Effects of intramuscular injections of vitamin E-selenium and a gonadotropin releasing hormone analogue (GnRHa) on reproductive performance and blood metabolites of post-molt male broiler breeders
2016-10-18AhmadHezarjaribiVahidRezaeipourRohullahAbdollahpour
Ahmad Hezarjaribi, Vahid Rezaeipour, Rohullah Abdollahpour
1Department of Animal Science, Qaemshahr Branch, Islamic Azad University, Qaemshahr, PO. Box 163, Iran
Effects of intramuscular injections of vitamin E-selenium and a gonadotropin releasing hormone analogue (GnRHa) on reproductive performance and blood metabolites of post-molt male broiler breeders
Ahmad Hezarjaribi, Vahid Rezaeipour*, Rohullah Abdollahpour
1Department of Animal Science, Qaemshahr Branch, Islamic Azad University, Qaemshahr, PO. Box 163, Iran
ARTICLE INFO
Article history:
Vitamin E
GnRH
Testosterone
Male broiler breeder
ABSTRACT
Objective: To investigate the effects of intramuscular injection of vitamin E-selenium and a GnRH analogue (GnRHa) on reproductive performance and serum biochemical parameters in post-molt male broiler breeders. Methods: A total of 32 ROSS 308 male broiler breeders (60 weeks of age) were induced to molt and then were randomly distributed into four groups: group 1 (control) without any injection, group 2 subjected to intramuscular of 0.1 mL/kg body weight of vitamin E-selenium, group 3 subjected to intramuscular of 0.3 mL Receptal solution as a GnRHa and group IV subjected to intramuscular both of vitamin E-selenium and GnRHa. Results: The results showed that the egg hatchability and fertility percentages were increased and the eggs infertility percentage declined significantly in groups 3 and 4 (P<0.05). However, eggs and chicks weight was not affected by experimental treatments (P>0.05). The results of blood biochemical parameters indicated that serum glucose was higher in group 3 which was injected with GnRHa (P<0.05). The experimental treatments did not alter hepatic enzymes activity including AST and ALT (P>0.05). The results showed that serum testosterone concentration was increased in groups 3 and 4 (P< 0.05). In addition, the serum concentration of T4 (tetraiodothyronie) was higher in groups 3 and 4 (P<0.05). However, the T3 (triiodothyronine) concentration was not influenced by experimental treatments (P>0.05). Conclusions: It is concluded that reproductive performance and serum testosterone in post-molt male broiler breeders were improved by treated with vitamin E-selenium and GnRHa.
Document heading doi: 10.1016/j.apjr.2016.01.013
1. Introduction
Age has an adverse effect on the reproductive success in birds. Fertility of domestic roosters kept under controlled conditions, peaks at about 37 weeks of age and then decreases rapidly at 45 weeks of age. The low-fertility roosters are characterized by: (1) a significant decline in concentration of ejaculated sperm [1]; (2) malformations of sertoli cell ectoplasmic specializations [2]; and (3) structural changes in the Leyding cells [3]. Force molting is an economical approach which improves the productive and reproductive life span of aged roosters. However, it has been previously reported that semen quality and quantity in molted males is affected by the process of molting [4]. Recently, it was shown that semen quality in molted male broiler breeders was improved when they were fed with different feed additives in the post-molt period [5]. Several reports have been published on the beneficial effects of Vitamin E on improving reproductive traits in male poultry [6-8]. However, most of these studies have been conducted on the male birds at the peak of production. Moreover, the effects of vitamin E were examined without consideration of other factors such as selenium. Selenium and vitamin E are involved in many biochemical and physiologicalprocesses in animal organism, including those related to reproduction [9]. Particularly relevant to semen quality is the antioxidant enzyme glutathione peroxidase (GSH-Px), a selenium dependent enzyme that serves to protect cellular membranes from peroxidative damages [10]. This enzyme has an important role in the maintenance of testicular function, spermatogenesis and spermatozoa functions [11]. Therefore, in the present experiment, we used a supplement in term of Selenovit premix containing vitamin E and selenium.
A number of hormones control sexual maturity, semen production, and the behavior connected with reproduction in male broiler breeders. In this regard, testosterone is the primary sex steroid in the avian testis. So, relative changes in the secretion of this hormone are likely to correlate with, or be a reliable index of, testicular activity[12]. Also, it is well known that testicular function in birds and human is controlled by gonadotropin releasing hormones (GnRH) secretion which is responsible of stimulating the gonadotropes of the pituitary to secrete luteinizing hormone (LH) and follicle stimulating hormone (FSH) [13]. Therefore, the administration of a synthetic GnRH may be resulted in the sustained release of LH from the anterior pituitary and the production Leydig cell enzymes capable of converting cholesterol into testosterone in post-molted male broiler breeders.
