Reproductive toxicity of aqueous wood-ash extract of Azadirachta indica (Neem) on male albino mice
2016-10-18AutaHassan
Auta T., Hassan A. T.
1Department of Biological Sciences, Federal University Dutsin-Ma, Katsina State, Nigeria
Reproductive toxicity of aqueous wood-ash extract of Azadirachta indica (Neem) on male albino mice
Auta T.1*, Hassan A. T.2
1Department of Biological Sciences, Federal University Dutsin-Ma, Katsina State, Nigeria
2Department of Zoology, University of Ibadan, Ibadan, Nigeria
ARTICLE INFO
Article history:
Reproductive toxicology
Wood-ash
Sperm parameters
Hormones
Azadirachta indica
ABSTRACT
Objective: To evaluate the reproductive toxicity of aqueous wood ash extract of Azadirachta indica (A. indica) in male albino mice. Methods: Four different dose levels of 0, 5, 50 and 100 mg/kg body weight were administered to 20 male mice, with five mice per group for seven days that were sacrificed 35 days thereafter. Gonadosomatic index, sperm motility, sperm count, sperm morphology, serum follicle stimulating hormone (FSH), leuitinizing hormone (LH) and testosterone assay, and histopathology of testes were carried out. Results: Though no toxic effect on testicular weight, FSH, LH and testosterone (P>0.05), significant decrease in sperm motility, live/dead sperm and sperm count, with significant increase of abnormal sperm were recorded (P<0.05). Dose dependent histopathological damage of testes were obtained (P<0.05). Conclusion: Aqueous wood ash extract of A. indica have damaging effects on sperms and testicular tissues, which could impair reproduction.
Document heading doi: 10.1016/j.apjr.2016.01.005
1. Introduction
Aqueous wood ash extract of Azadirachta indica (A. indica) and other plants have been used as food additive and for medicinal purposes, such as stomach ache treatment among some ethnic groups in Middle-Belt region of Nigeria [1]. A. indica, which belongs to the plants Family Meliaceae has been well known in the traditional system of medicine for more than 2 000 years as one of the most versatile medicinal plants having a wide range of biological activity [2]. Various useful products such as antimalarials, spermicidals, antituberculosis agents, antipyrrhetics, antiviral drugs, antiseborrhoeics, antiallergic medicines, antienzymic, and antifungal agents, have been extracted from the A. indica [3]. A. indica have antiseptic, anti-helminth, antifungal, antibacterial, antipyretic, antimalarial, anti-diabetic and anti-fertility properties [4].
In recent years, there has been growing concern about the deleterious effects of chemicals on developing male reproductive system [5]. In the male reproductive system, weight loss of the gonads as well as reduced sperm count and epididymal sperm motility are considered standard criteria for the characterization of toxic agents that may cause fertility problems in the treated subject[6-7].
Sperms morphology serves as an important and sensitive indicator of chemical toxicity on the reproductive cells. They can be used to evaluate the spermatogenic damage, fertility and heritable genetic changes, which provide a direct measure of the quality of sperm production in chemically treated animals [8-10].
The testis is surrounded by a dense connectival capsule, called the tunica albuginea. From the internal surface of the tunica albuginea, the connective tissue septa depart toward the mediastinum within which the anastomotic network of ducts, the testis, is located. Spermatogenesis occurs within the seminiferous tubules which are located within the network of testicular lobules. Control of the reproductive process is finely regulated by the neuroendocrine system through the hypothalamus and pituitary axis [11]. Though several researches have been reported on A. indica products, there is dearth of information on reproductive toxicity of aqueous wood ash of A. indica. Hence, this study has been able to report the damaging effects on sperms and testicular tissues of aqueous wood ash extract of A. indica, which could impair reproduction.
2. Materials and methods
2.1. Plants and study design
Fresh A. indica (Neem) wood was collected from Katsina State, North-Western Nigeria. Stalk of the plant, carrying leaves and flowers, were collected and taken to the herbarium of Department of Biological Sciences, Ahmadu Bello University, Zaria for authentication and voucher number of 90051 was obtained. The wood processing to ash and sub sequent analysis was carried out as described by [1].
