咪唑基表面活性离子液体在气/液界面的扩张粘弹性
2016-09-06柴金岭
李 燕 柴金岭
(山东师范大学化学化工与材料科学学院,山东高校化学成像功能探针协同创新中心,济南250014)
咪唑基表面活性离子液体在气/液界面的扩张粘弹性
李燕柴金岭*
(山东师范大学化学化工与材料科学学院,山东高校化学成像功能探针协同创新中心,济南250014)
合成了两种咪唑基表面活性离子液体,通过界面膨胀流变法研究了其在气/液界面的聚集行为,考察了咪唑基表面活性离子液体浓度、无机盐和温度对聚集行为的影响。研究发现,咪唑基表面活性离子液体在吸附过程中吸附控制占主导作用,而弛豫过程不是单一指数函数;加入无机盐或升高温度可以提高咪唑基表面活性离子液体的表面活性、增强其在界面的吸附能力、降低表面张力。扩张流变结果显示扩张模量、弹性模量和粘性模量随震荡频率增加而增加;随表面活性离子液体浓度增大,扩张模量先增大后减小。扩张模量随温度升高或无机盐(NaBr或CaBr2)的加入而降低。表面活性离子液在气/液界面形成的吸附膜以弹性模量为主,而且C14mimBr的界面膜弹性模量大于C12mimBr的界面膜弹性模量。
吸附动力学;扩张模量;气/液界面;咪唑基表面活性离子液体
Long alkyl chain based ILs perform the characteristics of surfactants(SAILs)13,for instance,S-3-hexadecyl-1-(1-hydroxypropan-2-yl)imidazolium bromide([C16hpim]Br)shows superior capacity in forming micelle,higher micropolarity,small counterion bonding degree and mean aggregation number compared to traditional ionic surfactants14.1-Alkyl-3-methylimidazolium bromide(CnmimBr,n=10,12,14,16)and N-alkyl-N-methylpyrrolidinium bromide(CnmpbBr,n=12,14,16)could form surfactant-polymer aggregates with L64 or F68 through the hydrophobic interaction.The intensity of surfactant-copolymer interaction increases with an increase in hydrophobicity15.1-(2,4,6-Trimethylphenyl)-3-alkylimidazolium bromide(CnpimBr,n=10, 12,14)shows lower critical micelle concentration than CnmimBr, due to the weakened steric hindrance of head groups and the ππ interactions among the adjacent 2,4,6-trimethylphenyl groups16. The micelle formation process of CnpimBr is enthalpy-driven.On the contrary,the micelle formation process of butyl-α,β-bis(dodecylimidazolium bromide)([C12-2-C12im]Br2and[Cn-4-Cnim]Br2, n=10,12,14)is entropy-driven.The process of[C16-2-C16im]Br2and CnmimBr is entropy-driven at low temperature and enthalpydriven at high temperature17,18.[Cn-4-Cnim]Br2also shows strong dispersing and stabilizing ability for multiwalled carbon nanotubes (MWCNTs).The longer the hydrocarbon chain of[Cn-4-Cnim]Br2, the stronger its ability in dispersing and stabilizing MWCNTs19. In our previous work,one of the SAILs,CnmimBr(n=12,14,16), has been used to form microemulsions.It is revealed that the solubilization power of the CnmimBr based microemulsions increases with the increase in alkyl chain length,and with adding of inorganic salts20.
The interfacial layer that is formed by surfactants plays key roles in practical industrial utilization,such as in pharmaceuticals, food,mining,oil recovery and other daily life application21.There are several methods in investigating the properties of interfacial layer including equilibrium surface tension,dynamic surface tension,dilational rheological,shear rheological,Langmuir trough and so on22,23.A large number of literatures focused on the equilibrium surface tension,from which the surface tension reducing ability of a surfactant could be obtained.The adsorption kinetics is also very important for many technological applications,such as foaming and emulsification.The dynamic interfacial behavior provides important information about the interaction between molecules,change of conformation and aggregation of molecules, kinetics of formation and disintegration of micelles24.The interfacial rheology(including dilational rheology and shear rheology) is based on the adsorption process of the surfactants at or near the interface.It provides information on the resistance of the interface to deformation and the interfacial layer strength25,26.
Du et al.27-29studied the equilibrium and dynamic properties of biopolymers/C16mimBr at the decane/water interface.They found that C16mimBr affected the molecular structure of biopolymers through electrostatic or hydrophobic interaction.According to our literature review,studies on adsorption dynamics and dilational rheology of SAILs are lacking.In this paper,we have synthesized two SAILs,1-dodecyl-3-methyl-imidazolium bromide(C12mimBr) and 1-tetradecyl-3-methyl imidazolium bromide(C14mimBr),and studied their adsorption dynamical and dilational rheological properties using an oscillating bubble method.The influencing factors,such as concentration of CnmimBr,inorganic salts and temperature on the adsorption dynamics of CnmimBr and dilational rheology of the interfacial layer were investigated.The changing rules of dynamic surface tension and dilational rheological modulus were obtained.The paper aims to provide new insight in exploring the air/water interfacial properties of SAILs.