The objectives of this study were to investigate the effects of vitamin E and a GnRHa (Receptal solution) on reproductive traits and some blood biochemical parameters of post-molted male broiler breeders.
2. Materials and methods
2.1. Birds and treatments
This experiment was performed in a commercial broiler breeder farm. All procedures followed in this experiment were approved by Islamic Azad University, Qaemshahr branch, Qaemshahr, Iran. Thirty two ROSS-308 male broiler breeders at the age of 60 weeks were induced to molt with ZnO at the rate of 3 000 mg/kg of feed with a moderate decrease in lighting schedule from 16 to 12 h and they were offered 50 g/bird feed on the daily basis [5]. After completion of molting roosters were randomly assigned to four groups with four replicates (pens) per group in a completely randomized design. Each pen was equipped with a drinker, two separate-sex feeders, and a nest box. The birds were reared on floor pens and average temperature of day and night was 24 曟.
Treatment groups were as following: group I: male broiler breeders without any injection; group II: male broiler breeders were injected with 0.1 mL/kg body weight of vitamin E-selenium; group III: male broiler breeders were injected with receptal solution (0.2 mL) as a GnRHa; and group IV: male broiler breeders were injected with both of vitamin E-Selenium and GnRHa. Receptal solution used in this experiment is a GnRH analogue with a chemical entity of Buserelin acetate (0.0042 mg/mL). This solution was injected to male broiler breeder of each pen, weekly. The injectable solution of vitamin E-Selenium was purchased from Makian Daru Company (Iran). This solution contained 50 and 0.5 mg/mL of vitamin E and Sodium Selenite, respectively.
2.2. Reproductive traits
In order to study reproductive traits in this experiment, eight hens at the age of 40 weeks were subjected to each pen. The separatesex feeding was applied for hens. Therefore, the hens did not access to male broiler breeders diets. The reproductive data including hatchability, fertility, infertility, egg weight and chick weight were recorded during eight weeks. Eggs were collected and weighted daily and incubated for 10 days. Then, the eggs were examined for fertility. The fertile eggs were subjected to hatchery machine and hatchability percentage was measured at the end of hatchery time. In addition, the weight of hatched chicks was recorded. The overall means of these parameters for eight weeks were calculated and statistically analyzed.
2.3. Blood parameters
Heparinized blood samples (5 mL) were taken from each male broiler breeder via the brachial wing vein at the end of the experiment. The blood sample was drawn and allowed to clot at room temperature (18 曟) for 2 h prior to serum collection. Serum was separated by centrifugation and stored at -20 曟 for further analysis. A part of blood sample was prepared to measure the serum glucose, cholesterol and total lipids concentrations using commercial diagnostic kits (Pars Azmon, Tehran, Iran). Also, sera samples were used to measure the activities of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) as the indicators of liver health. The liver enzymes activity was assayed by auto analyzer (ALCYON 300). The concentration of triiodothyronine (T3) and thyroxine (T4) were determined using kits which provided from Tabeshyarnoor Company in Iran. Serum testosterone was also assayed by ELISA procedure described by Sauer MJ[14].
2.4. Statistical analysis
Data were subjected to ANOVA in a completely randomized design with four treatments and statistically analyzed using SAS (v. 9.1, SAS Inst. Inc., Cary, NC, USA). Pen was the experimental unit. Statistical significance of differences among treatments was done using the Duncan’s multiple range test at (P <0.05).
3. Results
The results of reproductive performance of post-molt male broiler breeders in response to experimental treatments are shown in Table 1. The weight of eggs and chicks were not influenced by treatments. The results indicated that infertile eggs (%) were greater in control group (treatment I) compared with other groups (P<0.05).In this regard, the percentage of fertile eggs and hatchability was significantly increased in treatment III and IV (P<0.05).
Effects of experimental treatments on blood biochemical parameters are presented in Table 2. These results showed that the serum concentration of cholesterol, total lipids and calcium did not alter by treatments. However, the glucose level was affected by experimental treatments (P<0.05). The male birds treated by GnRHa (III) and control group (I) had the greatest and lowest serum glucose level, respectively.

Table 1 Effects of treatments on reproductive traits of male broiler breeders.
