深层发酵灵芝胞内多糖的组成与结构分析
2016-10-10隋晓辰郭庆彬崔武卫刘艳芳夏咏梅
隋晓辰, 张 珏, 郭庆彬, 崔武卫, 刘艳芳, 夏咏梅*
(1.食品科学与技术国家重点实验室/化学与材料工程学院,江南大学,江苏 无锡 214122;2.卫生部核医学重点实验室,江苏省原子医学研究所,江苏无锡2140631;3.圭尔夫食品研究中心,加拿大农业部-农业食品部,安达略N1G5C9)
深层发酵灵芝胞内多糖的组成与结构分析
隋晓辰1, 张 珏2, 郭庆彬3, 崔武卫3, 刘艳芳1, 夏咏梅*1
(1.食品科学与技术国家重点实验室/化学与材料工程学院,江南大学,江苏 无锡 214122;2.卫生部核医学重点实验室,江苏省原子医学研究所,江苏无锡2140631;3.圭尔夫食品研究中心,加拿大农业部-农业食品部,安达略N1G5C9)
测定了深层发酵灵芝胞内多糖的分子量和单糖组成,首次详细分析了深层发酵灵芝胞内多糖的结构特性,为深入研究深层发酵灵芝多糖的生物活性及其构效关系奠定了基础。经单糖组成分析和光谱初步解析,灵芝胞内多糖是由5种单糖组成,其摩尔比为葡萄糖∶半乳糖∶甘露糖∶阿拉伯糖∶木糖=83.75∶4.76∶4.15∶2.04∶5.30;通过IR、GC-MS、HPAE-PAD、NMR等分析其是以α-(1→4)位键合吡喃葡萄糖主链为主,以T-Glcp为主要的残基,同时存在半乳糖、甘露糖、阿拉伯糖和木糖的分支残基。
灵芝;深层发酵;单糖组成;结构分析
多糖是由多个单糖分子缩合、失水以糖苷键结合而成的大分子化合物,广泛存在于生命体的细胞中,承担着能量存储、运输以及结构支撑等作用。许多多糖已被认为是免疫调节剂[1]和抗氧化剂[2],同时它们的体外抗肿瘤活性已成为近年来的研究热点[3-10]。灵芝多糖由于其具有抗肿瘤、抗病毒、抗氧化和提高免疫调节等多种生物活性,两千多年来一直受到人们的高度关注。很多研究已经报道了不同结构和来源的灵芝多糖的抗癌和免疫调节活性,例如子实体[11-13]、孢子[14-16]、固态发酵的菌丝体[17-20]、液态发酵的菌丝体[21-22]等。
