鹅不同繁殖时期GnRH和GnIH基因表达和激素浓度变化分析
2016-09-07张克山胡彦竞科韩笑哲高广亮王启贵
张克山,胡彦竞科,韩笑哲,高广亮,钟 航,王启贵*
(1.重庆市畜牧科学院,重庆 402460;2.重庆市肉鹅遗传改良工程技术研究中心,重庆 402460;3.西南大学,重庆 402460)
鹅不同繁殖时期GnRH和GnIH基因表达和激素浓度变化分析
张克山1,2,胡彦竞科3,韩笑哲3,高广亮1,2,钟航1,2,王启贵1,2*
(1.重庆市畜牧科学院,重庆 402460;2.重庆市肉鹅遗传改良工程技术研究中心,重庆 402460;3.西南大学,重庆 402460)
旨在研究GnRH和GnIH基因和激素在鹅繁殖过程中的重要作用,本研究以产蛋性能差异显著的四川白鹅和溆浦鹅为试验材料,利用Real-time RCR方法检测产蛋前期、产蛋期和休产期两种鹅的下丘脑、垂体和卵巢组织中GnRH和GnIHmRNA的表达量;利用放射性免疫和酶联免疫吸附测定法测定血清中的GnRH和GnIH激素变化情况。结果表明:GnRH和GnIH基因在四川白鹅和溆浦鹅的下丘脑、垂体和卵巢组织中均有表达;产蛋前期和产蛋高峰期的下丘脑和卵巢组织中,四川白鹅的GnRHmRNA表达量显著(P<0.05)或极显著(P<0.01)高于溆浦鹅,在产蛋高峰期和休产期四川白鹅GnRH激素浓度也极显著(P<0.01)或显著高于(P<0.05)溆浦鹅,在高峰期和休产期四川白鹅GnRH血清浓度分别为51.13和51.10 pg·mL-1,溆浦鹅分别为49.52和49.94 pg·mL-1;GnIH在两种鹅的变化比较一致,仅在产蛋高峰期,四川白鹅下丘脑组织中GnIHmRNA表达水平极显著低于(P<0.01)溆浦鹅,而两种鹅之间的GnIH激素在各个时期均无显著差异。这提示随着繁殖阶段的改变,在调控两种鹅产蛋性能的作用过程中GnRH可能较GnIH发挥着更为重要的作用,为进一步研究鹅繁殖分子机制奠定基础。
四川白鹅;溆浦鹅;GnRH基因;GnIH基因;生殖激素
中国是世界上养鹅最多的国家,但鹅的低繁殖力严重制约着养鹅业发展[1-2]。影响禽类繁殖性能的因素有很多,其中下丘脑-垂体-性腺轴(Hypothalamic-pituitary-gonadal axis,HPG)对繁殖机能的调控起到重要的作用[3]。促性腺激素释放激素(Gonadotropin-releasing hormone,GnRH)和促性腺激素抑制激素(Gonadotropin-inhibitory hormone,GnIH) 已被证明是HPG的关键信号分子,下丘脑接受经中枢神经系统分析和整合后的各种信息,在生殖调控中起着关键作用。GnRH选择性调节促卵泡素(Follicle-stimulating hormone,FSH)和黄体生成素(Luteinizing hormone,LH)的释放,FSH和LH能促进卵泡发育成熟和排卵[4]。GnIH在动物生殖轴中起着相反的调控作用,它能抑制LH和FSH的释放,并作用于PRL的释放[5-7],可抑制繁殖行为,并在维持性腺的稳定和刺激采食具有重要作用。GnIH可能在动物的季节性繁殖方面扮演着重要角色。禽类的GnRH和GnIH血清浓度和基因表达量会随着繁殖周期的变化而发生周期变化[8-10]。研究表明,GnRH和GnIH基因突变与禽类的产蛋性能有显著或极显著的关联[11-14]。杨海明等[15]发现产蛋高峰期鹅下丘脑和垂体GnRH基因相对表达量显著高于休产期鹅;X.Luan等[16]比较了产蛋期与休产期豁眼鹅卵巢组织的转录组数据,发现两个时期存在688个表达差异显著的基因,其中8个基因分布在GnRH信号通路上,这提示GnRH在调控生殖阶段的转变过程中起着重要作用。 G.L.Gao等[17]比较产蛋前期和产蛋期四川白鹅垂体组织的转录组数据,进一步证实垂体组织中的GnRH和GnIH基因在不同繁殖阶段差异表达,提示GnRH和GnIH基因和激素在鹅繁殖过程中发挥重要的作用。