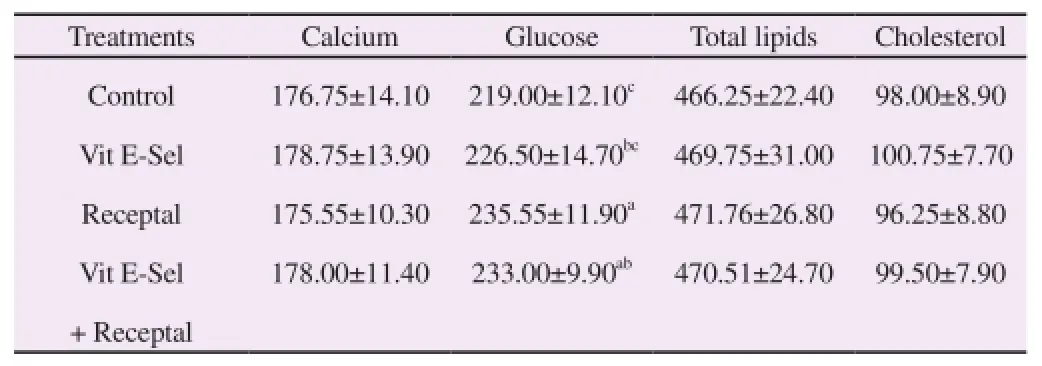
Table 2 Effects of treatments on blood biochemical parameters in male broiler breeders (mg/L).
The means of blood hormones including T3, T4and testosterone with serum concentration of liver enzymes (ALT and AST) are shown in Table 3. Results showed that the liver enzymes activities and T3concentration were not influenced by experimental treatments. In contrast, the serum concentration of T4and testosterone altered by treatments (P<0.05). In this regard, the levels of T4and testosterone were higher in male birds of treatment IV.
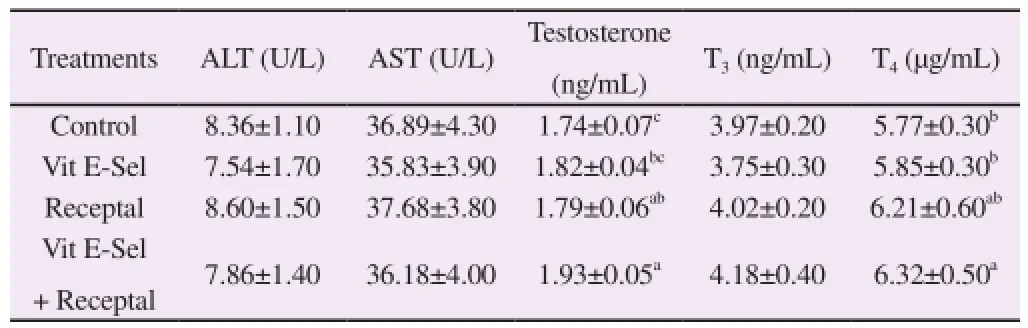
Table 3 Effects dietary treatments on blood hormones and liver enzymes in male broiler breeders.
4. Discussion
The reproductive traits of post-molt male broiler breeders were improved by using vitamin E-selenium injection in present experiment. Effects of vitamin E and selenium on reproductive traits of male birds were studied previously [15-17]. It is reported that vitamin E combined with selenium provided the best protection against lipid peroxidation in chicken semen compared with vitamin E or selenium standing alone in a diet [18]. Seleniumdependent glutathione peroxidase is an essential component of the antioxidant system in avian semen [9]. In this regard, Flohe L[19] suggested that the main problem to arise in selenium deficient spermatozoa is an imprecise architecture of the sperm midpiece. Experiments with broiler breeders showed that semen quality can be achieved by supplementation of selenium and vitamin E in diet[20, 21]. According to the results of Long and Kramer [22], Lipid peroxidation is a significant factor affecting the fertility of stored turkey sperm. Besides, they stated that addition of vitamin E alone was not sufficient to deter lipid peroxidation during storage of turkey semen. According to this literature review, it is suggested that vitamin E combined with selenium had an important role to increase the fertility traits in post-molt male broiler breeders in present experiment.
