D-1,2,4-丁三醇的绿色合成
2016-10-12杨萌董润安
杨萌,董润安
(北京理工大学 生命学院,北京 100081)
D-1,2,4-丁三醇的绿色合成
杨萌,董润安*
(北京理工大学 生命学院,北京 100081)
D-1,2,4-丁三醇(D-1,2,4-butanetriol,BT)是四碳的手性多羟基醇,同时也是极具价值的有机合成中间体,应用十分广泛,特别是在医药和军事工业领域。目前D-1,2,4-丁三醇的工业化生产仍以化学合成法为主,该方法存在反应条件苛刻、产率低、副产物多,易引发环境污染等弊端。目前,利用合成生物学技术,安全高效的生物合成该化学品成为研究的热点。本文评述了D-1,2,4-丁三醇的化学合成和生物合成,以期为D-1,2,4-丁三醇的绿色合成提供理论基础。
D-1,2,4-丁三醇;生物合成;绿色合成
1 D-1,2,4-丁三醇
D-1,2,4-丁三醇是无色无味、透明、粘稠的四碳多元醇[1],其分子式为C4H10O3,结构式如图1所示,由于第二位碳是手性碳原子,具有旋光性,在水和醇类物质中溶解度较高,具有吸湿性。工业用的D-1,2,4-丁三醇呈草黄色或褐色[2]。D-1,2,4-丁三醇是重要的有机合成中间体,广泛应用于医药、农业、化妆品、造纸、高分子材料、烟草、军工等领域[3-6]。D-1,2,4-丁三醇的硝基化合物冲击敏感性低、热稳定性好、低毒性、吸湿性好,与其它含能增塑剂混合使用,可显著提高以硝化纤维素为基火药的低温力学性能[7-9]。D-1,2,4-丁三醇用作缓释剂,是制备抗病毒化合物等药物的关键中间体[10-12],添加在烟草中,降低了硝基化合物的毒害作用,可用作高分子材料的交联剂,增加材料的强度和硬度,还可用作高级墨水的防干剂、高级服装表面处理剂、陶瓷加工助剂、特殊用途包装与储运等[13,14]。D-1,2,4-丁三醇能抑制微生物,是抗微生物制剂(如防腐剂)的组成部分[2]。有巨大的市场潜力。

图1 D-1,2,4-丁三醇的分子结构Fig. 1 The structure of D-1,2,4-butanetrion
2 D-1,2,4-丁三醇的化学合成
自Wagner以3-丁烯醇为原料合成D-1,2,4-丁三醇以来,合成方法不断改进,先后有苹果酸还原法[14]、丙烯醇法[15]、环醚的开环反应[1]、2-丁烯-1,4-二醇硼烷-氧化法[16]、2-丁烯-1,4-二醇的汞氧化水合法[17]、乙炔-硫酸汞法[18,19]、2-丁烯-1,4-二醇的酸催化法[20]、1,4-二羟基环氧丁烷的氢化法[21]、马来酸及其酯的氢化法[22]、3-丁烯-1-醇的氧化水合法[23-25]等。
从生产成本、污染程度和安全性评价,以下四条合成路线具有工业化生产的潜力[26]。2-丁烯-1,4-二醇(BTD)酸催化法[20]:BTD在H2SO4作用下水合,生成硫酸氢酯中间体,加热水解生成D-1,2,4-丁三醇,收率不足30%,成本约5300~6200美元/吨。该工艺需要进一步改进BTD酯化水解条件,提高收率。BTD氧化法[23-25]:在H2WO4催化下用H2O2氧化BTD,生成2-环氧基-1,4-二醇,然后以RaneyNi-Pd/C或硼化镍为催化剂,高压催化氢化,合成D-1,2,4-丁三醇,收率约60 %~ 80 %,成本价约4130~5500美元/吨[27]。马来酸及其酯的氢化法[22]:马来酸二乙酯溶于乙醇,以酮-铬为催化剂,在150℃,21MPa条件下发生氢化,D-1,2,4-丁三醇的转化率为50%~60%,该工艺条件比较苛刻,收率低。丙烯醇法[15]:由多聚甲醛、醋酸和硫酸组成混合液,将丙烯醇和醋酸酐、甲醛加入其中进行反应,反应温度控制在70~75 ℃,Na2CO3中和,收率在45%~50%,反应路线原料廉价、易得、成本低,反应条件温和,成本约3965~4400美元/吨[1],是目前工业化生产较好的工艺,该方法副反应多,产品提纯困难,所用溶剂醋酸对环境有一定的污染。
3. D-1,2,4-丁三醇的生物合成
3.1木糖/阿拉伯糖—D-1,2,4-丁三醇的转化
Niu等[3]2003年提出D-1,2,4-丁三醇的微生物合成法。在大肠杆菌(Escherichia coli)中,利用来自Pseudomonas putida的氧化酶氧化D-木糖生成D-木糖酸,产率70%,D-木糖酸再经Escherichia coli DH5α/pWN6.186A催化生成D-1,2,4-丁三醇,产率为25%,由木糖到丁三醇的总产率为17.5%。Pseudomonas fragi氧化L-阿拉伯糖生成L-阿拉伯-1,4-内酯和L-阿糖酸的混合物,内酯水解成L-阿糖酸后,再由Escherichia. coli BL21(DE3)/pWN6.222A转化L-阿糖酸生成L-1,2,4-丁三醇,产率为35%,总转化率19%[3]。