本研究检测产蛋前期、产蛋期和休产期的四川白鹅和溆浦鹅在下丘脑、垂体和卵巢组织中GnRH和GnIH基因mRNA表达量以及血清中GnRH和GnIH激素含量,并比较两种鹅GnRH和GnIH基因表达量和激素水平的差异,为进一步揭示动物繁殖的分子机制奠定基础。
1 材料与方法
1.1材料
1.1.1 试验动物与组织采集四川白鹅与溆浦鹅均选自重庆市畜牧科学院安富水禽养殖基地,均为自然光照下予以全价日粮自由采食的健康鹅;采集产蛋前期(22周)、产蛋高峰期(44周)和休产期(64周)四川白鹅(5只)与溆浦鹅(5只)母鹅的下丘脑、垂体和卵巢组织,-80 ℃保存;在产蛋前期、产蛋期和休产期,随机抽取四川白鹅和溆浦鹅种群中血样各20份血液样本,并分离出血清存储于-20 ℃备用。
1.1.2主要试剂RNA抽提试剂盒购自QIAGEN生物科技有限公司(73404);RNA反转录试剂盒购自Promega生物科技公司(A5001);荧光定量试剂盒购自TaKaRa公司(RR820);引物由上海英骏生物技术有限公司合成;GnRH放射性免疫测定试剂盒(LBTR-10035)和GnIH ELISA检测试剂盒(LBTR-10023)均购自北京莱博特瑞科技有限公司。
1.2方法
1.2.1Real-time PCR使用引物的设计与合成以甘油醛-3-磷酸脱氢酶基因(GAPDH)为内参,根据GenBank提供的GnRH和GnIH基因的mRNA序列,利用Primer Premier 5.0设计引物见表1。
1.2.2组织RNA的抽提与cDNA的合成将组织加入液氮研磨成粉状后,取适量粉末参照Qiagen试剂盒说明书提取组织总RNA;抽提的RNA取500 ng,参照Promega反转录试剂盒说明书进行反转录,获得各样品的cDNA,将cDNA稀释10倍后,置于-20 ℃备用。
表1Real-time PCR 引物序列
Table 1Primer sequences of real-time PCR

基因GeneGenBank登录号AccessionNo.引物序列(5'-3')Primersequence产物大小/bpProductlengthGnRHEF495207CTGGGACCCTTGCTGTTTTGAGGGGACTTCCAACCATCAC232GnIHKC514473TATGTGCCTAGATGAACTGATGTAATGCTTCTTTCTTCTGGGTAG148GAPDHKO1458GTGGTGCTAAGCGTGTCAGGCTGGGATAATGTTCTGG303
1.2.3荧光定量PCRReal-time RT-PCR按照SYBR®Premix Ex TaqTM(Perfect Real time)试剂盒说明书配制反应体系,SYBR®Premix Ex TaqTM(2×),5 μL;正反向引物(10 μmol·L-1)各0.2 μL;ROX Reference Dye Ⅱ(50×)0.2 μL;dH2O 2.4 μL;cDNA模板2 μL,共10 μL;反应条件:95 ℃预变性30 s,95 ℃变性5 s,60 ℃延伸34 s,40个循环。以GAPDH基因作为内参,每个组织设置3个重复。将该体系置于荧光定量PCR仪(型号:ABI7500)检测。