Few studies have been conducted on the use of GnRH analogues in male broiler breeders. However, we found several studies that demonstrate the mechanism action of GnRH analogues in mammals and birds [12, 23, 24]. GnRH analogues stimulate GnRH receptors in the anterior pituitary gonadotropes, causing FSH and LH production and release [25]. Our results are in accordance with findings of Elnagar SA[26] who reported that the synthetic GnRH (receptal solution) was capable of improving 40 week old cockerel’s reproductive status. It is suggested that the endocrine response to GnRH agonist treatment can be characterized by two phases, including acute and chronic phases, which during at acute phase there is an initial hyperstimulation of LH and FSH, followed by an increase in testosterone [23]. Previous studies have shown a reduction in the amplitude of release and concentration of hypothalamic GnRH following a reduction in the synthesis of pituitary FSH and LH in aging male Japanese quails [25, 27]. These results are in agreement with Avital-Cohen N[28] who reported that low fertility was accompanied by a reduction in hypothalamic GnRH expression in white leghorn roosters. Ottinger MA[29] described the Neuroendocrine regulation of GnRH and behavior during aging in quails. They noted that qualitative and quantitative alterations in aromatase enzyme system following the change in estradiol secretion regulate the sexual behavior or the control the secretion of GnRH in male quails. Furthermore, aging roosters had lower semen-quality variables, plasma testosterone concentration and mRNA expression of hypothalamic GnRH than young roosters [30]. Therefore, itconcluded that the use of GnRH analogue increased the reproductive activity of post-molt male broiler breeders in our experiment.
The serum glucose concentration increased in male broiler breeders injected with GnRHa in the present research. Weil S[31] indicated that plasma insulin levels were higher in low-fertility, aging rooster than in high-fertility roosters. Reduced glucose uptake, in part, appears to be responsible for reduced metabolism and poor motility of sperm in roosters [32]. This study supported the results of Jutte NHPM[33], who reported that Insulin as an endocrine regulator, like FSH, is essential in the regulation of glucose transport and lactate production by sertoli cells. Thus, it can be stated that the increase in blood glucose levels in this experiment could be related to increase male broiler breeder’s fertility.
Results of present experiment showed that liver enzymes activity of post-molt male broiler breeders was not affected by both of vitamin E-selenium and GnRHa injection. In contrast, [4] reported that serum AST and ALT decreased significantly in post-molt male broiler breeders fed vitamin E compared with those fed vitamin C, probiotic and proteins. They stated that lower AST and ALT levels are the indicator of better liver health in animals. A significant reduction in AST and ALT enzymes activities was observed in broiler chicks fed 0.3 ppm selenium [34]. The overall knowledge about the effect of GnRH analogues on the liver enzymes activities in male broiler breeders is limited. Therefore, direct comparisons cannot be made.
The levels of T4 and testosterone were increased in post-molt male broiler breeders injected with either vitamin E-selenium or GnRHa in present research. Khan RU[4] indicated that the serum thyroid hormone concentration increased in post-molt male broiler breeders fed diets supplemented with vitamin E. Thyroid hormones are involved in controlling metabolic rate, and the concentration of circulating T3 is positively correlated with oxygen consumption in broilers [35]. The effects of thyroid hormones alterations on the reproductive system have been studied in animals and have generally shown that changes from normal thyroid function resulted in decreased sexual activity and fertility [36]. Direct effect of T4 resulted in minimal oxygen consumption changes in testes following increased amount of testosterone biosynthesis [37]. The underlying reason for increased of T4 in response to GnRH analogues administration in male broiler breeders is not known.
The serum testosterone hormone was increased significantly in post-molt male broiler breeders treated with vitamin E-selenium combined with GnRHa. The positive effect of vitamin E-selenium on testosterone secretion may be associated with testes better utilization of selenium and vitamin E [15]. According to these authors, the increased levels of these antioxidants contributed to the maintenance of the seminiferous tubules and testosterone biosynthesis that is consistent with observations of Golzar-Adabi SH[38] who reported that the plasma testosterone concentration did not alter by using vitamin E supplementation in Japanese quails diet.
In conclusions, the results of this experiment showed that fertility characteristics were improved in post-molt male broiler breeders treated by both vitamin E-selenium and GnRHa. In addition, the levels of serum T4 and testosterone were increased in male birds received vitamin E-selenium and GnRHa. However, more research is needed to clear the mechanism action of GnRH analogues on liver enzymes activities and thyroid hormones in domestic birds.
Conflict of inertest statement
We declare that we have no conflict of interest.
References
[1] Rosenstrauch A, Degen AA, Friedlander M. Spermatozoa retention by srtoli cells during the decline in fertility in aging roosters. Biol Reprod 1994; 50: 129.
[2] Weil S, Degen AA, Rosenstrauch A, Friedlander SM. Intratesticular spermatozoa retention in low fertility aging roosters is rated to malformations of sertoli cell ectoplasmic specializations. J Exp Zool 1996; 275: 317.
