A new nucleotide variant G1358A potentially change growth differentiation factor 9 profile that may affect the reproduction performance of Friesian Holstein cattle
2016-10-18AfifiInayahSriRahayuWidodoWidyaAyuPrasdini
Afifi Inayah, Sri Rahayu*, Widodo, Widya Ayu Prasdini
1Biology Department, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang East Java, Indonesia
2Training Center for Animal Husbandry, Batu, East Java, Indonesia
A new nucleotide variant G1358A potentially change growth differentiation factor 9 profile that may affect the reproduction performance of Friesian Holstein cattle
Afifi Inayah1, Sri Rahayu1*, Widodo1, Widya Ayu Prasdini2
1Biology Department, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang East Java, Indonesia
2Training Center for Animal Husbandry, Batu, East Java, Indonesia
ARTICLE INFO
Article history:
BMP-15
Folliculogenesis
GDF-9
In silico
Docking molecule
ABSTRACT
Objective: To determine the polymorphism of GDF-9 gene in FH cattle that may affect its interaction with the BMP-15 protein. Methods: Blood was taken from the jugular vein using 10 mL sterile syringes from ten Friesian Holstein cattle. The DNA were isolated from the whole blood and used as a template to amplify GDF-9 gene. The Amplicon (PCR product) was sequenced to identify the new SNP. The three-dimensional structure of GDF-9 protein was modeled by SWISS-MODEL. The characteristic of the protein structure was analyzed by using projectHOPE. The binding affinity GDF-9 into MBP was examined by PatchDock and FireDock. Results: The result indicates a new variant G1358A changed amino acid residue at position 453 from Arginine into Histidine (R453H). In silico analysis using projectHOPE predicted that the variant altered side chain of GDF-9 and changed its interaction with BMP-15. Further study suggested that the polymorphism R453H also reduce the binding affinity of the GDF-9 into BMP-15 from -85.58 kcal/mol (R453) into -80.79 kcal/mol (H453). Conclusion: The new variant G1358A at anestrous FH cattle has potency alter GDF-9 profile that may affect to reproduction performance.
Document heading doi: 10.1016/j.apjr.2016.01.010
1. Introduction
GDF-9 is a member of Transforming Growth Factor β (TGF-β) superfamily[1,2] that has a significant role in follicle growth and development at all stages of folliculogenesis[3]. GDF-9 could influence the initiation of primordial follicle[4] in rat[5] and human ovaries[6]. GDF-9 promotes follicular survival by suppressing granulosa cell apoptosis and follicular atresia[7]. Therefore, GDF-9 regulates proliferation, differentiation and cumulus expansion of granulosa cells[8,9] which can influence oocyte maturation. Also, GDF-9 may also involve luteinization of the follicle at ovulation [10]. Knockout mice are lacking GDF-9 lead to infertility[11].
GDF-9 gene consist of 2 exons and 1 intron[1,12,13]. Exon 2 encoding an entire mature peptide of protein[14] and mutation in the region may affect the reproduction performance in sheep. Polymorphism of GDF-9 has lower ovulation rate in Moghani and Ghezel sheep[15]. Homozygote mutation S395F can lead to sterility in Cambridge and Belclare sheep[16]. Mutation S109R also has an effect on the fecundity of Thoka sheep[17]. Also, a mutation at position V371M has associated with litter size in Norwegian White Sheep[18,19].
The SNP is likely to affect the three-dimensional structure of the GDF-9 protein[18] and reduce its binding capacity with other protein that may influence the phenotype[19]. GDF-9 is important to interact with receptor or dimer protein which is BMP-15 protein[20] that related to fertility performance. The GDF-9 gene can be a candidate gene for determining the fertility of livestock[12]. Therefore, polymorphism of GDF-9 in exon 2 is important to be evaluated to explore biomarker of cattle fertility.
2. Material and methods
2.1. Amplification GDF-9 gene
This blood samples were collected from 10 Friesian Holstein(FH) cattle from Training Center for Animal Husbandry, Batu-Indonesia. Three of samples have low performance that is anestrous while the rest of samples have normal performance. Then blood was taken from each FH cattle from the jugular vein using 10 mL sterile syringes. Obtained blood is placed in an EDTA-vacutainer and stored at -20 曟. The DNA were isolated using NORGEN kit (#46300). Amplification of GDF-9 gene exon 2 using forward primer 5’AAGACTCTCCCTAGAGCTCCATACTC3’ and reverse primer 5’TAGAACTGCAATTCCACCCAAG3’ [21]. The PCR product was purified and sequenced in First Base co.Ltd., Malaysia.