2 Experimental
2.1Reagents and apparatus
N-methylimidazole(purity≥99%),dodecane(purity≥99%), tetradecane(purity≥96%),toluene(purity≥98.5%),ethyl acetate(purity≥99.5%),ether(purity≥99%),sodium bromide (NaBr,purity≥99%)and calcium bromide(CaBr2,≥98%)were all A.R.grades and purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).The N-methylimidazole,dodecane, and tetradecane were purified by vacuum distillation method; toluene was purified by atmospheric distillation method before experiments.Water was triply distilled by a quartz water purification system.
DZF-6050 vacuum oven thermostat(Shanghai Boxun Industry &Commerce Co.,Ltd.),FA1104 electronic balance(Shanghai Precision&Scientific Instrument Co.,Ltd.),HAAKE DC 30 thermostatic bath(Karlsruhe,Germany),Bruker EQUINOX 55 Fourier transform infrared(FTIR)spectrometer(Bruker Optics, Germany),Krüss K100 processor tensiometer(Krüss Co.Germany),and Oscillating bubble rheometer(Tracker,Teclis Co., France)were used.
2.2Synthesis and characterization of ionic liquidtype imidazolium surfactants
Both C12mimBr and C14mimBr were synthesized by one-step synthesis method,according to literature30.In a typical experiment, 16.4 g(0.2 mol)of N-methylimidazole,49.8 g(0.2 mol)of ndodecane,and 20 mL of toluene were added into a three-necked flask with a reflux device.The mixture was refluxed at 80°C for 48 h under the protection of nitrogen atmosphere.The crude product was mixed with certain amount of ether and cooled in icewater bath for 2-3 h.After being recrystallized by ether,and then dried in vacuum for 12 h at room temperature,the pure C12mimBr was obtained.The ethyl acetate was used in recrystallizing C14mimBr.
2.3Characterization of CnmimBr
FTIR spectra of C12mimBr and C14mimBr were recorded from 4000 to 400 cm-1at room temperature.The samples were scanned 16 times and the resolution ratio was 2 cm-1.
1H NMR spectrum of C14mimBr was performed using D2O as solvent.
The contents of C,H,N elements in the products were analyzed with Vario EL III(Elementar Analysen Syetem GmbH,Germany) elemental analyzer for further confirmation of the composition of the target compounds.
2.4Surface tension measurements
The surface tensions of CnmimBr solutions were measured using a ring method.The temperature was controlled at(25.0± 0.1)°C with a HAAKE DC 30 thermostatic bath.The CnmimBr solutions were kept for 15 min to reach an equilibrium.
2.5Measurements of dynamic surface tension and
interfacial dilational rheology
Dynamic surface tension(γt)and interfacial dilational rheology were measured using an oscillating bubble rheometer.The method was the same as that used by Deng et al.31The surface was created by injecting certain volume of air into an inverted stainless steel needle attached to an airtight syringe.The tip of the needle was placed into a quartz cuvette containing the CnmimBr solution.The image of the drop formed at the needle tip was captured by a CCD camera,digitized and analyzed by software.The Laplace equation was employed to obtain the surface tension.The surface tension was monitored as a function of adsorption time during the dynamic surface tension measurement.
Interfacial oscillating viscoelasticity measurements were performed by oscillating the bubble volume to a maximum change of 10%of the original drop volume.Data for all oscillation cycles were collected and averaged.
The rate of change in interfacial tension(γ)with respect to a change of interfacial area(A)was defined as interfacial oscillating modulus(ε),