1.2.4血清GnRH和GnIH激素浓度测定将收集的血清送北京莱博特瑞科技有限公司检测血清GnRH和GnIH浓度。
2 结 果
2.1四川白鹅和溆浦鹅GnRH和GnIH基因时空表达检测结果
GnRH和GnIH基因在四川白鹅和溆浦鹅的下丘脑、垂体和卵巢组织中均有表达(图1);在下丘脑、垂体和卵巢组织中,GnRH基因表达总体呈先上升后下降的趋势,且四川白鹅中的表达量均高于溆浦鹅,但休产期在四川白鹅卵巢组织中,该基因表达量极显著低于溆浦鹅(图1 A~C)。产蛋前期和产蛋高峰期,四川白鹅的下丘脑和卵巢组织中GnRH基因mRNA表达量均显著或极显著高于溆浦鹅(P<0.05 或P<0.01)(图1 A,C);在休产期,垂体中的四川白鹅GnRH基因表达量显著高于溆浦鹅(P<0.05)(图1 B),四川白鹅卵巢组织中GnRH基因表达量极显著低于溆浦鹅(P<0.01)(图1C)。
GnIH基因表达量在下丘脑和垂体组织中,总体呈上升趋势,且四川白鹅基因表达量均高于溆浦鹅,除产蛋前期四川白鹅卵巢组织中该基因表达量低于溆浦鹅(图1E,F);四川白鹅卵巢组织中该基因在两种鹅中没有一致规律,在四川白鹅中,该基因表达量先下降后上升,在溆浦鹅中该基因表达量先上升后下降(图1D)。除产蛋高峰期的下丘脑组织中四川白鹅GnIH基因表达量极显著低于溆浦鹅之外(P<0.01)(图1 D),在产蛋前期、高峰期和休产期,四川白鹅和溆浦鹅GnIH基因无显著差异(图1D~F)。
2.2四川白鹅和溆浦鹅GnRH和GnIH激素检测
四川白鹅与溆浦鹅血清GnRH浓度在产蛋前期存在较大差异,分别为47.915和53.976 pg·mL-1,溆浦鹅较四川白鹅高出约12.65%,但在产蛋高峰期和休产期比较两种鹅血清GnRH浓度时,四川白鹅分别极显著(P<0.01)和显著(P<0.05)高于溆浦鹅,四川白鹅产蛋高峰期和休产期GnRH血清浓度分别为51.13、51.10 pg·mL-1,溆浦鹅分别为49.52、49.94 pg·mL-1(图2 A)。四川白鹅与溆浦鹅的血清GnIH浓度都有相似的变化趋势,先上升后下降,在产蛋高峰时达到峰值,分别为232.315、233.230 ng·L-1,比产蛋前期的147.981和143.143 ng·L-1,分别上升了56.99%和62.93%;在休产期的浓度分别为52.525和45.637 ng·L-1,但在产蛋前期、产蛋期和产蛋后期,两种鹅之间比较GnIH激素浓度无显著差异(图2 B)。
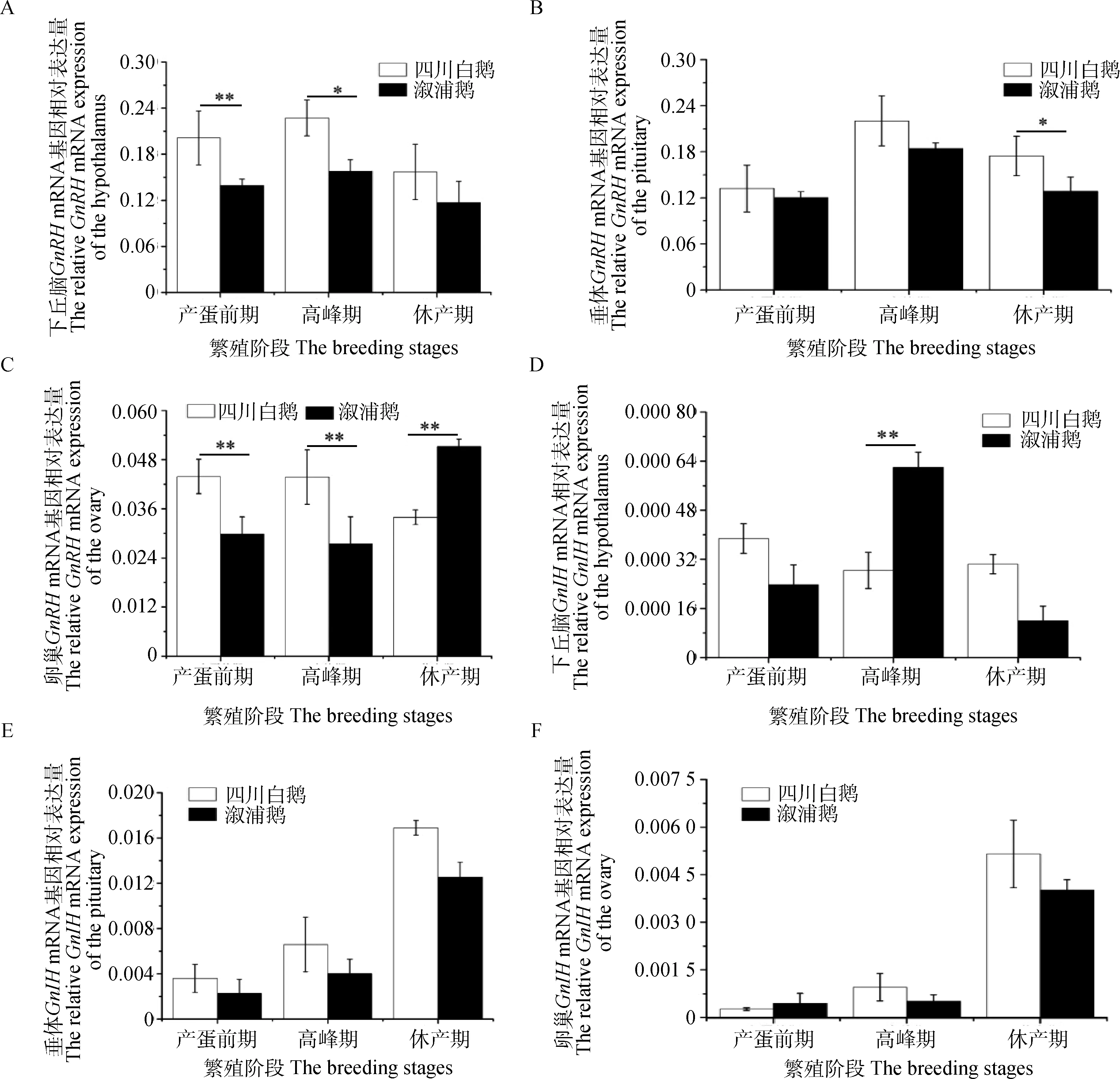
未标注为差异不显著;*代表差异显著(P<0.05); **代表差异极显著(P<0.01),下同No mark represents no significant difference; * represents significant difference(P<0.05); ** represents great significant difference (P<0.01).The same as below图1 四川白鹅和溆浦鹅在繁殖各阶段GnRH和GnIH的mRNA相对表达情况(n=5)Fig.1 The relative expression levels of GnRH and GnIH in Sichuan White geese and Xupu geese in different phases of breeding(n=5)