Reproductive toxicity study in male was carried out using methods described by [10, 12-14]. A total of 20 apparently healthy adult male mice were divided into 4 groups with 5 mice in each group. Group 1, serving as control was orally administered distilled water while 2, 3 & 4 received 5, 50 and 100 mg/kg bw aqueous wood ash extract of A. indica. They were kept in plastic cages with five 5 mice per cage in an environment of approximately 12 hour light/dark cycle, a temperature of (24 ±3 ) 曟. The mice were supplied with a standard diet and water ad-libitum.
2.2. Biochemical and morphological analysis
At 5 weeks (35 days) from the first administration, the mice were sacrificed by cervical dislocation and their caudal epididymis surgically removed, sperm smears were prepared from the epididymis. Gonadosomatic index, sperm motility and sperm count[12], Sperm morphology [15] FSH, LH and testosterone hormones were measured using microplate immunoenzymometric assay method (ELISA). Histopathology of testes was also carried out.
Immediately after sacrificing, the bloods were collected into plain 1.5 mL eppendough tubes. The blood were left to coagulate and then centrifuged at 3 000 rpm for 30 minutes to separate the serum. The separated serums were stored at -20 曟 for subsequent hormonal analyses. The circulating levels of testosterone, FSH and LH were determined using radioimmunoassay kits. TAC was also determined. The body weight of each mouse was determined immediately before sacrificing. After sacrifice and dissection, the testes were removed and weighed to determine the gonadosomatic index:
Immediately after sacrificing, the caudal epididymis was collected from each mouse to assess sperm motility, count and viability (morphology). The assessment of sperm was carried out according to [12] protocol and data were expressed as the number of sperm per mL. Samples from the testes of the four groups were processed histologically for paraffin sections. 5-7 μm sections was prepared and stained by haematoxylin and eosin stain.
Ethical approval was obtained from the University of Ibadan Animal Care and Use for Research Ethical Committee (ACUREC).
2.3. Statistical analysis
The values are expressed as mean ± Standard error (SE). An analysis of variance (one way ANOVA) was used to determine the significance between different doses of exposure and was followed by Duncan Multiple Range (DMR) test. The significant difference between the groups will be considered significant at P<0.05 level.
3. Results
The results obtained showed significant difference in the serum concentration of leautinizing (LH) and follicle stimulating hormones (FSH), while there was no significant difference in the gonadosomatic index and testosterone concentration among male mice exposed to different doses of aqueous wood ash extract of A. indica (Table 1).
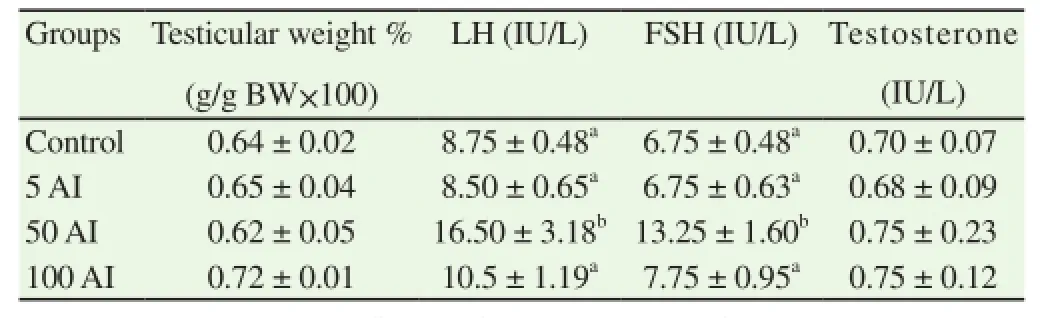
Table 1 Gonadosomatic index and hormonal assay of mice exposed to aqueous wood ash extract of A. indica.
Results obtained from sperm count showed significant decrease in percentage motility, live/dead and total sperm count with increase in dose concentration of aqueous wood ash extract of A. indica among male mice (Table 2). In the study of the sperm morphology among mice administered aqueous extract of A. indica wood ash, the results revealed a significant increase in number of curved tail and bent tail sperms with increase in the dose concentration. The total percentage of abnormal sperm cells also increased significantly with dose concentrations (Table 3).
As presented in Figure 1, histopathology of mice testes exposed to aqueous extract of A. indica shows control (A) with numerous regular variably sized STs (seminiferous tubules) packed (with normal spermatogenic cells.: Mice administered 5 mg/kg (B) had closely packed numerous regular STs with moderate to markedly depleted amounts of spermatogenic cells. For 50 mg/kg (C), thickened tunica albuginea; variably sized STs, some are packed full with spermatogenic cells while others are empty. A few STs contain necrotic debris. Mice given 100 mg/kg (D) showed testes with closely packed STs with irregular outlines and moderately depleted amounts of spermatogenic cells.
