冷诱导RNA结合蛋白参与小鼠H1N1甲型流感病毒感染的应答
2016-09-07聂培婷
聂培婷,汤 承,岳 华*
(1.西南民族大学生命科学与技术学院,成都 610041;2.新疆天康畜牧生物技术股份有限公司制药事业部,乌鲁木齐 830011)
冷诱导RNA结合蛋白参与小鼠H1N1甲型流感病毒感染的应答
聂培婷1,2,汤承1,岳华1*
(1.西南民族大学生命科学与技术学院,成都 610041;2.新疆天康畜牧生物技术股份有限公司制药事业部,乌鲁木齐 830011)
冷诱导RNA结合蛋白(CIRP)是哺乳动物间高度保守的多功能蛋白,但其在病毒感染中的作用尚不清楚,本研究旨在探讨CIRP对甲型流感病毒感染的应答。用H1N1甲型流感病毒PR-8株滴鼻接种成年BALB/c小鼠,分别于感染后24、48、72、96、120 h随机处死3只,用荧光定量RT-PCR和免疫组化法分别检测小鼠心、肝、脾和肺中CIRP基因和蛋白质的表达水平。基因定量检测结果显示,感染小鼠各被检器官中CirpmRNA的转录量显著升高;免疫组化试验结果显示,CIRP在感染小鼠心肌细胞、肺泡上皮细胞、肺支气管上皮细胞和脾淋巴细胞的细胞质中表达量均有不同程度的升高。综上可见,CIRP参与机体对H1N1甲型流感病毒感染的应答,由于CIRP对炎性因子产生过程具有显著的调节作用,其在流感病毒感染过程中的作用值得深入研究。
冷诱导RNA结合蛋白;甲型流感病毒;荧光定量RT-PCR;免疫组化
冷诱导RNA结合蛋白(cold induced RNA binding protein,CIRP)是RNA结合蛋白家族的异源核糖核蛋白(hnRNP)亚群成员[1],是哺乳动物中最早鉴定出的与冷应激相关的蛋白质[2],在多种应激状态下发挥细胞保护作用[2-7]。