2.2. Variant analysis
The sequences of each sample were aligned with GDF-9 Bos taurus (M_174681.2) sequence to find SNP in the sequence of samples. Nucleotide sequence translated into an amino acid sequence by using MEGA5 software to determine the SNP may result in amino acid alteration.
2.3. Prediction of polymorphism’s effect
Tertiary structure of GDF-9 protein was modeled by using homology method (SWISS-MODEL) based on BMP-3 (2QCQ) as a template. The protein model was validated by using Ramachandran Plot. The effect of polymorphic on a characteristic of protein structure was analyzed by using projectHOPE web server, pyMol and YASARA software. The binding affinity of the protein with its protein partner (MBP) was done by PatchDock and FireDock web server.
3. Results
Amplification of GDF-9 gene exon 2 using the primers yielded an 1100 bp DNA. Sequencing results of the PCR product of the tens samples have 99%-100% similarity to GDF-9 gene of Bos taurus (GQ922451.1; NM_174681.2). Further sequence Alignment analysis indicated that one of the ten samples has variant in position 1358 (Figure 1). The variant is not recorded yet in the Gene Bank (NCBI) and occur in the anestrus cattle.
In silico analysis revealed the substitution of guanine into adenine (G1358A) has changed amino acid Arginine into Histidine at position 453. The amino acid residue located at the surface of protein and lies on the relevant domain to carry out the primary activity of the GDF-9 protein. The alteration of arginine into histidine residue (R453H) may result in a shift rotation some residues and this effect to the shape or difference protrusions of the protein surface (Figure 2). Also, the projectHOPE analysis predicted the arginine residue involved in the ionic reaction, the alteration of the residue likely to interfere protein activity.
Further docking analysis showed that the substitution of Arginine into Histidine reduce the binding affinity of GDF-9 into BMP-15 (Figure 3). The binding energy of GDF-9 wild type (R453) and mutant (H453) with BMP-15 are -85.88 and -80.79, respectively. The docking protein analysis suggested that Histidine residue altered the binding pattern of GDF-9 with BMP-15 and caused loosing 24 binding sites.
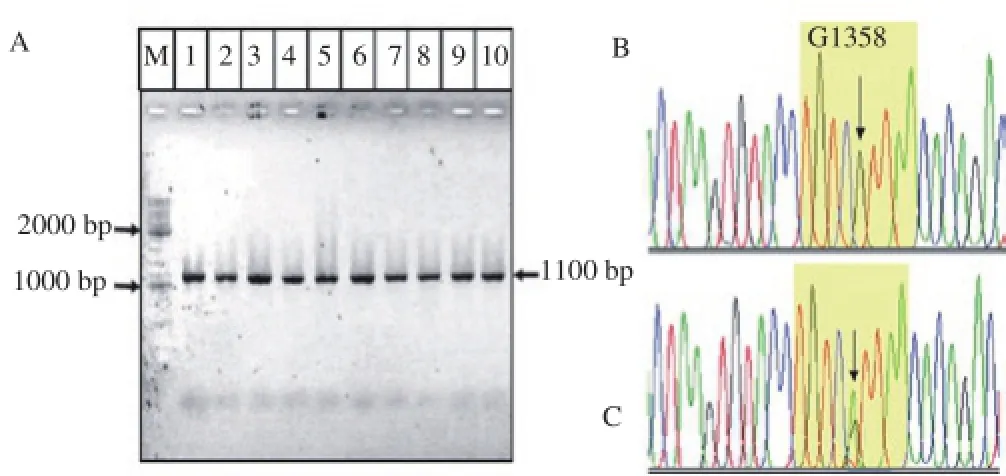
Figure 1. One of ten samples has new variant G1358A of GDF-9 gene. The PCR product of GDF-9 gene (A) that have wild-type sequence (B) and new variant A1358 (C). M=DNA ladder 100-10000bp. Lane 1-10= PCR samples.
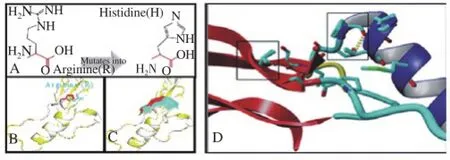
Figure 2. The variant G1358A of GDF-9 gene changes Amino acid from Arginine into Histidine (A), that altered side chain (B) and the surface shape (C) of GDF-9 wild type (blue) into new variant (red). The amino acid residues substitution shifted the rotation of the two side chain (black box in panel D).