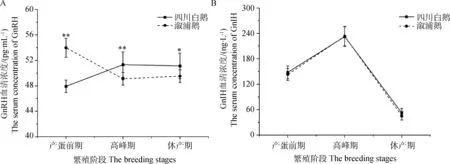
图2 不同时期四川白鹅和溆浦鹅血清GnRH和GnIH激素含量变化(n=20)Fig.2 The serum concentrations of GnRH and GnIH in Sichuan White goose and Xupu goose in different reproductive periods(n=20)
3 讨 论
四川白鹅与溆浦鹅均为地方优良水禽品种,两种鹅均为体型中等、短日照繁殖类型,但前者年产蛋量60~80枚,后者年产蛋30枚左右[18]。下丘脑激素GnRH是启动卵巢组织快速发育、卵泡生长成熟和排卵的首要促进因子,G.H.Son等[19]发现在GnRH功能缺失的小鼠会表现性激素大幅度降低和性腺发育障碍],T.D.Schirman-Hildesheim等[20]发现在小鼠排卵前的LH峰之前会出现一GnRH峰;刘自逵等研究表明,22~28周龄鹅的卵巢和输卵管形态结构生育系统进入快速发育时期[21],本试验检测到鹅在产蛋前期和高峰期的下丘脑和卵巢组织中GnRHmRNA出现持续性的高表达,前期高水平表达可能与GnRH促进卵巢的发育和卵泡成熟有关,而后期高水平表达可能与维持鹅的排卵有关;在进行两种鹅GnRH激素含量比较时,笔者发现:在产蛋前期、产蛋高峰期和休产期,四川白鹅中GnRH激素浓度与溆浦鹅中的激素浓度呈显著或极显著差异(P<0.01或P<0.05),尤其是在产蛋期阶段,四川白鹅的激素水平显著高于溆浦鹅(P<0.05),这预示着两种鹅GnRH激素浓度的差异可能与其产蛋量有关;鹅是一种光周期繁殖动物,有报道显示,短光照可以使光周期动物褪黑素含量增加,同时促进与其有着互作关系的GnIH基因的表达量增加,进而影响动物的繁殖性能[22-23]。在本试验中,GnIH在产蛋高峰期,无论是在mRNA水平还是在激素水平,GnIH的含量都处于高水平表达,这可能一方面与短日照刺激有关,而另一方面与产蛋期的鹅需抑制性行为与好斗行为有关;R.M.Calisi等[24]用免疫荧光的方法证实欧洲椋鸟繁殖期较非繁殖期有更高水平的GnIH肽,GnIHmRNA细胞丰度和GnIH细胞密度;T.Tachibana等[25]发现GnIH可通过刺激大脑中的细胞色素P450芳香化酶的活性抑制鸟类繁殖期的性行为;在比较两种鹅GnIH的表达差异时只有在产蛋高峰期的下丘脑组织中表现出极显著差异(P<0.01),而在繁殖阶段的各个时期GnIH激素浓度并未出现显著性差异。随着繁殖阶段的改变,两种鹅GnRH改变的幅度相较于GnIH变化更大,这提示在调控鹅产蛋性能的过程中GnRH发挥的作用可能较GnIH更为重要。
家禽产蛋性能是由排出的卵泡数量以及在输卵管中的卵转化成硬壳蛋的数量所决定的。在产蛋过程中,从卵泡形成到排卵,是由激素和其他生理条件所决定的,包括能量代谢、卵细胞和卵母细胞卵泡相关细胞的凋亡[26]。维持和活化正常卵泡功能是依靠促性腺激素,例如下丘脑和垂体分泌的GnRH、GnRH、LH 和PRL[27]。下丘脑和垂体中功能的细微差别影响繁殖性能,例如卵泡发育、排卵、产卵和孵化等行为[28]。本研究发现在产蛋前期、产蛋高峰期及休产期中GnRH和GnIH基因及其激素的阶段性变化,并在两种鹅之间的基因表达量和激素浓度的差异,调控着鹅产蛋和休产等行为,并与四川白鹅和溆浦鹅产蛋性能差异相关。