最近的研究表明,在LPS刺激和败血症情况下,CIRP通过调节NF-κB信号通路影响多种炎性因子的产生[8],并通过调节TRL4促进炎症发生[9],是一种新的促炎介质[10],在细菌感染中发挥重要作用。甲型流感病毒可引起人和多种动物严重的呼吸道感染[11],其引起的细胞因子风暴是引起重症肺炎和致死的主要原因[12],肺上皮细胞和炎性浸润在肺的病理性损伤中起重要作用[13]。CIRP与病毒感染的联系尚不清楚,本研究旨在探讨CIRP对甲型流感病毒感染的应答。
1 材料与方法
1.1材料
30只5周龄的雌性BALB/c小鼠,体重18 g±2 g,购自华西实验动物中心;H1N1甲型流感病毒(PR/8)由四川省疾病预防控制中心惠赠,HA效价为28;RNA提取试剂盒购自美国Invitrogen公司;反转录试剂盒购自日本TaKaRa公司;兔抗人CIRP多克隆抗体购自美国Proteintech公司;生物素羊抗兔抗体、免疫组化ABC检测试剂盒购自美国VECTOR公司;DAB显色试剂购自武汉博士德公司。
1.2病毒感染模型的建立及样本采集
将小鼠随机等分为两组,用干冰吸入麻醉后,感染组滴鼻接种PR/8,50 μL·只-1,对照组用等体积生理盐水代替病毒液滴鼻,两组小鼠隔离饲养。各组分别于感染后24、48、72、96、120 h随机处死3只小鼠,采集心、肝、脾和肺,每个样本分为两份,一份用焦炭酸二乙酯溶液清洗后立即放入液氮中过夜,随后转入-80 ℃冰箱冻存,用于mRNA转录量的检测,另一份用4%多聚甲醛固定,石蜡包埋切片。
1.3组织样本总RNA的提取及cDNA合成
按照 RNA提取试剂盒说明书推荐程序提取各组织样本的总RNA,用反转录试剂盒反转录合成cDNA。
1.4流感病毒感染的检测
采用RT-PCR[14]检测PR/8感染后48 h肺中H1N1甲型流感病毒M基因,以确定小鼠是否感染成功。