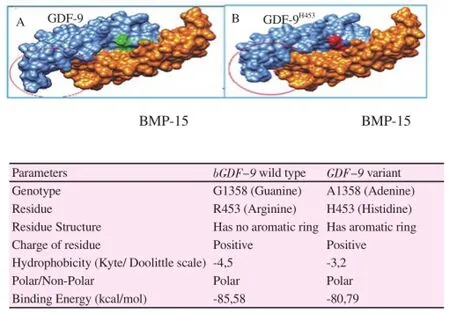
Figure 3. The variant R453H changed binding pattern and affinity of GDF-9 with BMP-15. GDF-9 wild type bound to BMP-15 (A) and GDF-9H453 (variant) bound to BMP-15 (B) that changed binding pattern (red circle) and energy.
4. Discussion
The new variant G1358A at gene GDF-9 was found in one among three samples of anestrous cattle. The variant altered amino acid residue 453 from Arginine into histidine (R453H) of GDF-9. The arginine usually involved in the ionic reaction, alteration of the residue into histidine may disrupt GDF-9 function. The histidine reduced protein hydrophobicity and shifted rotation side chain of some amino acid that detracted protrusion on the protein surface.Therefore, the amino acid substitution possibly changed character and function of GDF-9. The GDF9 mutation has an effect on fertility and ovulation rate of Moghani and Ghezel sheep in Iran and Turkey[15]. The GDF-9 was expressed in ovaries that regulate folliculogenesis in domestic ruminants[1].
Further investigated, we found that the variation is resided in heterodimer binding domain of GDF-9, which is important to bind with BMP-15. Study on protein-protein interaction using molecular docking showed that variation R453H changed the binding affinity and pattern of the GDF-9 and BMP-15 complex. The Histidine residue caused loosing 24 bonds between GDF-9 and BMP-15 compared to Arginine. So the alteration Arginine into Histidine reduced the binding affinity of GDF-9 with BMP-15 from -85.88 kcal/mol into -80.79 kcal/mol, respectively.
The changing of binding affinity and pattern of the complex protein may interfere cellular cascade on oocyte maturation. This is due to the heterodimer of the GDF-9 and BMP-15 complex, which is required for normal follicular development and ovulation that that necessary to regulate of mammals fertility[20-22]. Moreover, GDF-9 promote granulosa cell to produce luteinizing hormone receptor that is essential to pre-ovulatory process[23]. The bone BMP-15 and GDF-9 play crucial roles in determining folliculogenesis, and oocyte-secreted factors, ovulation rate in sheep and mice[25]. GDF9 and BMP15 form a complex intrafollicular regulatory system during folliculogenesis[24]. So the alteration on the BMP-15 binding domain of GDF-9 may affect the fertility of FH.
The illustration is consistent with the data that all of normal FH in this study have not nucleotide variant 1358A. Whereas the variant 1358A was found in anestrous FH, although only in one among three samples. The study suggested that the new variant G1358A has potentially influenced the fertility performance of FH cattle. The result warranted to be explored further for examining the potential of the variant as a biomarker in FH cattle reproduction performance.
The new variant G1358A at an estrous FH a cattle more likely alter GDF-9 profile and function that may affected to reproduction performance of FH cattle.
Conflict of interest statement
The authors declare that there is no conflict of interests.
Acknowledgment
The author would like to thanks to Didik Huswo Utomo, who have helped in bioinformatics analysis.
References
[1] Bodensteiner KJ, Clay CM, Moeller CL, Sawyer HR. Molecular cloning of the ovine Growth/Differentiation factor-9 gene and expression of growth/differentiation factor-9 in ovine and bovine ovaries. Biol Reprod 1999; 60: 381–386.
[2] McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/ differentiation factor-9. Mol Endocrinol 1995; 9: 131–136.
[3] Otsuka F, McTavish KJ, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev 2011; 78: 9–21.
[4] Vitt UA, McGee EA, Hayashi M, Hsueh AJW. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats 1. Endocrinology 2000; 141: 3814–3820.
[5] Hayashi M, McGee EA, Min G, Klein C, Rose UM, van Duin M, et al. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology 1999; 140: 1236–1244.
[6] Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJW, Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab 2002; 87: 316–321.
[7] Orisaka M, Jiang JY, Orisaka S, Kotsuji F, Tsang BK. Growth differentiation factor 9 promotes rat preantral follicle growth by upregulating follicular androgen biosynthesis. Endocrinology 2009; 150: 2740–2748.
[8] Vitt UA, Hsueh AJ. Stage-dependent role of growth differentiation factor-9 in ovarian follicle development. Mol Cell Endocrinol 2001; 183: 171–177.