综上表明,在生殖调控过程中,GnRH和GnIH的基因和激素在鹅繁殖阶段的转变过程中发挥着重要作用。GnRH在产蛋前期促进鹅的性成熟,在繁殖期维持其排卵;而GnIH在繁殖阶段的后期维持高水平表达,促进鹅由繁殖期向休产期转变。
[1]侯水生.国家水禽产业技术体系取得的重要成果及其应用[J].水禽世界,2014(4):6-9.
HOU S S.Important achievements in national waterfowl industry technology system and its application[J].WaterfowlWorld,2014(4):6-9.(in Chinese)
[2]侯水生.我国水禽产业发展面临的挑战[J].中国家禽,2013,35(10):36-37.
HOU S S.The challenge for the waterfowl industry development in our country[J].ChinaPoutry, 2013,35(10):36-37.(in Chinese)
[3]SPICER L J.Proteolytic degradation of insulin-like growth factor binding proteins by ovarian follicles:a control mechanism for selection of dominant follicles[J].BiolReprod,2004,70(5):1223-1230.
[4]SCHALLY A V,ARIMURA A,KASTIN A J,et al.Gonadotropin-releasing hormone:one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones[J].Science,1971,173(4001):1036-1038.
[5]OSUGI T,UKENA K,BENTLEY G E,et al.Gonadotropin-inhibitory hormone in Gambel’s white-crowned sparrow (Zonotrichialeucophrysgambelii):cDNA identification,transcript localization and functional effects in laboratory and field experiments[J].JEndocrinol,2004,182(1):33-42.
[6]SATAKE H,HISADA M,KAWADA T,et al.Characterization of a cDNA encoding a novel avian hypothalamic neuropeptide exerting an inhibitory effect on gonadotropin release[J].BiochemJ,2001,354(Pt 2):379-385.
[7]OHTA M,KADOTA C,KONISHI H.A role of melatonin in the initial stage of photoperiodism in the Japanese quail[J].BiolReprod,1989,40(5):935-941.
[8]刘丽平.番鸭GnRH基因的cDNA克隆及就巢相关基因的差异表达研究[D].福州:福建农林大学,2012.