1.5小鼠组织器官中CirpmRNA的表达水平的测定
以“1.3”合成的组织cDNA为模板,荧光定量PCR[15]检测CirpmRNA在感染小鼠组织器官中的时空表达水平,以在流感病毒感染过程中稳定表达的Ppia作为内参基因[16],用2-△△Ct法[17]进行相对定量,每个样本重复测定3次,用SPSS软件进行统计学分析。
1.6小鼠组织器官中CIRP的分布及表达水平测定
按照免疫组化试剂盒说明书对石蜡切片进行染色,用Nikon ECLIPSE 6200显微数码图像采集系统采集免疫组化图像信息,在每张切片随机选取5个视野,用ImagePro-Plus6.0 (IPP6.0) 图像分析软件测定其阳性颗粒的累积光密度值(integrated optical density,IOD),用SPSS软件对数据进行统计学处理。图像分析判定标准:IOD≤100为阴性;100≤IOD≤2 000为弱阳性;2 000≤IOD≤5 000为阳性;IOD≥5 000为强阳性[18]。
2 结 果
2.1H1N1甲型流感病毒成功感染小鼠
RT-PCR从感染小鼠肺中扩增出一条约238 bp的条带,大小与预期相符(图1),说明PR-8感染了小鼠,成功建立了N1N1甲型流感病毒感染小鼠模型。
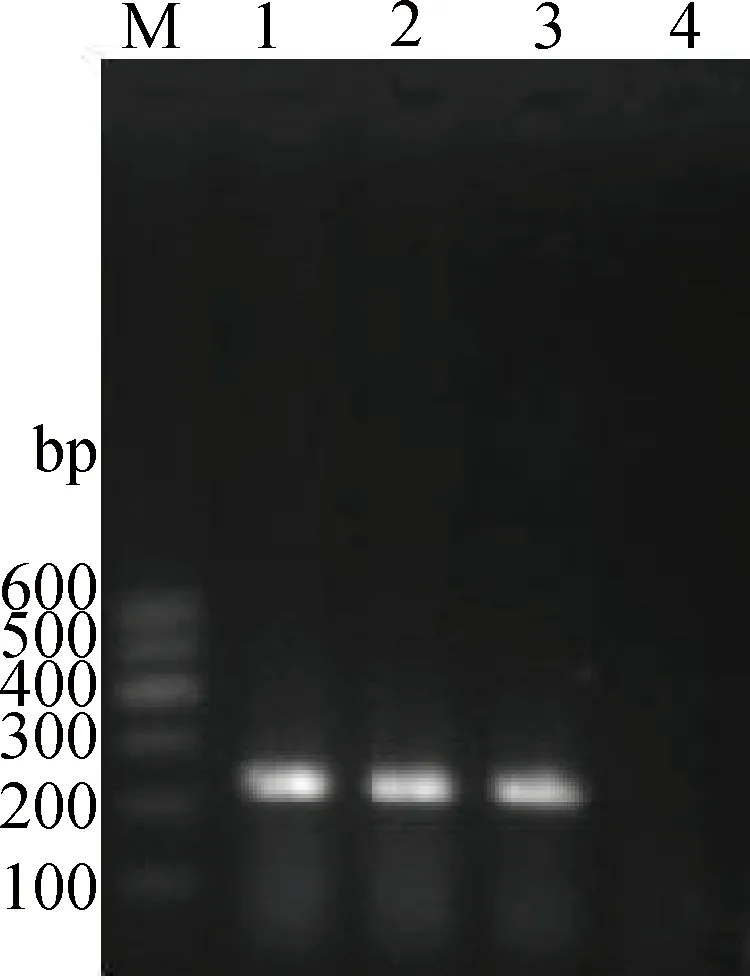
M.DNA相对分子质量标准Marker Ⅰ;1~3.感染小鼠肺组织样本;4.阴性模板对照M.DNA marker Ⅰ;1-3.Lung samples of infected mice;4.Negative control图1 RT-PCR检测感染后48 h小鼠肺中PR-8 M基因的结果Fig.1 The result of PR-8 M gene in infected lungs 48 h PI detected by RT-PCR
