(3):317-327.
[29] Mousavi ES, Behbahani M, Hadavi E, Miri SM. Callus induction and plant regeneration in lisianthus (Eustoma grandiflorium). Trakia J Sci 2012; 10(1) 22-25.
[30] Bagheri A, Safari M. In vitro culture of higher plants. Translate. 4th ed. Iran: Ferdowsi University of Mashhad; 2009 ,p.406.
[31] Lei Z, Chenghong L, Ling L, Wanshing C. Callus induction and adventitious shoot regeneration from petiole of Erigeron breviscapus. Plant Prod Sci 2007; 10(3): 343-345.
[32] Yesil-Celiktas O, Nartop P, Gure lA, Bedir E, Fazilet Vardar-Sukan. Determination of phenolic content and antioxidant activity of extracts obtained from Rosmarinus officinalis calli. J Plant Physiol 2007; 164: 1536-1542.
[33] Vinterhalter B, Janković T, Šavikin K, Nikolić R, Vinterhalter D. Propagation and xanthone content of Gentianella austriaca shoot cultures. Plant Cell, Tissue & Organ Culture 2008; 97: 329–335.
[34] Malik SK, Chaudhary R, Kalia RK. Rapid in vitro multiplication and conservation of Garcinia indica: A tropical medicinal three species. Sci Hort 2005; 106: 539–553.
[35] Pandey S, Singh M, Jaiswal U, Jaiswal VS. Shoot initiation and multiplication from a tree of Terminalia arjuna Roxb. In Vitro Cell Dev Biol Plant 2006; 42: 389–393.
[36] Mahendran G, Narmatha Bai V. Micropropagation, antioxidant properties and phytochemical assessment of Swertia corymbosa (Griseb.) Wight ex CB Clarke: a medicinal plant. Acta Physiologiae Plantarum 2014; 36(3):589-603.
[37] Kaviani B. Micropropagation of ten weeks (Matthiola incana) and Lisianthus (Eustoma grandiflorum) (Two ornamental plants) by using kinetin (Kin), naphthalene acetic acid (NAA) and 2,4-Dichlorophenoxyacetic acid (2,4-D)’, Acta Sci Pol Hortorum Cultus 2014; 13(1): 141-154.
[38] Das NP, Pereira TA. Effects of flavonoids on thermal auto oxidation of palm oil: structure–activity relationship. J Am Oil Chem Soc 1990; 67: 255–258.
[39] De Gaulejac NSC, Glories Y, Vivas N. Free radical scavenging effect of anthocyanins in red wines. Food Res Int 1999; 32: 327–333.
[40] Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y. Effects of the interaction of tannins with co-existing substances. VI: effects of tannins and related polyphenols on superoxide anion radical and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull 1989; 37: 2016–2021.
[41] Canadanovic-Brunet JM, Djilas SM, Cetkovic GS. Free-radical scavenging activity of wormwood (Artemisia absinthium) extracts. J Sci Food Agric 2005; 85: 265–272.
[42] Sengul M, Yildiz H, Gungor N, Cetin B, Eser Z, Ercisli S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak J Pharm Sci 2009; 22; 102–106.
[43] Jayasinghe C, Jayasinghe C, Goto N, Aoki T, Wada S. Phenolics compositionand antioxidant activity of sweet basil. J Agric Food Chem 2003; 51:4442–4449.
[44] Ali MB, Khatun S, Hahn EJ, Paek KY. Enhancement of phenylpropanoidenzymes and lignin in Phalaenopsis orchid and their influence on plant acclima-tisation at different levels of photosynthetic photon flux. Plant Growth Regul 2006; 49: 137–146.
[45] Kim HJ, Chen F, Wang Xi Choi JH. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). J Agric Food Chem 2006; 54: 7263–7269.
[46] Ali MB, Hahn EJ, Paek KY. Methyl jasmonate and salicylic acid inducedoxidative stress and accumulation of phenolics in Panax ginseng bioreactor rootsuspension cultures. Molecules 2007;12 (3): 607–621.
[47] Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J Agr Food Chem 1992; 40: 945–948.
[48] Fukumoto L, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J Agr Food Chem 2000; 48: 3597-3604.
[49] Baskar R, Lavanya R, Mayilvizhi S, Rajasekaran P. Free radical scavenging activity of antitumor polysaccharide fractions isolated from Ganoderma lucidum (Fr.) P. Karst. Nat Prod Rad 2008; 7(4): 320-325.
[50] Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem 2005; 90: 743–749.
[51] Giri L, Dhyani P, Rawat S, Bhatt ID, Nandi SK, Rawal RS, et al. In vitro production of phenolic compounds and antioxidant activity in callus suspensioncultures of Habenaria edgeworthii: a rare Himalayan medicinal orchid. Ind Crops Prod 2012; 39: 1–6.
[52] Diwan R, Shinde A, Malpathak N. Phytochemical composition andantioxidant otential of Ruta graveolens L. in vitro culture lines. J Bot 2012; http://dx.doi.org/10.1155/2012/685427,.
27 October 2015
Loganathan Kousalya, Plant Tissue Culture Laboratory, Department. of Botany, Bharathiar University, Coimbatore-641046, India.
E-mail: lkousalya25@gmail.com
Received in revised form 10 December 2015 Accepted 2 January 2015
Available online 1 March 2015
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Effect of heparin, caffeine and calcium ionophore A 23187 on in vitro induction of the acrosome reaction of fresh ram spermatozoa
- The characterisation and cryopreservation of Venda chicken semen
- A new nucleotide variant G1358A potentially change growth differentiation factor 9 profile that may affect the reproduction performance of Friesian Holstein cattle
- Pregnancy rate in Bulgarian White milk goats with natural and synchronized estrus after artificial insemination by frozen semen during breeding season
- Pregnancy outcomes of using ICSI with frozen-thawed spermatozoa in Riyadh, Saudi Arabia
- Effects of intramuscular injections of vitamin E-selenium and a gonadotropin releasing hormone analogue (GnRHa) on reproductive performance and blood metabolites of post-molt male broiler breeders