灵芝多糖的生物活性强弱与其溶解度、相对分子质量、分子组成、分子构型、支化度、黏度等密切相关[23]。但是由于多糖的化学结构本身就很复杂、微观不均一性等使多糖的化学结构难以得出完全正确的结构式。大多数灵芝多糖主要通过 (1→3)、(1→4)和/或(1→6)-α/β糖苷键连接的葡聚糖,主要的单糖组分为葡萄糖,甘露糖,半乳糖,木糖,果糖和阿拉伯糖,相对分子质量从几千到上百万不等[24]。目前对灵芝多糖的生物活性研究报道较多,但对其多糖结构作全面深入的分析研究报道并不多见。深层发酵的胞外灵芝多糖比胞内灵芝多糖分子量低的多,然而目前没有报道胞内灵芝多糖的详细结构特征。作者将灵芝多糖甲基化、乙酰基化反应,通过高效液相色谱(HPLC)、脉冲安培-高效阴离子交换色谱(HPAE-PAD)、气相色谱-质谱联用(GCMS)、核磁共振(NMR)等对胞内灵芝多糖的结构特性进行了全面的研究,为研究灵芝多糖的生物活性及其构效关系提供理论基础。
1 材料与方法
1.1 材料与仪器
1.1.1 材料 发酵灵芝多糖(GLPs):实验室自制;乙醇、硫酸、碳酸钠、苯酚等:均为分析纯,购自中国医药集团上海化学试剂公司。透析袋(截留相对分子质量500):购自华美生物工程公司上海分公司;葡聚糖标准品:购于中国食品药品检验所;灵芝子实体多糖(GLP-Fr,总糖含量95.9%):上海农科院食用菌研究所提供。
1.1.2 仪器 Waters 600高效液相色谱仪,配置Waters 600四元泵,2410示差折光检测器:美国Waters公司产品;Dionex ICS-5000脉冲安培-高效阴离子交换色谱(HPAE-PAD):美国Dionex公司产品;Finnigan trace DSQII气相色谱-质谱联用仪:美国热电公司产品;SP-2330色谱柱;Bruker ARX500核磁共振仪:瑞士Bruker公司产品。
1.2 实验方法
1.2.1 灵芝多糖的提取 灵芝深层发酵液经高速离心后取菌丝体固体沉淀,将菌丝体洗净、烘干;用组织捣碎机破壁,在95℃热水提取3.5 h,离心取上清液;上清液超滤分离,再减压蒸馏浓缩。用savage试剂除蛋白,用体积分数95%的乙醇分级醇沉,收集乙醇体积分数为80%的醇沉组分,经过冷冻干燥后,即为胞内多糖(GLP-In)。胞外(GLP-Ex)的制备参照文献[21,25],稍作修改,其中GLP-Ex仅是低相对分子质量的多糖,GLP-Ex1是用体积分数80%乙醇溶液沉淀透析后得到的胞外多糖,两者都用透析袋透析。
1.2.2 灵芝多糖总糖含量的测定 用苯酚-硫酸法检测灵芝多糖的总糖含量[26]。
1.2.3 灵芝多糖的单糖组分和相对分子质量测定