[3] Rosenstrauch A, Weil S, Degen AA, Friedlander M. Leydig cell functional structure and plasma androgen level during the decline in fertility in aging roosters. Gen Comp Endocrinol 1998; 109: 251–258.
[4] Khan RU, Rahman Z, Javed I, Muhammad F. Supplementation of vitamins, protein and probiotics on semen traits and immunohistochemical study of pituitary hormones in zinc-induced molted broiler breeders. Acta Histochem 2013; 115: 698.
[5] Khan RU, Rahman Z, Javed I, Muhammad F. Effects of vitamins, probiotics and protein on semen traits in post-molt male broiler breeders. Anim Rep Sci 2012; 135: 85.
[6] Lin YF, Chang SJ, Yang JR, Lee YP, Hsu L. Effects of supplemental vitamin E during the mature period on the reproduction performance of Taiwan native chicken cockerels. Br Poutl Sci 2005; 46: 366.
[7] Ceroloni S, Zaniboni L, Maldjian A, Gliozzi T. Effect of decosahexaenoic acid and aplpha tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology 2006; 66: 877.
[8] Biswas A, Mohana J, Sastrya KVH. Effect of high dietary vitamin E concentrations on physical and biochemical characteristics of semen in Kadaknath cockerels. Br Poult Sci 2009; 50: 733.
[9] Surai PE, Blesbois E, Grasseau I, Chalah T, Brillard JP, Wishart G, et al. Fatty acid composition, glutathione peroxidase and superoxide dismutase activity and total antioxidant activity of avian semen. Comp Bioch Physiol 1998; 120(B): 527.
[10] Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: effect on semen parameters and pregnancy rate. Int J Gen Med 2011; 4: 99.
[11] Marin-Guzman J, Mahan DC, Pate JL. Effect of dietary selenium and vitamin E on spermatogenic development in boars. J Anim Sci 2000; 78:1536.
[12] Lovas EM, Johnston SD, Filippich LJ. Using a GnRH agonist to obtain an index of testosterone secretory capacity in the cockatiel (Nymphicus hollandicus) and sulphur-crested cockatoo (Cacatua galerita). Aust Vet J 2010; 88: 52.
[13] Sharp PJ, Gow CB. Neuroendocrine control of reproduction in the cockerel. Poult Sci 1983; 62: 1671-1675.
[14] Sauer MJ, Cookson AD, McDonald BJ, Foulkes JA. The use of enzyme immunoassay for the measurement of hormones with particular reference to the determination of progesterone in unextracted whole milk. In: The ELISA. 1st ed. Netherlands: Martinus Nijhoff Publishers; 1982,p.271-296.
[15] Jerysz A, Lukaszewicz E. Effect of dietary selenium and vitamin E on ganders’ response to semen collection and ejaculate characteristics. Biol Trace Elem Res 2013; 153: 196.
[16] Tabatabaei S, Batavani R, Ayen E. Effects of vitamin E addition to chicken semen on sperm quality during in vitro storage of semen. Vet Res Forum 2011; 2: 103.
[17] Barber SJ, Parker HM, McDaniel CD. Broiler breeder semen quality as affected by trace minerals in vitro. Poult Sci 2005; 84: 100.
[18] Adebiyi OA, Aliu OT, Majekodunmi BC, Adeniji OA. Effect of vitamin E and selenium on fertility, hatchability and survivability of turkeys. J Anim Sci Adv 2014; 4:955.
[19] Flohe L, Brigelius-Flohe R, Maiorino M, Roveri A, Wissing J, Ursini F, et al. Selenium and male reproduction. Dordrecht: Kluwer Academic Publisher; 2001, p.273-281.
[20] Edens FW, Sefton AE. Sel-Plex improves spermatozoa morphology in broiler breeder males. Int J Poult Sci 2009; 8: 853.
[21] Gallo R, Veronico M, Nacucchi O, Tafaro E, Barile P, Nicastro F, el al. The effect of selenium, zinc and vitamin E supplementation on performance of broiler breeder males. Ital J Anim Sci 2003; 2(supplement): 471.
[22] Long JA, Kramer M. Effect of vitamin D on lipid peroxidation and fertility after artificial insemination with liquid-stored turkey semen. Poult Sci 2003; 82: 1802.