LIU L P.cDNA cloning of Gonadotropin-releasing hormone (GnRH) and differentially expression of the nesting related genes in Muscovy duck[D].Fuzhou:Fujian Agriculture and Forestry University,2012.(in Chinese)
[9]何宗亮.鸡GnRH、GnIH和VIP基因表达与繁殖性能间关系的研究[D].南京:南京农业大学,2009.
HE Z L.Relationship in expression of laying hen GnRH,GnIH,VIP gene and laying performance[D].Nanjing:Nanjing Agricultural University,2009.(in Chinese)
[10]张利.树麻雀GnRH和GnIH在脑中的定位及其季节性表达[D].石家庄:河北师范大学,2013.
ZHANG L.The identification and seasonal expression of GnRH and GnIH in the brain of eurasian tree sparrows[D].Shijiazhuang:Hebei Normal University,2013.(in Chinese)
[11]黄钦柯.地方蛋鸡新品系基础群产蛋性能及相关候选基因(GnIH)多态性的研究[D].雅安:四川农业大学,2013.
HUANG Q K.Studies on egg-laying performance and polymorphism of relavent candidate genes (GnIH) in the underlying group of new local hens[D].Ya’an:Sichuan Agricultural University,2013.(in Chinese)
[12]杨涛.鹅GnRH基因5′端调控区和外显子1 SNPs检测及与产蛋量的关系[D].合肥:安徽农业大学,2007.
YANG T.SNPs in geese GnRH genes 5′-flank region and exon l and association with egg production[D].Heifei:Anhui Agricultural University,2007.(in Chinese)
[13]魏茹华,耿照玉,姜润深,等.鹅GnRH基因5′端调控区单核苷酸多态性分析[J].畜牧与饲料科学,2009,30(2):127-129.
WEI R H,GENG Z Y,JIANG R S,et al.Analysis of the single nucleotide polymorphisms in the 5′ regulating region of goose GnRH gene[J].AnimalHusbandryandFeedScience,2009,30(2):127-129.(in Chinese)
[14]赵兴涛,杨国锋,孙燕,等.五龙鹅GnRH、PRL和FSHβ基因多态性与产蛋性状相关研究[J].中国家禽,2011,33(16):26-28.
ZHAO X T,YANG G F,SUN Y,et al.Association of polymorphisms ofGnRH,PRLandFSHβgenes with egg production traits in Wulong goose[J].ChinaPoultry,2011,33(16):26-28.(in Chinese)
[15]杨海明,巨晓军,王志跃,等.光照时间和环境温度对种鹅繁殖系统及相关激素mRNA表达、分泌的影响[J].中国农业科学,2015,48(13):2635-2644.
YANG H M,JU X J,WANG Z Y,et al.Effects of illumination time and ambient temperature on reproductive system and gene expression and secretion of hormone in breeding geese[J].ScientiaAgriculturaSinica, 2015,48(13):2635-2644.(in Chinese)
[16]LUAN X,LIU D,CAO Z,et al.Transcriptome profiling identifies differentially expressed genes in Huoyan goose ovaries between the laying period and ceased period[J].PLoSOne,2014,9(11):e113211.
[17]GAO G L,ZHAO X Z,LI Q,et al.Gene expression profiles in the pituitary glands of Sichuan White geese during prelaying and laying periods[J].GenetMolRes,2015,14(4):12636-12645.
[18]王光英,陈宽维,李宁.中国畜禽遗传资源志·家禽志[M].北京:中国农业出版社,2011.
WANG G Y,CHEN K W,LI N.Animal genetic resources in China· poutry[M].Beijing:China Agriculture Press,2011.(in Chinese)
[19]SON G H,PARK E,JUNG H,et al.GnRH pre-mRNA splicing:solving the mystery of a nature’s knockout,hpg mouse[J].BiochemBiophysResCommun,2005,326(2):261-267.
[20]SCHIRMAN-HILDESHEIM T D,BAR T,BEN-AROYA N,et al.Differential gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acid expression patterns in different tissues of the female rat across the estrous cycle[J].Endocrinology,2005,146(8):3401-3408.
[21]刘自逵,刘进辉,康顺之,等.溆浦母鹅产蛋前后卵巢和输卵管发育的形态观察[J].湖南农业大学学报,1996,22(4):381-385.