图2 重组大肠杆菌利用木糖/阿拉伯糖生产D/L-1,2,4-丁三醇的代谢途径[33]Fig 2 Metabolic pathway from D-xylose or L-arabinose to D/L-1,2,4-butanetrion
Frost 等[34]利用木糖途径(图2)生产丁三醇,不敲除任何副产物途径,丁三醇的产量仅有0.08 g/L。 Kris 等[35]敲除了木糖的副产物途径后,产量0.25 g/L,这两种情况丁三醇转化率较低。
Valdehuesa等[36]敲除了E.coli的木糖异构酶(xylA)、木酮糖激酶(xylB)、2-酮酸醛羧酶(yjhH和yagE),使菌体无法以木糖为碳源进行代谢,并过表达了来源于新月柄杆菌(Caulobacter crescentus)的木糖脱氢酶(xylB)和来自于恶臭假单胞菌(Pseudomonas putida)的2-酮酸脱羧酶(mdlC),并对其序列优化,重组大肠杆菌可直接利用D-木糖合成D-1,2,4-丁三醇,产量0.88g/L。江南大学的孙文龙等[37]与Valdehuesa类似的研究,丁三醇产量0.9g/L。
马鹏飞等[33]以E.coli MG1655为出发菌株,通过敲除消耗木糖和中间代谢产物的关键基因xylA、yjhH和yagE,并过表达Caulobacter Crescentus CB15 编码D-木糖脱氢酶的xylB基因和Pseudomonas putida ATCC12633编码2-酮酸脱羧酶的mdlC基因,以宿主菌自身的D-木糖酸脱水酶和醇脱氢酶[34,38-40]即MG1655△yjhH△yagE△xylA::Kanr/pTMX实现了D-木糖向D-1,2,4-丁三醇的转化,代谢途径如图3,产量2.05g/L。为保证该重组菌的繁殖代谢,应增加细胞的碳源供应,提出补加葡萄糖的策略,对影响葡萄糖和木糖共利用的相关基因进行分析发现ptsG、mgsA的敲出有利于丁三醇的合成,结果产量分别提高到3.05g/L和9.0g/L[33]。
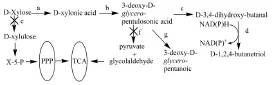
图3 D-木糖转化为D-1,2,4-丁三醇的代谢途径及主要分支途径[33]Fig 2.2 Metabolicpathway for D-1,2,4-butanetriol biosynthesis from D-xylose and main branch pathways.
3.2赤藓糖醇—D-1,2,4-丁三醇的转化
Man Kit Lau 等[35]构建工程菌E. coli W3110(PML5.200),过表达Klebsiella ATCC25955 的甘油脱水酶基因gldABC,用重组菌的裂解液催化赤藓糖醇脱水生成3,4-二羟基丁醛后,被NaBH4还原成1,2,4-丁三醇。但在培养基中添加赤藓糖醇时,在发酵液中无法检测到1,2,4-丁三醇。
3.3葡萄糖—D-1,2,4-丁三醇的转化
Li等[41]以大肠杆菌为基础菌,构建以葡萄糖为底物经苹果酸合成途径合成1,2,4-丁三醇的代谢通路。该途径是六个连续的酶促反应。选择苹果酸硫激酶,琥珀酸盐半醛脱氢酶,4-羟基丁酸酯脱氢酶,4-羟基丁酰-CoA转移酶和双功能醛/醇脱氢酶5种酶,酶的编码基因重组在大肠杆菌两个兼容性质粒上表达。在确定两个功能模块(即相容性质粒)导入宿主细胞后,在加入苹果酸的发酵液中检测到1,2,4-丁三醇。这种新的合成途径实现了从更为低廉的葡萄糖生产1,2,4-丁三醇。
孙雷等[42]人利用来自乳酸乳球菌(Lactococcus lactis)的α-酮异戊酸脱羧酶(KivD)替代 MdlC,表现出更高的催化效率,通过对 KivD的合理设计,进一步提高 KivD 对目标反应的催化活性,重组菌株的最高产量2.38g/L。
4 结论
化学合成1,2,4,-丁三醇反应条件苛刻、产率低、副产物多,易引发环境污染。生物合成成本低廉,环境友好,合成绿色。克服了化学合成的不足。但非天然合成途径与原有的代谢互相影响,导致1,2,4-丁三醇产量较低,生物法合成1,2,4-丁三醇需更为精细的研究,克服现有瓶颈,才能为实现大规模生产奠定基础。
[1] 李赤峰,徐保国. 1,2,4-丁三醇合成工艺述评[J]. 精细化工中间体,2000(03): 9-11.