[9] Wu X, Matzuk MM. GDF-9 and BMP-15: oocyte organizers. Rev Endocr Metab Disord 2002; 3: 27–32.
[10] Juengel JL, Hudson NL, Heath DA, Smith P, Reader KL, Lawrence SB, et al. Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biol Reprod 2002; 67: 1777–1789.
[11] Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996; 383: 531–535.
[12] Ghaffari M, Nejati-Javaremi A, Rahimi-Mianji G. Lack of polymorphism in the oocyte derived growth factor (GDF9) gene in the Shal breed of sheep. South African J Anim Sci 2009; 39: 355–360.
[13] Tang KQ, Yang WC, Li SJ, Yang LG. Polymorphisms of the bovine growth differentiation factor 9 gene associated with superovulation performance in Chinese Holstein cows. Genet Mol Res 2013; 12: 390–399.
[14] Aono T, Sugino H, Vale WW. (eds.) Inhibin, activin and follistatin: regulatory functions in system and cell biology [Online]. Springer Science & Business Media; 2012. Available at: https://books.google.com/books?i d=7Iu1BwAAQBAJ&pgis=1[Accessed on 15 August 2015].
[15] Barzegari A, Atashpaz S, Ghabili K, Nemati Z, Rustaei M, Azarbaijani R. Polymorphisms in GDF9 and BMP15 associated with fertility and ovulation rate in Moghani and Ghezel sheep in Iran. Reprod Domest Anim 2010; 45: 666–669.
[16] Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, et al. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod 2004; 70: 900–909.
[17] Nicol L, Bishop SC, Pong-Wong R, Bendixen C, Holm LE, Rhind SM, et al. Homozygosity for a single base-pair mutation in the oocyte-specific GDF9 gene results in sterility in Thoka sheep. Reproduction 2009; 138: 921–933.
[18] Wang TT, Ke ZH, Song Y, Chen LT, Chen XJ, Feng C, et al. Identification of a mutation in GDF9 as a novel cause of diminished ovarian reserve in young women. Hum Reprod 2013; 28: 2473–2481.
[19] Våge DI, Husdal M, Kent MP, Klemetsdal G, Boman IA. A missense mutation in growth differentiation factor 9 (GDF9) is strongly associated with litter size in sheep. BMC Genet 2013; 14: 1.
[20] Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, et al. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA 2013; 110: E776–E785.
[21] Santos-Biase WKF, Biase FH, Buratini J, Balieiro J, Watanabe YF, Accorsi MF, et al. Single nucleotide polymorphisms in the bovine genome are associated with the number of oocytes collected during ovum pick up. Anim Reprod Sci 2012; 134: 141–149.
[22] Ernst Knobil, Jimmy D. Neill. Knobil and Neill’s physiology of reproduction. Oxford:Gulf Professiopnal publishing;2006.
[23] Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 1999; 13: 1035–1048.
[24] Silva JR V, Van Den Hurk R, Van Tol HTA, Roelen BAJ, Figueiredo JR. Expression of growth differentiation factor 9 (GDF9), bone morphogenetic protein 15 (BMP15), and BMP receptors in the ovaries of goats. Mol Reprod Dev 2005; 70: 11–19.
[25] Moore RK, Erickson GF, Shimasaki S. Are BMP-15 and GDF-9 primary determinants of ovulation quota in mammals? Trends Endocrinol Metab 2004; 15: 356–361.
1 August 2015
Sri Rahayu, Biology Department, Faculty of Mathematics and Natural Sciences, Brawijaya University, Jl. Veteran, Malang-Indonesia 65145.
Tel: +62-341-575841
E-mail: srahayu@ub.ac.id
Received in revised form 10 October 2015 Accepted 12 November 2015
Available online 1 March 2016
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Effect of growth regulators on rapid micropropagation and antioxidant acitivity of Canscora decussata (Roxb.) Roem. & Schult.-A threatened medicinal plant
- The characterisation and cryopreservation of Venda chicken semen
- Pregnancy rate in Bulgarian White milk goats with natural and synchronized estrus after artificial insemination by frozen semen during breeding season
- Effect of heparin, caffeine and calcium ionophore A 23187 on in vitro induction of the acrosome reaction of fresh ram spermatozoa
- Pregnancy outcomes of using ICSI with frozen-thawed spermatozoa in Riyadh, Saudi Arabia
- Effects of intramuscular injections of vitamin E-selenium and a gonadotropin releasing hormone analogue (GnRHa) on reproductive performance and blood metabolites of post-molt male broiler breeders