LIU Z K,LIU J H,KANG S Z,et al.Morphological observation of the ovary and oviduct development of Xupu geese before and after laying[J].JournalofHunanAgriculturalUniversity,1996,22(4):381-385.(in Chinese)
[22]UBUKA T,BENTLEY G E,UKENA K,et al.Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain[J].ProcNatlAcadSciUSA,2005,102(8):3052-3057.
[23]UBUKA T,SON Y L,TSUTSUI K.Molecular,cellular,morphological,physiological and behavioral aspects of gonadotropin-inhibitory hormone[J].GenCompEndocrinol,2016,227:27-50.
[24]CALISI R M,DIAZ-MUOZ S L,WINGFIELD J C,et al.Social and breeding status are associated with the expression of GnIH[J].GenesBrainBehav,2011,10(5):557-564.
[25]TACHIBANA T,SATO M,TAKAHASHI H,et al.Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks[J].BrainRes,2005,1050(1-2):94-100.
[26]LUAN X,CAO Z,XU W,et al.Gene expression profiling in the pituitary gland of laying period and ceased period huoyan geese[J].Asian-AustralasJAnimSci,2013,26(7):921-929.
[27]KUO Y M,SHIUE Y L,CHEN C F,et al.Proteomic analysis of hypothalamic proteins of high and low egg production strains of chickens[J].Theriogenology, 2005,64(7):1490-1502.
[28]ETCHES R J,PETITTE J N,ANDERSON-LANGMUIR C E.Interrelationships between the hypothalamus,pituitary gland,ovary,adrenal gland,and the open period for LH release in the hen (Gallusdomesticus)[J].JExpZool,1984,232(3):501-511.
(编辑程金华)
Analysis of the Serum Concentrations and mRNA Expression Levels ofGnRHandGnIHin Geese during Different Reproductive Periods
ZHANG Ke-shan1,2,HU Yan-jingke3,HAN Xiao-zhe3,GAO Guang-liang1,2,`ZHONG Hang1,2,WANG Qi-gui1,2*
(1.ChongqingAcademyofAnimalScience,Chongqing402460,China;2.ChongqingEngineeringResearchCenterofGooseGeneticImprovement,Chongqing402460,China;3.SouthwestUniversity,Chongqing402460,China)
To investigate the role of gonadotropin-releasing hormone and gonadotropin-inhibitory hormone genes (GnRHandGnIH,respectively)during the reproductive process of geese,the Sichuan White goose and Xupu goose were selected as experimental animals.We detected the mRNA expression profiles ofGnRHandGnIHin the hypothalamus,pituitary and ovary tissues using real-time reverse transcription PCR.Serum concentrations of GnRH and GnIH were respectively measured using radio immunoassay and ELISA during prelaying and laying periods and the period when laying ceased.GnIHandGnRHmRNA were expressed in the hypothalamus,pituitary and ovary tissues.GnRHmRNA expression of the hypothalamus and the ovary were significantly higher in Sichuan White geese than in Xupu geese in both prelaying and laying periods,particularly in the latter.Serum GnRH concentrations were significantly higher or higher in Sichuan White geese (51.13 and 51.10 pg·mL-1) than in Xupu geese(49.52 and 49.94 pg·mL-1) during the laying and cease period,respectively.During the laying period,GnIHmRNA expression of Sichuan White geese was significantly lower than in Xupu geese;however,there were no significant differences inserum GnIH concentrations between the 2 geese during prelaying,laying or cease periods.These results indicate thatGnRHis more important thanGnIHin regulating laying performance and changing the laying stages which establishes the foundation for understanding the breeding mechanisms of geese.
Sichuan White geese;Xupu geese;GnRH;GnIH;reproductive hormone
10.11843/j.issn.0366-6964.2016.08.025
2016-02-25
重庆市基础科学与前沿研究(cstc2014jcyjA80021);重庆市基本科研业务费(14305);国家自然科学基金(31572386)
张克山(1988 ),女,四川达州人,助理研究员,硕士,主要从事家禽遗传育种研究,E-mail:zhangksh1988@163.com
王启贵,教授,博士,主要从事家禽遗传育种研究,E-mail: wangqigui@hotmail.com
S835.2
A
0366-6964(2016)08-1720-07