2.2Cirp基因对H1N1甲型流感病毒感染的转录应答
荧光定量RT-PCR检测结果显示,CirpmRNA在健康小鼠的心、肝、脾和肺等器官中均有转录,在肝中转录量最低,其在心、脾和肺中的转录量分别为肝转录量的7.6、7.4和2.2倍(图2)。CirpmRNA在感染H1N1甲型流感病毒后显著升高,但在不同器官上升的时间点不同(图3)。
2.3CIRP在小鼠组织器官中的定位及对H1N1甲型流感病毒感染的应答
免疫组化结果显示,CIRP在健康小鼠和感染小鼠心肌细胞、肝细胞、肺泡上皮细胞、肺支气管黏膜上皮细胞和脾淋巴细胞的细胞质中表达,甲型流感病毒感染未引起CIRP细胞定位的改变(图4)。
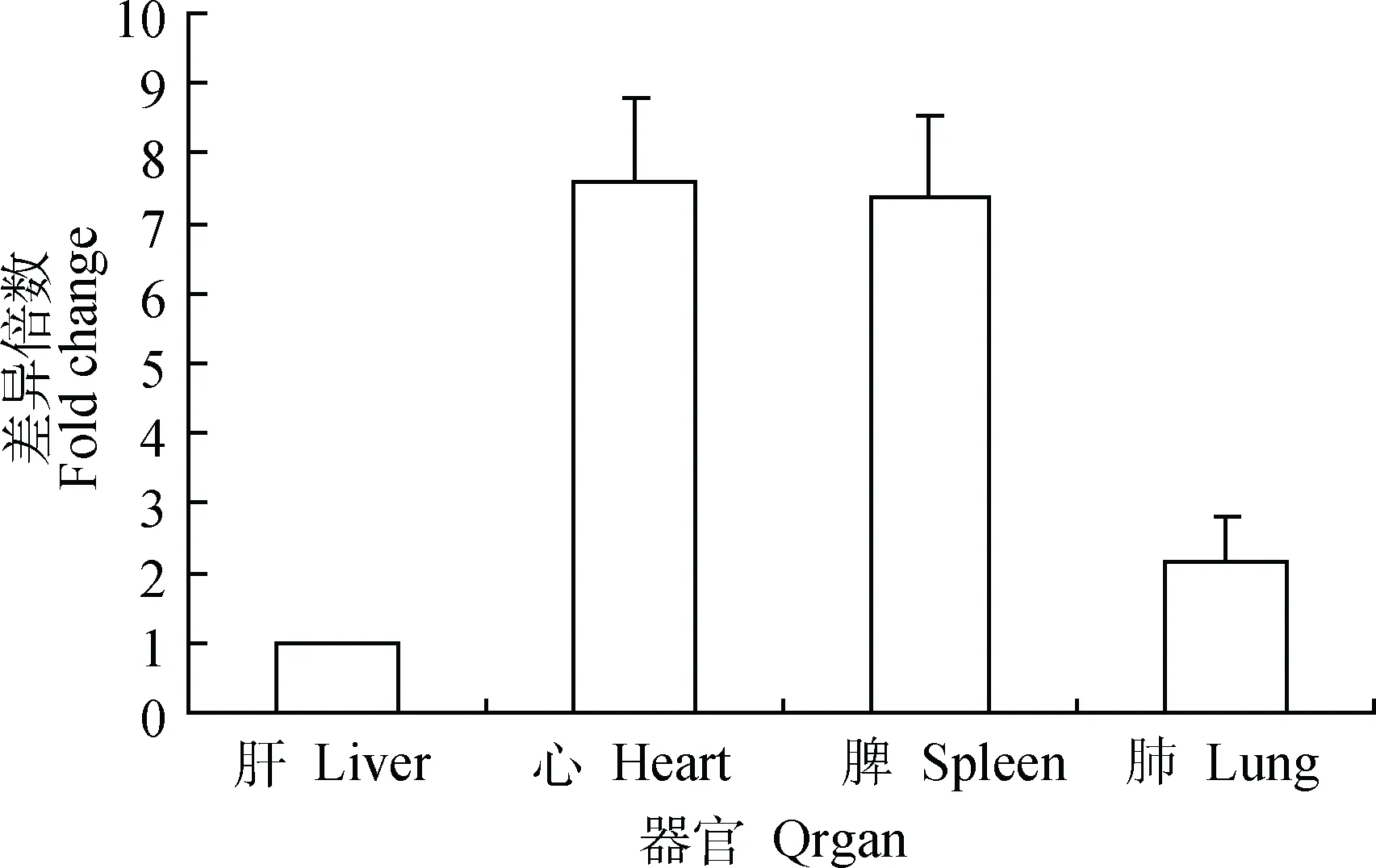
图2 健康小鼠各组织器官中Cirp基因的相对转录水平Fig.2 The relative transcription level of Cirp gene in the organs of healthy mice
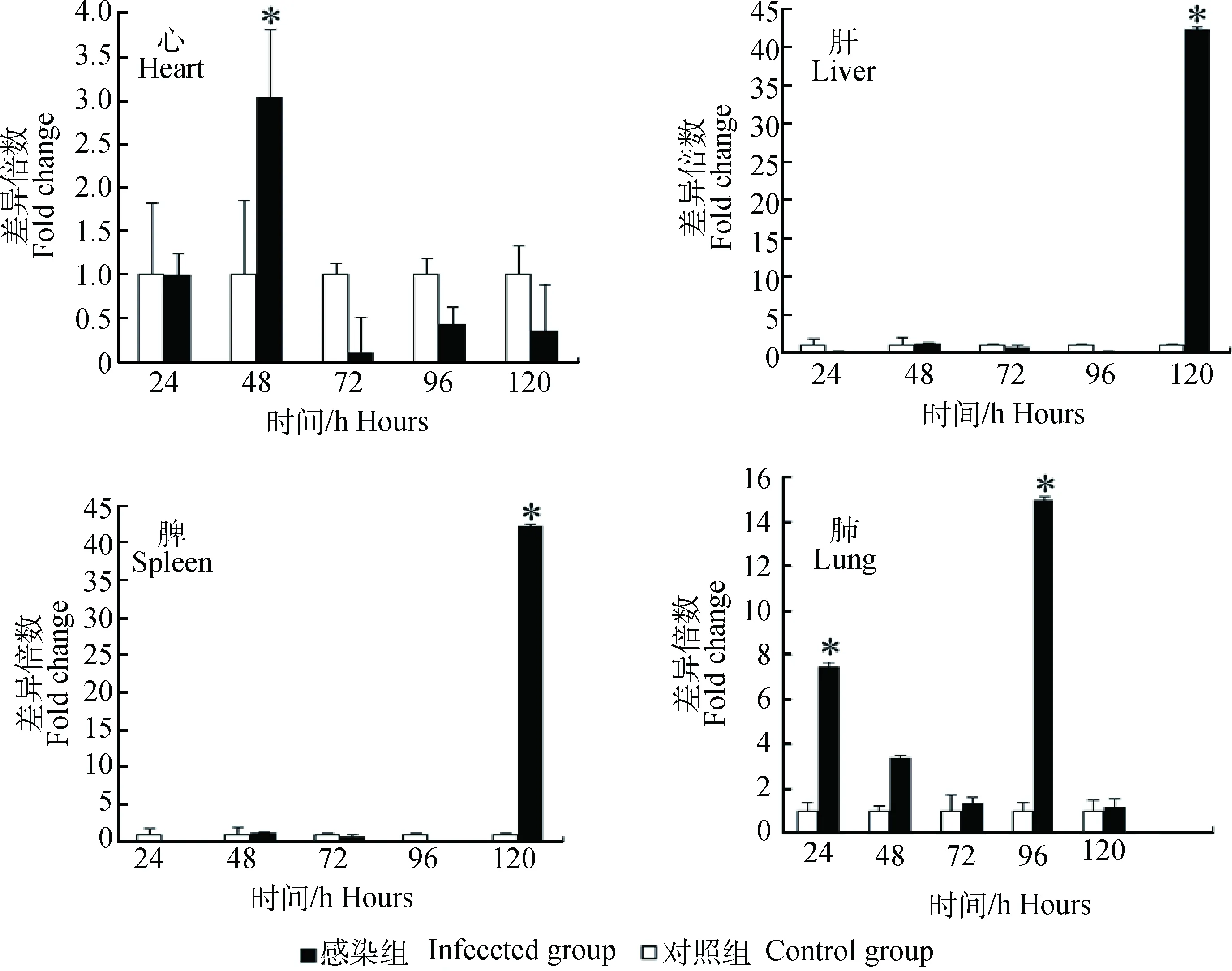
*.差异显著(P<0.05)*.Significant difference (P<0.05)图3 小鼠各脏器Cirp基因对H1N1甲型流感病毒感染的转录应答Fig.3 The transcription response of Cirp in mouse organs infected Influenza A Virus