多糖的水解:称取2 mg灵芝多糖,加入1 mL三氟乙酸(2 mol/L),封管,110℃下水解4 h。用碳酸钠中和水液,过滤,冷冻干燥。蒸干后的残留物在60℃下真空干燥8 h,然后置干燥瓶中冷却备用。
灵芝多糖的单糖组成使用脉冲安培-高效阴离子交换色谱检测(HPAE-PAD),检测条件:色谱柱CarboPac PA20(3 mm×150 mm),淋洗液为5 mmol/ L NaOH,柱温30℃,流量0.5 mL/min。根据标准单糖、GLPs中单糖的保留时间和出峰面积及内标峰面积确定GLPs的单糖组成种类及物质的量比。
相对分子质量测定采用HPLC凝胶过滤法。Water 600Controller,2410RefractiveIndex检测器,Waters UltrahydrogelTMLinear 7.8 mm×300 mm柱;流动相为0.1 mol/L NaNO3;流量0.9mL/min,进样量10 μL。
1.2.4 IR分析胞内灵芝多糖 取3 mg样品,用溴化钾压片,测定范围为4 000~400 cm-1。
1.2.5 GC-MS分析胞内灵芝多糖 灵芝多糖经过甲基化反应、水解、NaBH4还原、乙酰基化等反应处理后供GC-MS分析。检测条件为:SP-2330色谱柱(Supelco公司;30 m×0.25 mm液膜厚度0.2 μm),程序升温,160~210℃保持2℃/min,210~240℃保持5℃/min,氦气流量1 mL/min。
1.2.6 NMR分析胞内灵芝多糖 灵芝多糖溶解于Me2SO-d6/D2O(30 mg/mL)中,用BrukerARX500核磁共振仪测试。Me2SO作为内标 (δH=2.50×10-6for1H)。
2 结果与讨论
2.1 灵芝多糖的总糖质量分数和单糖组分
灵芝多糖GLP-In、GLP-Ex和GLP-Ex1总糖质量分数分别为93.9%,94.96%和93.55%。不同来源的灵芝多糖它们的结构特点是不同的,它们的相对分子质量从几千到百万之间不等(表1)。
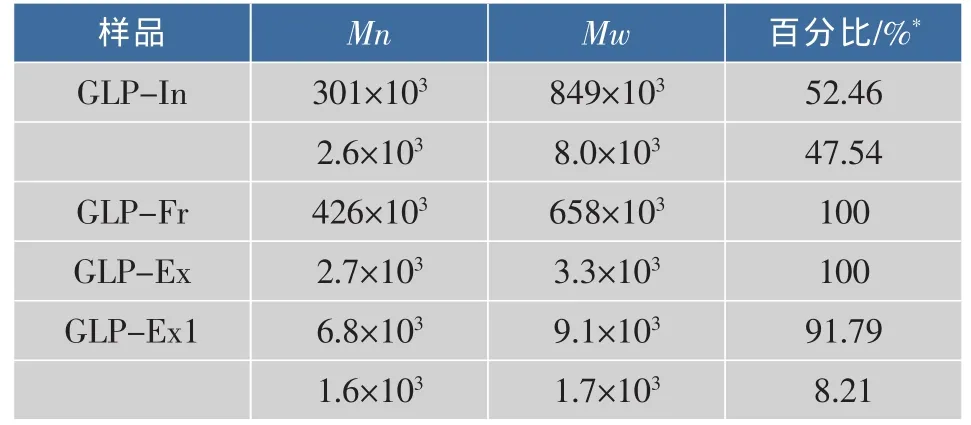
表1 灵芝多糖的相对分子质量和相对分子质量分布Table1 Molecular weights and distribution of the GLPs