[23] Herbert CA, Trigg TE, Renfree MB, Shaw G, Eckery DC, Cooper DW. Effect of a gonadotropin-releasing hormone agonist implant on reproduction in a male marsupial, Macropus eugenii. Biol Rep 2004; 70: 1836.
[24] Shimizu M, Bedecarrats GY. Activation of the chicken gonadotropininhibitory hormone receptors reduces gonadotropin-releasing hormone receptor signaling. Gen Comp Endocrinol 2010; 167: 331.
[25] Ottinger MA, Abdelnabi M, Li Q, Chen K, Thompson M, Harada N, et al. The Japanese quail: a model for studying reproductive aging of hypothalamic system. Exp Gerontol 2004; 39: 1679.
[26] Elnagar SA. Response of Alexandria cockerel’s reproductive status to GnRH (Receptal) injection. Int J Poult Sci 2009; 8: 242.
[27] Ishii S. The molecular biology of avian gonadotropin. Poult Sci 1993; 7: 856.
[28] Avital-Cohen N, Heiblum R, Argov-Argaman N, Rosenstrauch A, Chaiseha Y, Mobarkey N, et al. The effect of active immunization against vasoactive intestinal peptide (VIP) and inhibin on reproductive performance of aging white leghorn roosters. Poult Sci 2012; 91: 161.
[29] Ottinger MA, Thompson M, Viglietti-Panzica C, Panzica GC. Neuroendocrine regulation of GnRH and behavior during aging in birds. Brain Cell Bull 1997; 44: 471.
[30] Avital-Cohen N, Heiblum R, Argov-Argaman N, Rosenstrauch A, Chaiseha Y, Mobarkey N, et al. Age-related change in gonadal and serotonergic axes of broiler breeder roosters. Dom Anim Endocrinol 2013; 44: 145.
[31] Weil S, Degen AA, Friedlander SM, Rosenstrauch A. Low fertility in aging roosters is related to a high plasma concentration of insulin and low testicular contents of ACTH and lactate. Gen Comp Endocrinol 1999; 115: 110.
[32] McLean DJ, Jones LG, Froman DP. Reduced glucose transport in sperm from roosters (Gallus domesticus) with heritable subfertility. Biol Rep 1997; 57: 791.
[33] Jutte NHPM, Grootegoed JA, Rommerts FFG, Van Der Molen HJ. FSH stimulation of the production of pyruvate and lactate may be involved in normal regulation of spermatogenesis. J Rep Fertil 1983; 68: 219.
[34] Peric L, Milosevic N, Zikic D, Kanacki Z, Dzinic N, Nollet L, et al. Effect of selenium sources on performance and meat characteristics of broiler chickens. J Appl Poult Res 2009; 18: 403.
[35] Gabarrou JF, Duchump C, Williams J, Geraert PA. A role of thyroid hormones in the regulation of dietinduced thermogenesis in birds. Br J Nutr 1997; 78: 963.
[36] Johnson CA. Thyroid issues in reproduction. Clin Tech Small Anim Pract 2002; 17: 129.
[37] Aruldhas MM, Valivullah HM, Srinivasan N, Govindarajulu P. Role of thyroid in testicular lipids in prepubertal, pubertal and adult rats.I. hyperthyroidism. Biochim Biophys Acta 1986; 881: 462.
[38] Golzar-Adabi SH, Cooper RG, Kamali MA, Hajibabaei A. The influence of inclusions of vitamin E and corn oil on semen traits of japanes quail. Anim Reprod Sci 2010; 123: 119.
6 October 2015
Vahid Rezaeipour, Department of Animal Science, Qaemshahr Branch, Islamic Azad University, Qaemshahr, PO. Box 163, Iran.
Tel: +98-912-4157511
E-mail: vrezaeipour@gmail.com
Received in revised form 5 January 2016 Accepted 10 January 2016
Available online 1 March 2016
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Effect of heparin, caffeine and calcium ionophore A 23187 on in vitro induction of the acrosome reaction of fresh ram spermatozoa
- The characterisation and cryopreservation of Venda chicken semen
- A new nucleotide variant G1358A potentially change growth differentiation factor 9 profile that may affect the reproduction performance of Friesian Holstein cattle
- Pregnancy rate in Bulgarian White milk goats with natural and synchronized estrus after artificial insemination by frozen semen during breeding season
- Pregnancy outcomes of using ICSI with frozen-thawed spermatozoa in Riyadh, Saudi Arabia
- Effect of growth regulators on rapid micropropagation and antioxidant acitivity of Canscora decussata (Roxb.) Roem. & Schult.-A threatened medicinal plant