Interfacial oscillating modulus can also be expressed as the summation of elasticity and viscosity contribution,

whereεdand ηdare storage modulus and expanding viscosity, respectively.They can be calculated by the equations,

where||εand θ are absolute modulus and phase angle,respectively.ω is the frequency of the sinusoidal variation for droplets (ω=2πf,where f is the oscillation frequency,and its unit is Hz).
3 Results and discussion
3.1Characterization of CnmimBr
FTIR spectra of C12mimBr and C14mimBr and1H NMR spectra of C14mimBr are shown in Fig.S1(Supporting Information).The adsorption peaks at 1381,1462,1570,1168,and 3146 cm-1belong to the symmetric bending vibration peak of C―H in substituent, the asymmetric bending vibration peak of C―H in substituent,the stretching vibration of C=N in imidazole ring,the asymmetric stretching vibration of C=N in imidazole ring,and the stretching vibration of=C―H in imidazole ring,respectively.The1H NMR peaks at δ 0.756,1.166(and 1.266,double peaks),3.873,4.194, 7.505,and 8.95 are assigned to―CH3in tetradecyl,―(CH2)12―in tetradecyl,―CH3in imidazole ring,CH=CH in imidazole ring,―CH2―in imidazole ring,CH that is linked with two N atoms,respectively.Data of FTIR,1H NMR as well as elemental contents of N,C,H(Table S1(Supporting Information))demonstrate the synthesis of CnmimBr.The melting points of C12mimBr and C14mimBr were 39.6 and 48.4°C20,respectively.
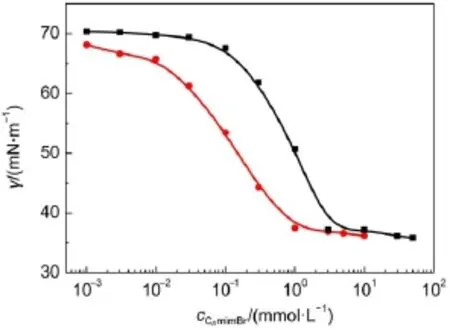
Fig.1 Plots of surface tension vs concentration of C12mimBr(■) or C14mimBr(●)at 25°C

Table 1 Surface properties of C12mimBr and C14mimBr at 25°C
3.2Surface activity of CnmimBr
The plots of surface tension vs CnmimBr concentration at 25°C are given in Fig.1.The surface tension of CnmimBr decreases with the increase in concentration up to a plateau region.The break point and constant value of surface tension correspond to the critical micelle concentration(cmc)and surface tension at cmc (γcmc)of CnmimBr,respectively.It is worth mentioning that the absence of a minimum around the breakpoint confirms the high purities of CnmimBr.The values of cmc and γcmcare listed in Table 1.The two γcmcvalues are almost the same with those Dong et al.32obtained,indicating that the hydrocarbon chain length of SAIL shows small influence on γcmcvalue for homologues33.
The adsorption efficiency(pc20)and surface tension reduction effectiveness(πcmc)could be obtained from the surface tension isotherm.Greater value of pc20implies a higher adsorption effi-ciency of surfactant at the air/water interface.The πcmcindicates the reduction of surface tension.Alarger value of πcmccorresponds to a higher surface tension reduction effectiveness of surfactant.The values of pc20and πcmcfor C12mimBr and C14mimBr are summarized in Table 1.Both the values for C14mimBr are larger than those for C12mimBr,indicating that the surface activity of C14mimBr is superior to that of C12mimBr.
From the Gibbs adsorption isotherm within the concentration below and close to the cmc,the maximum surface excess concentration(Γmax(mmol∙m-2))and the minimum cross-sectional area of surfactant molecule(Amin)are obtained34.The Gibbs equation for monovalent ionic surfactants is as follows:

where R is the gas constant(8.314 J∙mol-1∙K-1),T is the absolute temperature,γ is the surface tension in N∙m-1,and c is the surfactant concentration in mol∙L-1.From Γmax,Amincould be estimated from the following equation:

where NAisAvogadro constant(6.022×1023mol-1).The values of ΓmaxandAminfor CnmimBr are almost the same(Table 1).It is well known that the size of hydrophilic head group is a dominant factor in determining Aminand Γmaxvalues33.The results indicate that the imidazolium positive ions in both C12mimBr and C14mimBr are arranged at the air/water interface with the same density.
3.3Dynamic surface tension
From both scientific and industrial perspectives,adsorption dynamics is an important property of surfactant35,36.To compare the surface activity of C12mimBr and C14mimBr,the concentrations at the same times of cmc were used,as shown in Table 2.The plots of dynamic surface tensions(γt)of C12mimBr and C14mimBr solutions vs adsorption time(t)are shown in Fig.2.
When the concentration of C12mimBr is larger than 10-3cmc (that of C14mimBr is larger than 10-2cmc),the dynamic surface tensions decrease rapidly at the initial stage of adsorption,and then slowly.The larger the concentrations of CnmimBr,the faster the dynamic surface tensions decrease.When the concentration of C12mimBr is smaller than 10-4cmc(that of C14mimBr is smaller than 10-3cmc),the dynamic surface tensions decrease slightly at the initial stage of adsorption,and then rapidly,until the end stage of adsorption,when the dynamic surface tensions decrease slowly.

Table 2 Concentrations of CnmimBr used in the experiments
As is known that the dynamic surface tension is controlled by two main processes—— diffusion controlled process and adsorption controlled process.The diffusion process is very fast and the process observed experimentally is a molecular reorganization at the air/water interface37.This is very similar to what one observes during protein adsorption38,and the process can be expressed by an exponential function39. where γ is the surface tension,γ0the initial surface tension,γeqthe equilibrium surface tension,and τ the characteristic time for the reorganization process.
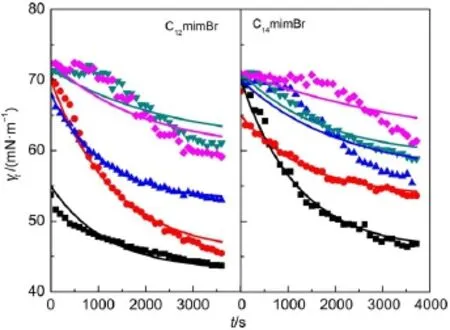
Fig.2 Plots of dynamic surface tension(γt)vs adsorption time(t) for CnmimBr solutions with different concentrations at 25°CcCnmimBr:10-1cmc(■),10-2cmc(●),10-3cmc(▲),10-4cmc(▼)and 10-5cmc(◆).The lines are fitted ones with Eq.(7).

The fitted lines with Eq.(7)are shown in Fig.2(the smooth lines).The quality of the fit is perfect when the concentration is larger than 10-3cmc for C12mimBr(that is larger than 10-2cmc for C14mimBr),although there are small deviations at short and long characteristic times.This indicates that the relaxation process is not purely mono-exponential.The value of τ gives a good idea of the characteristic time scale of the surface tension variation.The values of τ deduced from Eq.(7)decrease with increasing CnmimBr concentration(Fig.3),which is due to the diffusionprocess37.The result is consistent with that obtained by Miller and coworkers40.
3.4Effect of temperature and NaBr on the adsorption of C12mimBr
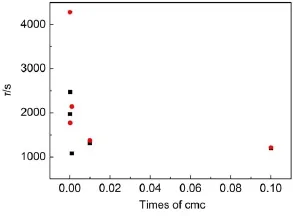
Fig.3 Plots of adsorption time(τ)vs C12mimBr(■)or C12mimBr(●)at different concentrations on the air/water interface
While increasing temperature,C12mimBr molecules are acti-vated and more of them adsorb at the air/water interface,resulting in the reduction of dynamic surface tensions(Fig.4).At the same adsorption time,the order of the dynamic surface tension is γt,45°C< γt,35°C<γt,25°C.The dynamic surface tension decreases more rapidly for high temperature.That is,increasing temperature is an alternative means to reduce dynamic surface tension.At the same adsorption time,the γt(0.103 mmol∙L-1)for C12mimBr solution is lower than that of γt(0.0103 mmol∙L-1).This is because more C12mimBr molecules in 0.103 mmol∙L-1solution could reach and adsorb at the air/water interface than those in 0.0103 mmol∙L-1solution.Meanwhile,the 0.103 mmol∙L-1C12mimBr solution needs long adsorption time to reach the equilibrium surface ten-