利用脉冲安培-高效阴离子交换色谱检测(HPAE-PAD)深层发酵灵芝多糖的单糖组成 (表2)。GLP-In由葡萄糖、半乳糖、甘露糖、阿拉伯糖和木糖5种单糖组成;其摩尔比为,葡萄糖∶半乳糖∶甘露糖∶阿拉伯糖∶木糖=83.75∶4.76∶4.15∶2.04∶5.30,因此GLP-In的单糖组成以葡萄糖为主。GLP-Ex则由葡萄糖、半乳糖、阿拉伯糖、木糖和岩藻糖等单糖组成,各单糖摩尔比为葡萄糖∶半乳糖∶阿拉伯糖∶木糖∶岩藻糖=91.76∶1.83∶1.85∶3.02∶1.54。GLP-Ex1的单糖组成和摩尔比为葡萄糖∶半乳糖∶甘露糖∶阿拉伯糖∶木糖∶岩藻糖=71.77∶10.40∶2.95∶5.33∶8.68∶0.86。与GLP-Ex一样,GLP-Ex1中葡萄糖摩尔比最高,因此它们都以葡萄糖为主,而GLP-Fr仅由葡萄糖组成。
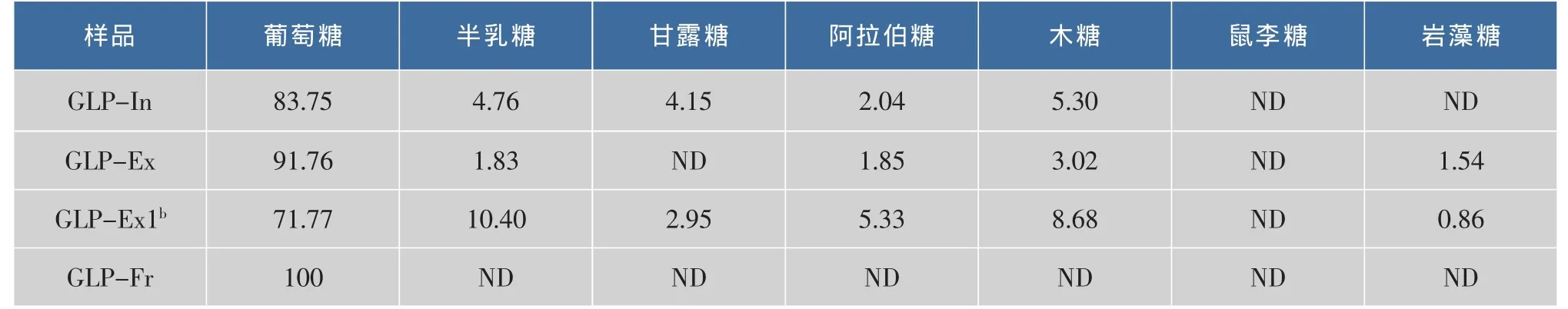
表2 灵芝多糖的单糖组成aTable2 Monosaccharide compositions of GLPs
2.2 灵芝多糖GLP-In的结构分析
Liu[11]报道了从灵芝子实体中提取的β-葡聚糖(GLP-Fr)。在众多深层发酵的灵芝多糖中GLP-In属于高分子量的多糖,并且它的组成和结构可能和GLP-Fr相似,但可能具有不同的生物活性。目前尚未见报道深层发酵的胞内灵芝多糖GLP-In结构特征,作者将利用IR、GC-MS、NMR手段来表征其结构。
2.2.1 IR分析 胞内灵芝多糖的红外光谱见图1。 3 400 cm-1处的宽峰为O-H伸缩振动峰,2 900 cm-1处出现的吸收峰为C-H的伸缩振动峰;1 643 cm-1处为O-H弯曲振动峰;1 369 cm-1处出现C-H变角振动峰;1 243 cm-1处吸收峰为C-O伸缩振动峰;1 153 cm-1处是C-O-C伸缩振动峰;1 024和1 079 cm-1处吸收峰为 O-H变角振动峰;1 153 cm-1为C-O-C伸缩振动峰。以上这些均为糖类的特征吸收峰。762 cm-1处为 D-吡喃葡糖糖的特征峰;929和857 cm-1处出现α-吡喃环糖苷键特征吸收峰,这表明GLP-In糖链由α-型糖苷键连接。

图1 GLP-In的红外光谱图Fig.1 FT-IR spectra of GLP-In
2.2.2 GC-MS分析 GLP-In经甲基化并水解后的GC-MS的主要离子峰如下(表3)。