A1.对照组小鼠心;A2.感染后96 h小鼠心;B1.对照组小鼠肝;B2.感染后120 h小鼠肝;C1.对照组小鼠脾;C2.感染后120 h小鼠脾;D1.对照组小鼠肺;D2.感染后24 h小鼠肺A1.Control group of heart tissue;A2 Experimental mice of 96 h-PI heart tissue;B1.Control group of liver tissue;B2.Experimental mice of 120 h-PI liver tissue;C1.Control group of spleen tissue;C2.Experimental mice of 120 h-PI spleen tissue;D1.Control group of lung tissue;D2.Experimental mice of 24 h-PI lung tissue图4 CIRP在健康及感染小鼠器官中的分布(免疫组化ABC法,20×)Fig.4 Distribution of CIRP in healthy and infected mice (ABC IHC staining,20×)
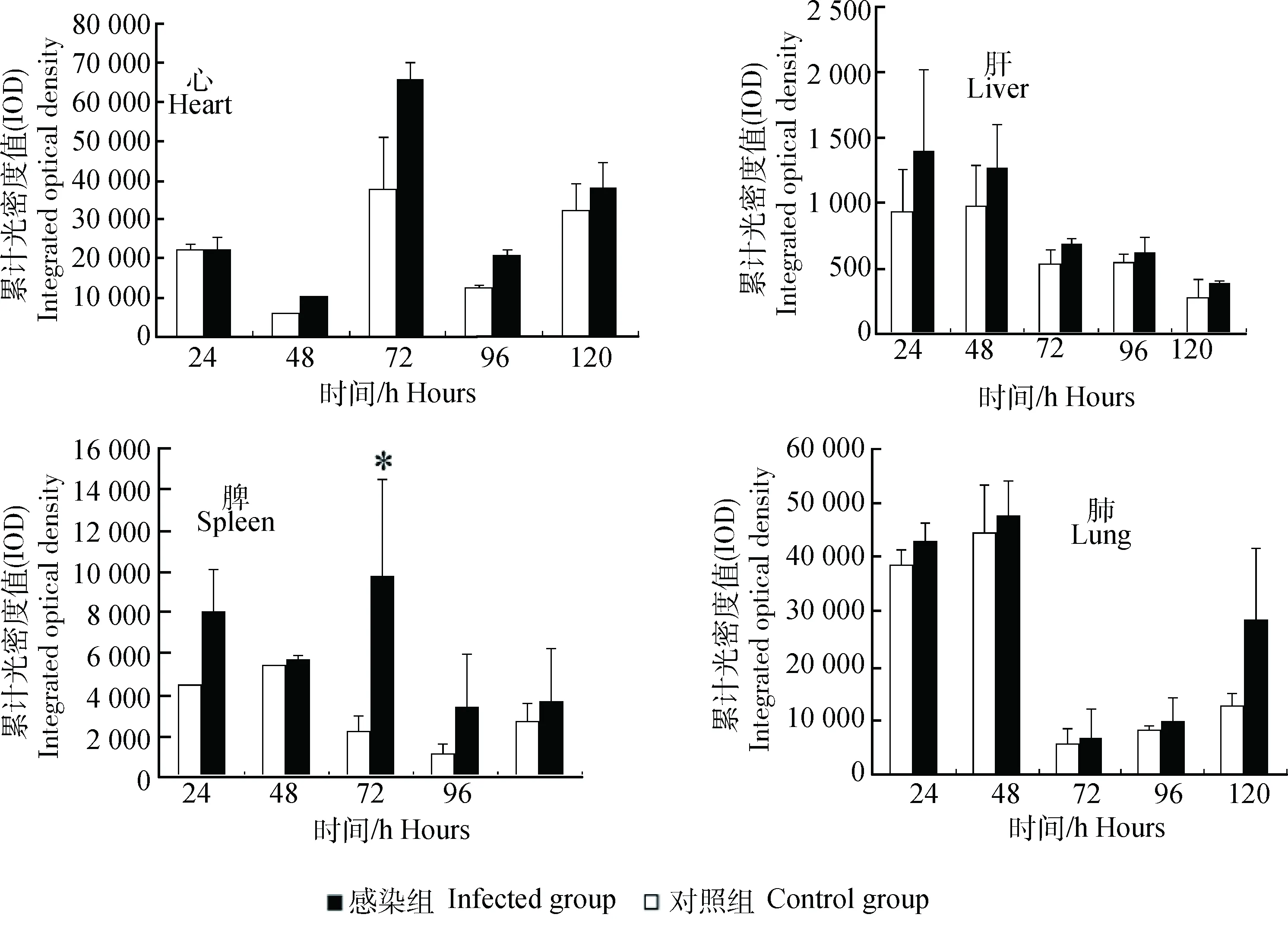
图5 CIRP对H1N1甲型流感病毒感染的应答Fig.5 Response of CIRP to H1N1 influenza A virus infection
IPP6.0图像定量分析结果显示,CIRP在健康小鼠心和肺的表达呈强阳性,在脾中的表达呈阳性,在健康小鼠肝中的表达量为弱阳性;CIRP在感染小鼠心、肝、脾和肺中的表达量有升高趋势,其中脾CIRP的表达量在感染后72 h显著升高(图5)。
3 讨 论