[2] 孙文龙. 生物法合成D-1,2,4-丁三醇关键基因的克隆及重组菌的构建[D]. 江南大学,2014.
[3] Niu W,Molefe M N,Frost J W. Microbial synthesis of the energetic material precursor 1,2,4-butanetriol[J]. Journal of the American Chemical Society,2003,125(43): 12998-12999.
[4] Sato T,Aoyagi S,Kibayashi C. Enantioselective total synthesis of(+)-azimine and(+)-carpaine[J]. Organic Letters,2003,5(21): 3839-3842.
[5] Xu Y,Qian L,Pontsler A V,et al. Synthesis of difluoromethyl substituted lysophosphatidic acid analogues[J]. Tetrahedron,2004,60(1): 43-49.
[6] Yamada O K,Norimoto A,Kawada N,et al. Production of optically active 1,2,4-butanetriol from corresponding racemate by microbial stereoinversion[J]. Journal of bioscience and bioengineering,2007,103(5): 494-496.
[7] Dai X Y,Yang K X. Minimum Signature Composite Solid Propellants Containing HEGH Energy-Density Materials[J]. Aerospace Shang Hai,1996,1(5): 9-14.
[8] Wang F,Zhang X P,Hu R Z,et al. Study on Combustion Properties of Nitrate Ester Plasticized Polyether Propellants at High Pressure[J]. Journal of Propulsion Technology,2004,19(5): 20-25.
[9] Li Z F,Feng Z G,Hou Z L. Mechanical Properties of Hydroxy-Terminated Glycidyl Azide Polymer(GAP)Polyurethanes[J]. Journal of Beijing Institute of Technology,1997,23(5): 469-472.
[10] Luo S G,Tan H M,Zhang J G. Thermal Degradation
Characteristics of Co-Polyether(EO-THF)and the Poly(etherurethane-urea)Cured by N-100[J]. Journal of Beijing Institute of Technology,1995,10(06): 10-12.
[11] Johansson K,Kovacs Z,Lindborg B,et al. Derivatives of Purine,US 5,036,071 1991,30(07).
[12] Chu G H,Zhou Q,Bai D L. Synthesis of Sarmentosin[J]. Acta Pharmaceutica Sinica,1999,7(1): 7-10.
[13] Xu X M,Tang X Z. The Study on 1,2,4-butantriol[N]. ID 9319201320 1989,06.
[14] Adkins H,Billica H R. The Hydrogenation of Esters to Alcohols at 25-150[J]. Journal of the American Chemical Society,1948,70(9): 3121-3125.
[15] Olsen S. Naturforsch[J]. Acta Chem Scand,1950(4): 375.
[16] Zweifel G,Brown H. Organic reactions[M]. New York: Wiley,1963(13).
[17] Pariselle H.d'une glycérine en C4: passage à la série du furfurane et aux érythrites,par M. Henri Pariselle[M]. Gauthier-Villars,1911.
[18] Reppe J W. Acetylene chemistry[M]. Meyer,1949: 418.
[19] Reppe J. Acetylene Chemistry. University Microfilms: PB Report[R]. Inc: Ann Arbor,Mich(London),1949.
[20] Taberner P,Pearce M. Hypothermic and toxic actions of 2-butyne-1,4-diol and other related diols in the rat[J]. Journal of Pharmacy and Pharmacology,1974,26: 597-604.
[21] Eggersdorfer D,Siegel D,Mueller D,et al. Method for the production of 1,2,4-butanetriol [P]. EP Patent,0297444,1989.