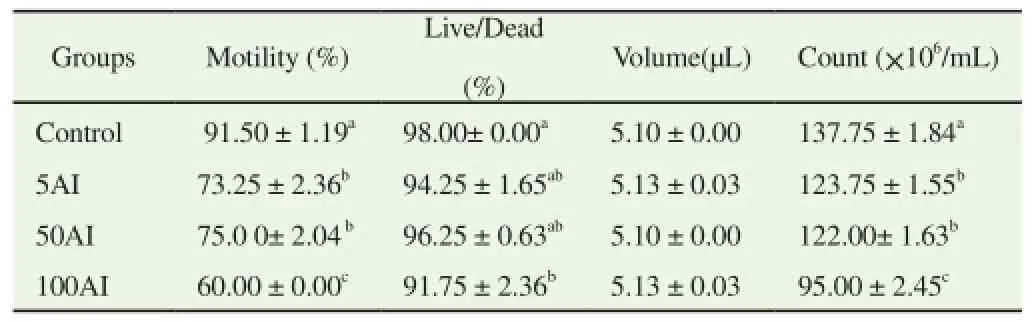
Table 2 Result of sperm count and motility assay of mice exposed to aqueous wood ash extract of A. indica.

Figure 1. Photomicrograph of mice testes exposed to aqueous wood ash extract of A. indica.
A (Control) showing numerous regular variably sized seminiferous tubules (STs) packed full with normal spermatogenic cells. B (5 mg/kg) with closely packed numerous regular STs (arrow) with moderate to markedly depleted amounts of spermatogenic cells. C (50 mg/kg) with variably-sized STs; some are packed full with spermatogenic cells while others are empty. A few STs (arrow) contain necrotic debris. D (100 mg/kg): There are closely packed STs (arrows) with irregular outlines and moderately depleted amounts of spermatogenic cells. H&E. 400伊. ST: seminiferous tubules.
4. Discussion
In this study, the luteinizing and follicle stimulating hormones showed significant increase in group 50 A. indica, male mice administered 50 mg/kg of the extract. The cause of such high increase, which almost double the concentration of the control group calls for further study to understand the mechanism behind that. The insignificant difference in concentration of serum testosterone implies the extract have no effect on it. Male mice orally administered aqueous extract of A. indica wood ash had significant decrease in sperm motility, live/dead and total sperm count. The decrease was with increase in the dose concentration of extract administered, when compared to the control group. The decrease in sperm viability (live/dead) agreed with reduction in the progressive sperm motility because immobile sperms were considered dead as they took up the Eosin/Nigrosin stain when the smear was examined. This result may also be due to the effect of this extract on the epididymis by acting as a spermatoxic agent on maturing or matured spermatozoa [16].

Table 3 Showing sperm morphology parameters of mice exposed to aqueous wood ash extract of A. indica.
According to [17] several kind of mutation can lead to abnormal sperm morphology. The abnormal sperm shape can be caused by protein abnormality, as sperm shape is partially imparted by structural protein. The significant increase of number of sperm head abnormalities in exposed mice, which increase with increase in dose suggest that aqueous wood ash extract of A. indica might have caused damage to the pre-meiotic stages of spermatogenesis since during spermatogenesis, DNA synthesis occurs before premeiotic phase and no further DNA synthesis occurs throughout spermatogenesis in the cell cycle [18,19]. Numerous reasons have been deduced to substantiate the increase in the frequency of occurrence of sperm head abnormalities in organisms exposed to some chemicals. In general, damage to the sperm cell is said to occur either by physiological, cytotoxic or genetic mechanism [20]. Exposure to the extracts could have produced pituitaryhypothalamic or sex hormonal effects which in turn affected spermatogenesis or exposure could have resulted to abnormalities in seminal fluid leading to functional or structural impairment of sperm [18]. Studies have shown that increased oxidative stress, enzymatic and nonenzymatic antioxidants reduced levels in Leydig cells and an important factor for impaired spermatogenesis and consequently a significant reduction in epididymal sperm count [21]. According to [22], the development of abnormal sperm head morphology and variations in DNA content of spermatozoa are often genetically controlled. These abnormalities have also been attributed to the chromosomal aberrations that occur during the packaging of genetic material in the sperm head or occurrence of point mutation in testicular DNA [23]. It may also arise as a consequence of naturally occurring level of mistakes in the spermatozoon differentiating process during spermatogenesis [24]. In this study, aqueous extract of A. indica wood ash acted in a similar pattern to other known chemical mutagens by increasing significantly the frequency of these mistakes during spermatogenesis. It indicates that the extracts exerted toxic impact during the process of sperm differentiation and caused sperm abnormalities. These extracts were not only capable of altering spermatogenesis, but also of reducing or destroying the viability of sperm cells. This therefore suggests that the extracts contained constituents which are not only able to produce damaged sperm cells which might be unable to fertilize ovum or produce mutated zygote but are also capable of reducing viable sperm cells which is a major factor leading to infertility [14].