已知CIRP存在于多种不同类型的细胞中,是哺乳动物种间高度保守的多功能蛋白,参与对温度[2]、H2O2[3]、缺氧[4]、渗透压[5]等多种应激的转录应答,多种应激因子诱导机体CIRP的表达量升高和/或发生核质迁移,发挥细胞保护作用[2-7,15]。此外还参与胚胎发育、神经调节[19]和生物钟调节等生理过程,并参与机体对细菌的应答,但CIRP对病毒感染的应答未见报道。本研究发现CIRP在甲型流感病毒感染小鼠心、肝、脾、肺等器官中无论是基因还是蛋白质的水平均显著升高,证明其具有参与机体对流感病毒感染应答的功能。研究证明机体在LPS刺激下CIRP表达量升高,通过激活NF-κB信号通路诱导产生炎性因子,流感病毒感染能引发机体的炎性损伤,H5N1高致病性禽流感病毒刺激肺上皮细胞和巨噬细胞释放大量细胞因子,在细胞因子风暴中起重要作用[12]。在本研究中,CIRP在流感病毒感染小鼠体内表达量升高,极有可能通过调节炎性因子的产生,进而参与机体对流感病毒感染的应答过程。
RNA结合蛋白家族的某些成员在流感病毒感染中发挥着重要作用。S.D.Shapira等[20]发现一个RNA结合蛋白调控网络,能通过影响IFN-β的合成来抵抗机体对流感病毒的免疫作用,而RNA结合蛋白3(RanBP3)可通过Ser58位点的磷酸化作用促进流感病毒vRNP的出核转运[21]。ERK和NF-κB信号通路是流感病毒复制和机体起始免疫不可或缺的重要环节[22~25],因此推测作为这两条信号通路的上游调控因子,CIRP在流感病毒感染过程中表达量升高也可能影响流感病毒的复制,其在流感病毒感染中的作用值得进一步研究。
[1]BURD C G,DREYFUSS G.Conserved structures and diversity of functions of RNA-binding proteins[J].Science,1994,265(5172):615-621.