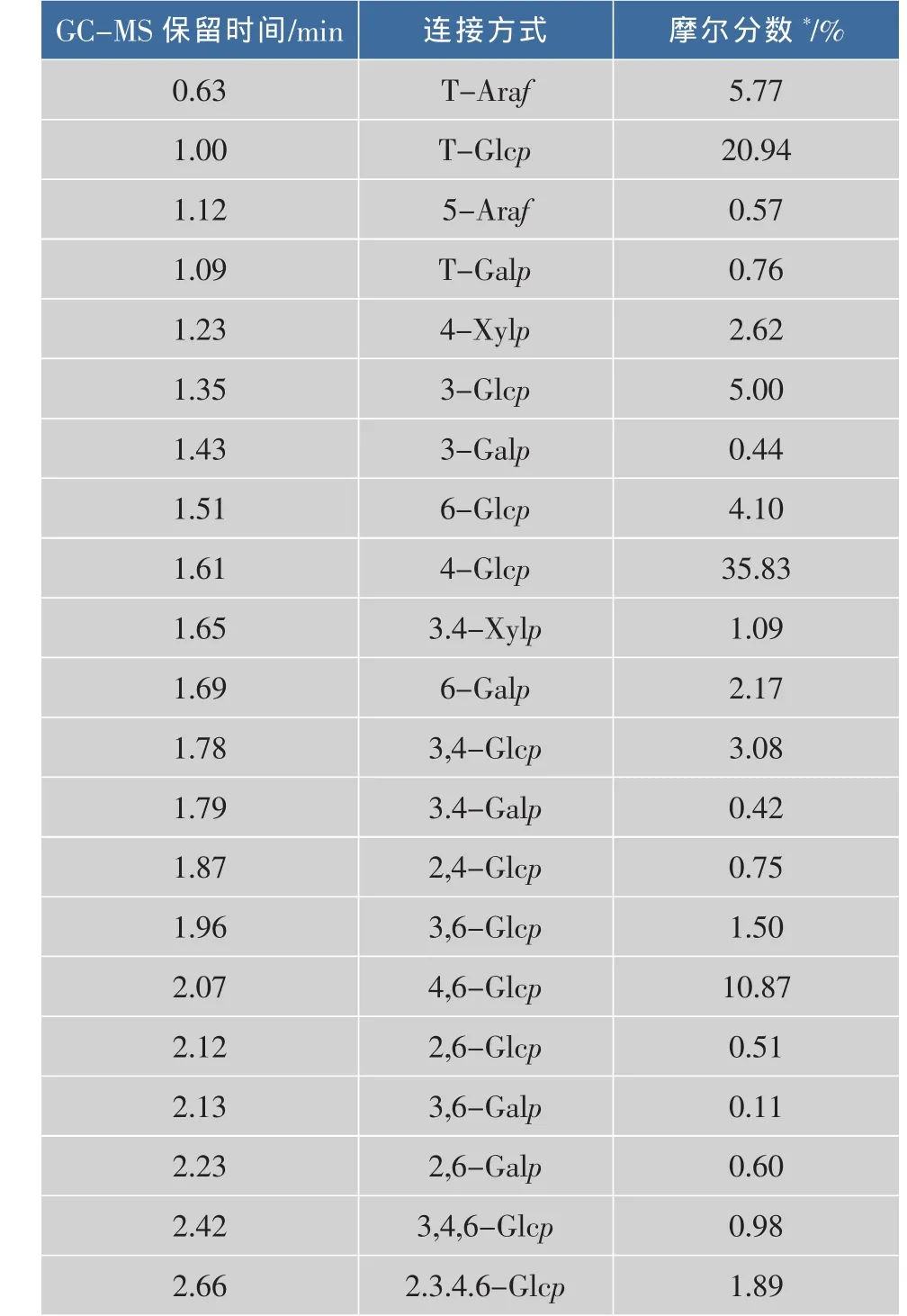
表3 胞内灵芝多糖GLP-In的主要连接方式Table3 Main linkage patterns of the submerge-cultured intracellular GLP
表3表明,在GLP-In的骨架结构主要的3个葡萄糖基连接型态为:T-Glcp(20.94%),4-Glcp (35.83%)和4,6-Glcp(10.87%),这和单糖组分的数据相吻合(见表2)。主要的糖残基是葡萄糖,也检测到少量的半乳糖、木糖和阿拉伯糖基的糖残基。末端糖基 (T-Araf,T-Glcp和T-Galp)百分比之和为27.5%,而分支部分(3,5-Araf,3,4-Glcp,2,4-Glcp,3,6-Glcp,4,6-Glcp,3,6-Glcp,2,6-Glcp 和-3,4,6-Glcp0)为26.56%。
2.2.3 核磁共振分析 GLP-In的核磁共振谱图更能清晰的表明其结构特点(图2)。1H-NMR谱显示,质子信号几乎都出现在δ 3.30-5.50区间内,这是典型的多糖信号,且H-1质子化学位移δ>4.95,可判断为α型-链接的吡喃糖苷键构型[28]。结合前面数据分析,作者提出一个α-葡聚糖状结构(图3)。
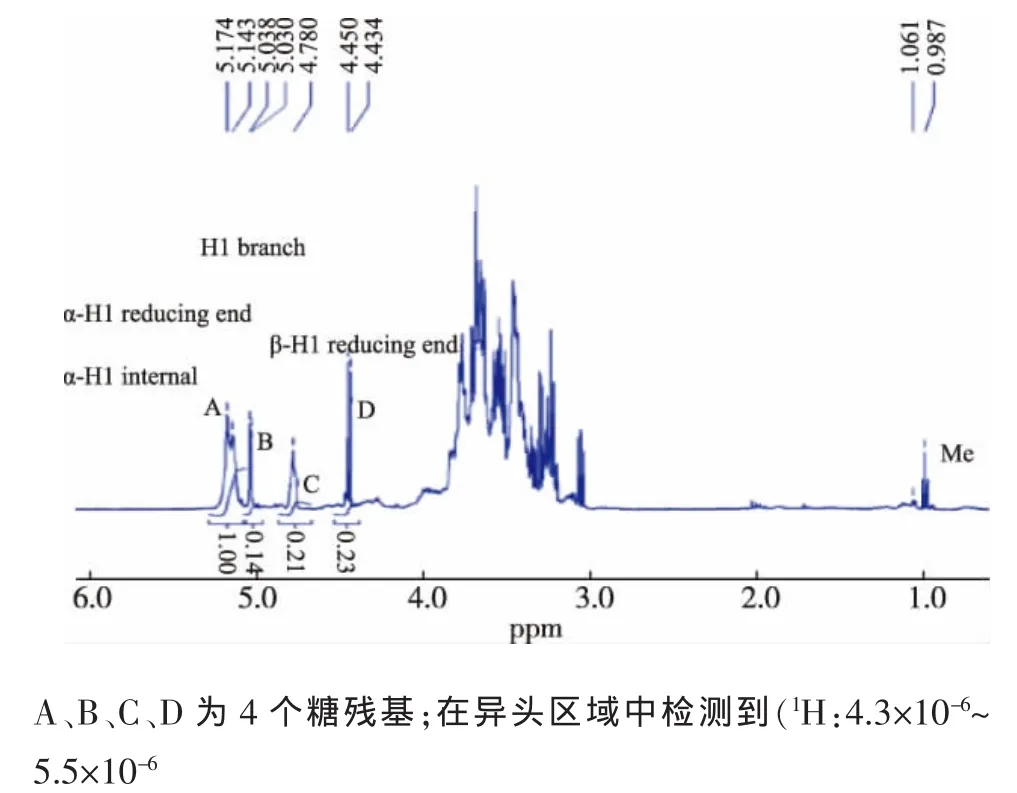
图2 胞内灵芝多糖GLP-In的1H-NMR谱图Fig.21H NMR spectrum of GLP-In,four sugar residues were detected in anomeric region

图3 胞内灵芝多糖GLP-In的预计结构Fig.3 Proposed structure of GLP-In
3 结语
从灵芝深层发酵液中获得的灵芝胞内外多糖和子实体多糖,经单糖组成分析和光谱初步解析,胞内外多糖的组成主要是葡萄糖,子实体多糖的单糖组成全部是葡萄糖,灵芝胞内多糖由葡萄糖、半乳糖、甘露糖、阿拉伯糖和木糖这5种单糖组成,其摩尔比为葡萄糖∶半乳糖∶甘露糖∶阿拉伯糖∶木糖= 83.75∶4.76∶4.15∶2.04∶5.30;通过IR、GC-MS、NMR等分析其是以α-(1→4)位键合吡喃葡萄糖主链为主,以T-Glcp为主要的残基,同时存在半乳糖、甘露糖、阿拉伯糖和木糖的分支残基。胞内多糖的组成和结构的确定,为灵芝多糖样本库的建立提供了相关数据,也将为后续构效关系的确立提供理论参考。
[1]HUYAN T,LI Q,YANG H,et al.Protective effect of polysaccharides on simulated microgravity-induced functional inhibition of human NK cells[J].Carbohydrate Polymers,2014,101(1)﹕819-827.
[2]PAN D,ZHANG D,WU J,et al.A novel proteoglycan from Ganoderma lucidum fruiting bodies protects kidney function and ameliorates diabetic nephropathy via its antioxidant activity in C57BL/6 db/db mice[J].Food and Chemical Toxicology,2014,63﹕111-118.
[3]LIN M,XIA B,YANG M,et al.Anti-ovarian cancer potential of two acidic polysaccharides from the rhizoma of Menispermum dauricum[J].Carbohydrate Polymers,2013,92(2)﹕2212-2217.
[4]LEE H J,LUCI J J,TANTAWY M N,et al.Detecting peritoneal dissemination of ovarian cancer in mice by DWIBS[J]. Magnetic Resonance Imaging,2013,31(2)﹕227-234.
[5]CHEN Y,HU M,WANG C,et al.Characterization and in vitro antitumor activity of polysaccharides from the mycelium of Sarcodon aspratus[J].International Journal of Biological Macromolecules,2013,52(1)﹕52-58.