[22] Frank. J Pisacane. 1,2,4-Buntanetriol Analysis And Synthesis[R]. NSWC TR 82-380,1983,20(7).
[23] Gallaghan J A. Butanetriol Trinit rate:Its Manufacture and Evaluation as a Propellant Ingredient[R]. Navord,Report 3014,Technical Report 41,1963-01-12.
[24] Gallaghan J A. The Synthesis of 1,2,4-Butanetriol and the Evaluation of It s Trinit rate [N].US Naval Powder Factory Technical Report,1948,19.
[25] Schaffuer I J. Synthesis of New Explosives and Propellants [R]. Quarterly Progress Report,1948,3: US Rubber Co.
[26] 罗阿力,乔建军,宋新潮. 1,2,4-丁三醇的合成工艺研究[J].西北药学杂志,2007,22(3): 144-145.
[27] 吴思忠,余正坤,刘韧,等. 一种1,2,4-丁三醇的合成方法: CN1803747[P],2006.
[28] Furukawa Y,Ho S,Ikai K,et al. Process for producing 1,2,4-butanetriol: EP,EP 1061060 B1[P]. 2005.
[29] Schofield D,Bailey M,Monteith M J. Process for thepreparation of butane triols: US,US 6479714 B1[P]. 2002.
[33] 马鹏飞. 重组大肠杆菌合成D-1,2,4-丁三醇代谢系统的理性改造[D]. 北京理工大学,2015.
[34] Frost J,Niu W. Microbial synthesis of d-1,2,4-butanetriol[P]. WO Patent,2008091288,2008.
[35] Kris NG. Valdehuesaa,Huaiwei Liu,et al. Direct bioconversion of d-xylose to 1,2,4-butanetriol in an engineered Escherichia coli[J]. Process Biochemistry,2013.
[36] Valdehuesa K N G,Liu H,Ramos K R M,et al. Direct bioconversion of d-xylose to 1,2,4-butanetriol in an engineered Escherichia coli[J]. Process Biochemistry,2014,49(1): 25-32.
[37] 孙文龙,陆信曜,宗红,等. 代谢工程改造大肠杆菌合成D-1,2,4-丁三醇[J]. 微生物学通报,2014,41(10): 1948-1954.
[38] Wang X,Miller E N,Yomano L P,et al. Increased furfural tolerance due to overexpression of NADH-dependent oxidoreductase FucO in Escherichia coli strains engineered for the production of ethanol and lactate[J]. Applied and environmental microbiology,2011,77(15): 5132-5140.
[39] Rodriguez G M,Atsumi S. Isobutyraldehyde production from Escherichia coli by removing aldehyde reductase activity[J]. Microb Cell Fact,2012,11(1): 90.
[40] Leonardo M R,Cunningham P R,Clark D P. Anaerobic regulation of the adhE gene,encoding the fermentative alcohol dehydrogenase of Escherichia coli[J]. Journal of bacteriology,1993,175(3): 870-878.
[41] Li X,Cai Z,Li Y,et al. Design and Construction of a Non-Natural Malate to 1,2,4-Butanetriol Pathway Creates Possibility to Produce 1,2,4-Butanetriol from Glucose[J]. Scientific reports,2014,4.
[42] 孙雷. 重组大肠杆菌中1,2,4-丁三醇合成途径的构建和优化[D]. 江南大学,2015.
Environmentally Friendly Synthesize of 1,2,4-Butanetriol
Yang Meng,Dong Runan*
(School of Life Science,Beijing Institute of Technology,Beijing 100081)
D-1,2,4- butanetriol(BT)is a chiral polyhydric alcohol with four carbon. As an important and valuable intermediate in organic synthesis,BT has a lot of industrial applications,particularly in the military and the medical field. Currently industrialized production of BT mainly employs chemical synthesis,which has many drawbacks,such as harsh reaction conditions,low yield,more byproducts,severe environment contamination and so on. In recent years,with the development of synthetic biology,the safe and efficient biosynthesis method to produce chemicals has become a research hotspot. The main synthetic methods for 1,2,4-butanetriol,including chemical synthesis and biosynthesis,were reviewed in this paper,in order to provide a theoretical basis for 1,2,4-butanetriol synthesis.
D-1,2,4-Butanetriol; Biosynthesis; Environmentally Friendly Synthesize
Q81 [Document Code] A
10. 11967/ 2016140402
中图分类法:Q81ADOI : 10. 11967/ 2016140402
杨萌(1988-),女,硕士研究生,主要研究方向:微生物代谢,Email:liuguangyexue1106@163.com。