[2]NISHIYAMA H,ITOH K,KANEKO Y,et al.A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth[J].JCellBiol,1997,137(4):899-908.
[3]XUE J H,NONOGUCHI K,FUKUMOTO M,et al.Effects of isehemia and H2O2:on the cold stress protein CIRP expression in rat neuronal cells[J].FreeRadicBiolMed,1999,27(11-12):1238-1244.
[4]WELLMANN S,BÜHRER C,MODEREGGER E,et al.Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-l-independent mechanism[J].JCellSci,2004,117(Pt 9):1785-1794.
[5]PAN F,ZARATE J,CHOUDHURY A,et al.Osmotic stress of salmon stimulates upregulation of a cold inducible RNA binding protein (CIRP) similar to that of mammals and amphibians[J].Biochimie,2004,86(7):451-461.
[6]YANG C,CARRIER F.The UV-inducible RNA-binding protein A18 (A18 hnRNP) plays a protective role in the genotoxic stress response[J].JBiolChem,2001,276(50):47277-47284.
[7]DE LEEUW F,ZHANG T,WAUQUIER C,et al.The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor[J].ExpCellRes,2007,313(20):4130-4144.
[8]BROCHU C,CABRITA M A,MELANSON B D,et al.NF-κb-dependent role for cold-inducible RNA binding protein in regulating interleukin 1β[J].PLoSOne,2013,8(2):e57426.
[9]QIANG X,YANG W L,WU R,et al.Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis[J].NatMed,2013,19(11):1489-1495.
[10]ZHOU M,YANG W L,JI Y,et al.Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia[J].BiochimBiophysActa,2014,1840(7):2253-2261.
[11]NEUMANN G,NODA T,KAWAOKA Y.Emergence and pandemic potential of swine-origin H1N1 influenza virus[J].Nature,2009,459(7249):931-939.
[12]TEIJARO J R,WALSH K B,RICE S,et al.Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection[J].ProcNatlAcadSciUSA,2014,111(10):3799-3804.
[13]LA GRUTA N L,KEDZIERSKA K,STAMBAS J,et al.A question of self-preservation:immunopathology in influenza virus infection[J].ImmunolCellBiol,2007,85(2):85-92.
[14]聂培婷,汤承,岳华.H1N1甲型流感病毒在BHK21中的增殖规律[J].西南民族大学学报·自然科学版,2012,38(5):764-769.
NIE P T,TANG C,YUE H.Study on the proliferation profile of influenza A virus in BHK21 cells[J].JournalofSouthwestUniversityforNationalities·NaturalScienceEdition,2012,38(5):764-769.(in Chinese)
[15]周鸿淼,汤承,岳华,等.冷诱导RNA结合蛋白参与小鼠对LPS的应答[J].畜牧兽医学报,2014,45(8):1348-1354.
ZHOU H M,TANG C,YUE H,et al.Cold-inducible RNA binding protein involves in response to LPS in mice[J].ActaVeterinariaetZootechnicaSinica,2014,45(8):1348-1354.(in Chinese)
[16]吴巧,张斌,汤承,等.H5N1禽流感病毒感染小鼠后内参基因的筛选[J].中国畜牧兽医,2013,40(9):55-60.