The increased depletion of seminiferous tubules observed in the histopathology of testes of mice orally exposed aqueous extract of A. indica wood ash might have been responsible for the significant increase in abnormal sperm cells recorded and the low motility and viability of the cells. According to [25], histopathological changes in the testis such as vacuolation and swelling of the round spermatids, necrosis of the late elongated spermatids, numerous apoptotic cells and formation of multinucleated giant cells in the seminiferoustubules affect the sperm parameters. Mutation in germ cells prior to or during the reproductive period can be transmitted to later generations resulting in reproductive defects. This may lead to carcinogenicity or teratogenicity in somatic cells. It may also alter a gene so that it contains a wrong code [26].
Aqueous wood ash extract of A. indica caused decrease in sperm motility, live/dead sperms and an increase in the number of abnormal sperm cells, which is an indication of infertility. Histopathological changes in the testis such as vacuolation and necrosis of the late elongated spermatids, numerous apoptotic cells and formation of multinucleated giant cells in the seminiferous tubules means the extracts have toxic effect on spermatogenesis.
Conflict of interest statement
The authors declare that there is no conflict of interest on this article.
Acknowledgements
The financial support from Federal University Dutsin-Ma, Katsina State, Nigeria is duly acknowledged. The Head, Zoology Department, University of Ibadan and members of technical staff are appreciated for making available the animal house and laboratory that were used for this research.
References
[1] Auta T, Otalu EJ. Hassan AT. Evaluation of chemical constituents in aqueous wood ash extracts of Azadirachta indica (Neem) and Parkia biglobosa (locust bean). J Environ Toxicol Pub Health 2015; 1(1): 36-40.
[2] Haque E, Baral R. Neem (Azadirachta indica) leaf preparation induces prophylactic growth inhibition of murine Ehrlich carcinoma in Swiss and C57BL/6 mice by activation of NK cells and NK-T cells. Immunobiology 2006; 211(9): 721-731.
[3] Sharma A, Bhattacharyya KG. Azadirachta indica (Neem) leaf powder as a biosorbent for removal of Cd(II) from aqueous medium. J Hazard Mater 2005; 125(1): 102-112.
[4] Habila N, Humphrey NC, Abel AS. Trypanocidal potentials of Azadirachta indica seeds against Trypanosoma evansi. Vet Parasitol 2011; 180(3-4): 173-178.
[5] Sharma R, Garu U. Effects of lead toxicity on developing testes in swiss mice. Universal J Environ Res Tech 2011; 1(4): 390-398.
[6] Queiroz-Neto A, Mataqueriro MI, Santana AE, Alessi AC. Toxic effects of Annona squamosa seed extract in rats and swine. Revista Brasileira de Toxicol 1997; 10: 11-15.
[7] Ban Y, Komatu KM, Inagaki S, Nakatsuka MH. Testicular spermatid and epididymalvsperm head count as an indicator for reproductive toxicity in rats. Exp Anim 1995; 44: 315-322.
[8] Dev KR, Yadamma K, Reddy KD. Protective effects of curcumin in cyclophosphamide induced sperm head abnormalities in male mice. Int J Pharm Biol Sci 2013; 4(1): 1131-1137.
[9] Gautam D, Sharma G, Goyal RP. Evaluation of toxic impact of Tartrazine on male swiss albino mice. Pharm Online 2010; 1: 133-140.