[6]ZONG A,CAO H,WANG F.Anticancer polysaccharides from natural resources:A review of recent research[J].Carbohydrate Polymers,2012,90(4)﹕1395-1410.
[7]SUN L X,LIN Z B,DUAN X S,et al.Ganoderma lucidum polysaccharides counteract inhibition on CD71 and FasL expression by culture supernatant of B16F10 cells upon lymphocyte activation[J].Experimental and Therapeutic Medicine,2013,5(4)﹕1117-1122.
[8]JOSEPH S,SABULAL B,GEORGE V,et al.Antitumor and anti-inflammatory activities of polysaccharides isolated from Ganoderma lucidum[J].Acta Pharmaceutica,2011,61(3)﹕335-342.
[9]JIN X,RUIZ Beguerie J,SZE D M,et al.Ganoderma lucidum(Reishi mushroom)for cancer treatment[J].Cochrane Database of Systematic Reviews(Online),2012,6:1-38.
[10]OLAKU O,WHITE J D.Herbal therapy use by cancer patients:A literature review on case reports[J].European Journal of Cancer,2011,47(4)﹕508-514.
[11]LIU Y,ZHANG J,TANG Q,et al.Physicochemical characterization of a high molecular weight bioactive β-d-glucan from the fruiting bodies of Ganoderma lucidum[J].Carbohydrate Polymers,2014,101(1)﹕968-974.
[12]WANG J,YUAN Y,YUE T.Immunostimulatory activities of β-d-glucan from Ganoderma Lucidum [J].Carbohydrate Polymers,2014,102(1)﹕47-54.
[13]ZHU L,LUO X,TANG Q,et al.Isolation,purification,and immunological activities of a low-molecular-weight polysaccharide from the lingzhi or reishi medicinal mushroom ganoderma lucidum (Higher Basidiomycetes)[J].International Journal of Medicinal Mushrooms,2013,15(4)﹕407-414.
[14]HELENO S A,BARROS L,MARTINS A,et al.Fruiting body,spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal:A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts[J].Food Research International,2012,46(1)﹕135-140.
[15]DONG Q,WANG Y,SHI L,et al.A novel water-soluble β-D-glucan isolated from the spores of Ganoderma lucidum[J]. Carbohydrate Research,2012,353﹕100-105.
[16]CHEN X,LIU X,SHENG D,et al.Distinction of broken cellular wall Ganoderma lucidum spores and G.lucidum spores using FTIR microspectroscopy[J].Spectrochimica Acta-Part A:Molecular and Biomolecular Spectroscopy,2012,97﹕667-672.
[17]BAI W F,GUO X Y,MA L Q,et al.Chemical Composition and Sensory Evaluation of Fermented Tea with Medicinal Mushrooms[J].Indian Journal of Microbiology,2013,53(1)﹕70-76.
[18]ZAPATA P,ROJAS D,ATEHORTUA L.Production of biomass,polysaccharides,and ganoderic acid using non-conventionalcarbon sources under submerged culture of the lingzhi or reishi medicinal mushroom,ganoderma lucidum(W.Curt.:Fr.)P.Karst. (Higher Basidiomycetes)[J].International Journal of Medicinal Mushrooms,2012,14(2)﹕197-203.
[19]DIAMANTOPOULOU P,PAPANIKOLAOU S,KAPOTI M,et al.Mushroom polysaccharides and lipids synthesized in liquid agitated and static cultures.Part I:Screening various mushroom species[J].Applied Biochemistry and Biotechnology,2012,167(3)﹕536-551.
[20]CAMARGO M R,KANENO R.Antitumor properties of Ganoderma lucidum polysaccharides and terpenoids[J].Annual Review of Biomedical Sciences,2011,13﹕1-8.
[21]ZHANG J,LIU Y J,PARK H S,et al.Antitumor activity of sulfated extracellular polysaccharides of Ganoderma lucidum from the submerged fermentation broth[J].Carbohydrate Polymers,2012,87(2)﹕1539-1544.