WU Q,ZHANG B,TANG C,et al.Selection of reference gene in mice infected with H5N1 avian influenza virus[J].ChinaAnimalHusbandry&VeterinaryMedicine,2013,40(9):55-60.(in Chinese)
[17]LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△Ctmethod[J].Methods,2001,25(4):402-408.
[18]蔡文琴,王伯沄.实用免疫细胞化学与核酸分子杂交技术[M].成都:四川科学技术出版社,1994.
CAI W Q,WANG B Y.Practical immunocytochemistry and nucleic acid hybridization techniques[M].Chengdu:Sichuan Science and Technology Press,1994.(in Chinese)
[19]SAITO K,FUKUDA N,MATSUMOTO T,et al.Moderate low temperature preserves the stemness of neural stem cells and suppresses apoptosis of the cells via activation of the cold-inducible RNA binding protein[J].BrainRes, 2010,1358:20-29.
[20]SHAPIRA S D,GAT-VIKS I,SHUM B O,et al.A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infections[J].Cell,2009,139(7):1255-1267.
[21]PREDICALA R,ZHOU Y.The role of Ran-binding protein 3 during influenza A virus replication[J].JGenVirol,2013,94(Pt 5):977-984.
[22]PLANZ O.Development of cellular signaling pathway inhibitors as new antivirals against influenza[J].AntiviralRes,2013,98(3):457-468.
[23]LUDWIG S,PLANZ O.Influenza viruses and the NF-κB signaling pathway—towards a novel concept of antiviral therapy[J].BiolChem,2008,389(10):1307-1312.
[24]CANNON G,CALLAHAN M A,GRONEMUS J Q,et al.Early activation of MAP kinases by influenza A virus X-31 in murine macrophage cell lines[J].PLoSOne,2014,9(8):e105385.
[25]NIMMERJAHN F,DUDZIAK D,DIRMEIER U,et al.Active NF- kappaB signalling is a prerequisite for influenza virus infection[J].JGenVirol,2004,85(Pt 8):2347-2356.
(编辑白永平)
Cold-inducible RNA Binding Protein of Mice Involved in Response to Influenza A Virus Infection in Mice
NIE Pei-ting1,2,TANG Cheng1,YUE Hua1*
(1.CollegeofLifeScienceandTechnology,SouthwestUniversityforNationalities,Chengdu610041,China;2.XinjiangTeconAnimalHusbandryBio-TechnologyCO.,LT,Urumqi830011,China)
Cold induced RNA binding protein (CIRP) is a highly conserved multifunctional protein among vertebrates,but its role in viral infection is unclear.The objective of this paper was to explore the response of CIRP to influenza virus infection.The BALB/c mice were intranasal inoculated with H1N1 influenza A virus.Three mice were randomly selected in each group and euthanized at 24,48,72,96,120 h post infection.The heart,liver,spleen and lung of each mouse were collected.Real-time RT-PCR and immunohistochemistry (IMH) were used to detect the transcription ofCirpmRNA and expression of CIRP in each organ.The results of Real-time RT-PCR exhibited that theCirpmRNA significantly increased in all detected organs.The results of IMH test showed that CIRP protein was highly expressed in cytoplasm of myocardial cells,alveolar epithelial cells,bronchial epithelium cells and spleen lymphocytes.In summary,CIRP is involved in the response to influenza A virus infection,because CIRP can significantly regulate the production of inflammatory factors,the role of CIRP in influenza virus infection should be further studied.
cold-inducible RNA binding protein;influenza A virus;real-time RT-PCR;immunohistochemistry
10.11843/j.issn.0366-6964.2016.08.016
2015-12-28
国家自然科学基金(31172307);四川省教育厅创新团队(13TD0057)
聂培婷(1989-),女,新疆霍城人,主要从事感染与免疫学研究,E-mail:540016852@qq.com
岳华,教授,E-mail:yhua900@163.com
S852.23
A
0366-6964(2016)08-1652-06