[10] Bakare AA, Okunola AA, Adetunji AO, Jenmi BH. Genotoxicity assessment of a pharmaceutical effluent using four bioassays. Gen Mol Biol 2009; 32(2): 373-381.
[11] Spano M, Evenson DP. Flow cytometric analysis for reproductive biology. Biol Cell 1993; 78: 53-62.
[12] World Health Organization (WHO). WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. New York: Cambridge University Press; 1999.
[13] Dong L, Zhang H, Duan L, Cheng X, Cui L. Genotoxicity of testicle cell of mice induced by microcystin-LR. Life Sci J 2008; 5(1): 43-45.
[14] Alabi OA, Bakare AA. Cytogenotoxic effects and reproductive abnormalities induced by e-waste contaminated underground water in mice. Cytology 2014; 79(3): 331-340.
[15] Talebi AR, Khorsandi, L, Moridian M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J Assisted Reprod Gen 2013; 30(9): 1203-1209.
[16] Pacifici R, Altieri I, Gandini L, Lenzi A, Passa AR, Pichini S. Environmental tobacco smoke: nicotine and cotinine concentration in semen. Environ Res 1995; 68(1): 69-72.
[17] Wyrobek AJ, Gordon LA, Burkhart JG, Francis MW, Kapp Jr RW, Letz G, et al. An evaluation of the mouse sperm morphology test and other sperm tests in non-human mammals. A report of the US environmental protection agency gene-toxicology program. Mut Res 1983; 115: 1-72.
[18] Odeigah PGC. Sperm head abnormalities and dominant lethal effects of formaldehyde in albino rats. Mutat Res 1997; 389: 141-148.
[19] Otubanjo OA, Mosuro AA. An in vivo evaluation of induction of abnormal sperm morphology by some antihelmintic drugs in mice. Mut Res 2001; 497: 131-138.
[20] Otitoloju AA, Obe IA, Adewale OA, Otubanjo OA, Osunkalu VO. Preliminary study on the induction of sperm head abnormalities in mice, mus musculus, exposed to radiofrequency radiations from global system for mobile communication base stations. Bull Environ Contam Toxicol 2010; 84: 51–54.
[21] Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and nonenzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol 2004; 88(1): 61-67.
[22] Beatty RA. The genetics of the mammalian gamete. Biol Rev 1970; 45: 2–119.
[23] Bruce WR, Heddle JA. The mutagenic activity of 61 agents as determined by the micronucleus, Salmonella and sperm abnormality assays. Can J Gen Cytol 1979; 21: 319–334.
[24] Bakare AA, Mosuro AA, Osibanjo O. An in vivo evaluation of induction of abnormal sperm morphology in mice by landfill leachates. Mut Res 2005; 582: 28–34.
[25] Yang HJ, Lee SH, Jin Y, Choi JH, Han CH, Lee MH. Genotoxicity and toxicological effects of acrylamide on reproductive system in male rats. J Vet Sci 2005; 6: 103–109.
[26] Aduloju RK, Otubanju OA, Odeigah PGC. An in vivo assay of the mutagenic potential of praziquantel (PZQ) using sperm head abnormality test. J Hum Ecol 2008; 23(1): 59-63.
15 October 2015
Auta T., Department of Biological Sciences, Federal University Dutsin-Ma, P.M.B 5001, Dutsin-Ma, Katsina State.
Tel: +234 8039213141
E-mail:autatimz@gmail.com Foundation project: This study was financially supported by Federal University Dutsin-Ma, Katsina State, Nigeria.
Received in revised form 6 January 2016 Accepted 12 January 2016
Available online 1 March 2016
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Effect of growth regulators on rapid micropropagation and antioxidant acitivity of Canscora decussata (Roxb.) Roem. & Schult.-A threatened medicinal plant
- A new nucleotide variant G1358A potentially change growth differentiation factor 9 profile that may affect the reproduction performance of Friesian Holstein cattle
- Pregnancy rate in Bulgarian White milk goats with natural and synchronized estrus after artificial insemination by frozen semen during breeding season
- Effect of heparin, caffeine and calcium ionophore A 23187 on in vitro induction of the acrosome reaction of fresh ram spermatozoa
- The characterisation and cryopreservation of Venda chicken semen
- Effects of intramuscular injections of vitamin E-selenium and a gonadotropin releasing hormone analogue (GnRHa) on reproductive performance and blood metabolites of post-molt male broiler breeders