[22]乔双逵,彭林,丁重阳,等.液体发酵条件对灵芝菌体形态及胞外多糖活性的影响[J].食品与生物技术学报,2014,33(10)﹕1070-1076. QIAO Shuangkui,PENG Lin,DING Zhongyang,et al.Effect of different culture conditions on mycelium morphology and activityof exopolysaccharides from Ganoderma lucidum in submerged culture[J].Journal of Food Science and Biotechnology. 2014,33(10)﹕1070-1076.(in Chinese)
[23]YE L,ZHANG J,YE X,et al.Structural elucidation of the polysaccharide moiety of a glycopeptide (GLPCW-II)from Ganoderma lucidum fruiting bodies[J].Carbohydrate Research,2008,343(4)﹕746-752.
[24]NIE S,ZHANG H,LI W,et al.Current development of polysaccharides from Ganoderma:Isolation,structure and bioactivities[J]. Bioactive Carbohydrates and Dietary Fibre,2013,1(1)﹕10-20.
[25]LIU Y J,SHEN J,XIA Y M,et al.The polysaccharides from Ganoderma lucidum:Are they always inhibitors on human hepatocarcinoma cells?[J].Carbohydrate Polymers,2012,90(3)﹕1210-1215.
[26]DUBOIS M,GILLES K A,HAMILTON J K,et al.Colorimetric method for determination of sugars and related substances[J]. Analytical chemistry,1956,28(3)﹕350-356.
[27]LI Y Q,FANG L,ZHANG K C.Structure and bioactivities of a galactose rich extracellular polysaccharide from submergedly cultured Ganoderma lucidum[J].Carbohydrate Polymers,2007,68(2)﹕323-328.
[28]刘玉红,王凤山.核磁共振波谱法在多糖结构分析中的应用[J].食品与药品,2007,9(8):39-43. LIU Yuhong,WANG Fengshan.Applications of nuclear magnetic resonance spectroscopy in structural analysis of polysaccharides[J].Food and Drug.2007,9(8):39-43.(in Chinese)
Structure Features and Monosaccharide Composition of the Intracellular Polysaccharide from Submerge-Cultured Ganoderma lucidum
SUI Xiaochen1, ZHANG Jue2, GUO Qingbin3, CUI Steve W3, LIU Yanfang1, XIA Yongmei*1
(1.State Key Laboratory of Food Science and Technology/School of College of Chemical&Material Engineering,Jiangnan University,Wuxi 214122,China;2.Key Laboratory of Nuclear Medicine of Ministry of Health,Jiangsu Institute of Nuclear Medicine,Wuxi 214063,China;3.Guelph Food Research Centre,Agriculture and Agri-Food Canada,Guelph N1G5C9,Canada)
The molecular weight and monosaccharide composition of intracellular polysaccharide from submerge-cultured Ganoderma lucidum(GLP)was investigated in this study,whose structure features was also thoroughly studied for the first time.This work could build a solid theoretical foundation for the further research on the biological activity and structure-activity relationship of GLP.The components of intracellular GLP were preliminary determined as glucose,galactose,mannose,arabinose and xylose with a molar ratio of 83.75∶4.76∶4.15∶2.04∶5.30 using monosaccharide composition analysis and spectrum analysis.The structure feature was confirmed with a backbone of 1,4-α-glucan and main terminal sugar residues of T-Glcp,and other branch residues containing galactose,mannose,xylose and arabinose by characterization of IR,GC-MS,HPAE-PAD and NMR,respectively.
Ganoderma lucidum,submerge-cultured,monosaccharide compositions,structure features
O 657.3
A
1673—1689(2016)07—0728—06
2015-01-04
国家自然科学基金项目(20875038,31371837);2014年美大地区科研合作项目(5922059999140060);食品科学与技术国家重点实验室2015自主研究课题(SKLF-ZZB-201504)。
夏咏梅(1965—),女,江苏南京人,工学博士,教授,主要从事精细化品合成及生物催化研究。
E-mail:ymxia@jiangnan.edu.cn