Fig.4 Plots of γtvs t for C12mimBr aqueous solutions at temperatures of 25°C(■),35°C(●),and 45°C(▲)The lines are the fitted ones with Eq.(7).
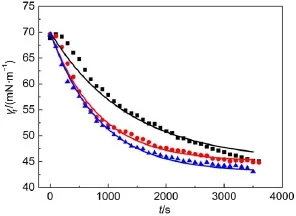
Fig.5 Plotsof γtvs t for1.03×10-2mmol∙L-1C12mimBrsolutions with cNaBrof 0mmol∙L-1(■),1 mmol∙L-1(●),and 10mmol∙L-1(▲) The lines are the fitted ones with Eq.(7).
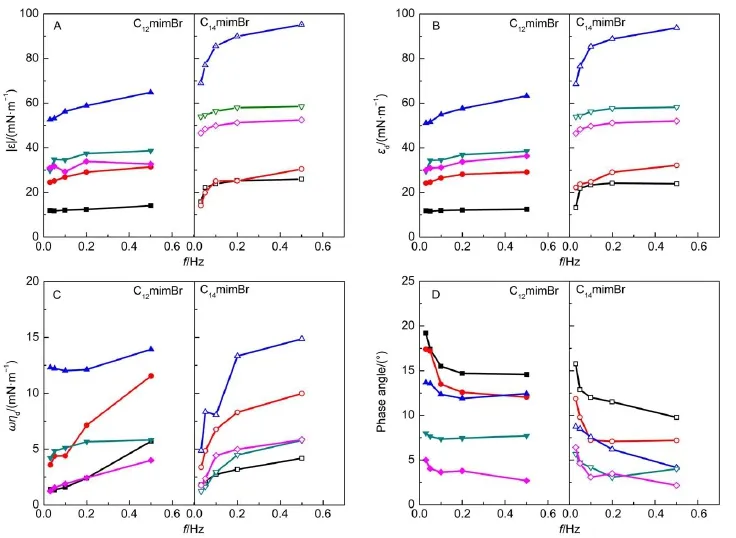
Fig.6Plots of complex modulus(A),oscillating elasticity(B),oscillating viscous modulus(C),and phase angle(D)of CnmimBr vs oscillating frequency at 25°C
cCnmimBr:10-1cmc(■,□),10-2cmc(●,○),10-3cmc(▲,△),10-4cmc(▼,▽);10-5cmc(◆,◇)sion than that of 0.0103 mmol∙L-1,for a large repulsion between the C12mimBr molecules at the end stage of adsorption.The fitted curves(smooth lines in Fig.4)indicate that the diffusion process is not purely mono-exponential,except for small deviations at short and long adsorption times.
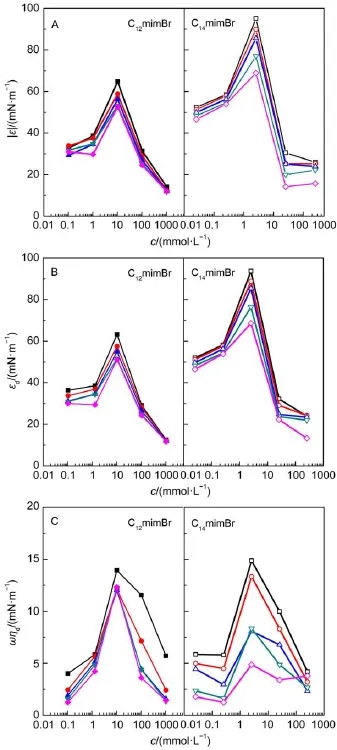
Fig.7 Plots of absolute modulus(A),oscillating elasticity(B),and oscillating viscous modulus(C)of CnmimBr vs oscillating frequency at 25°CThe frequencies are 0.5 Hz(■,□),0.2 Hz(●,○),0.1 Hz(▲,△), 0.05 Hz(▼,▽),and 0.03 Hz(◆,◇).
To avoid the introduction of impurity elements,NaBr was used. The effect of NaBr on the adsorption of C12mimBr at the air/water interface has also been investigated.Fig.5 shows that the presence of NaBr decreases γt,and the higher concentration of NaBr,the lower γt.The C12mimBr ionizes totally into C12mim+and Brions in aqueous solutions.The added Na+ions compress the electric double layer around C12mim+and shield the repulsion among C12mim+ions,inducing a close arrangement of C12mim+hydrophobic tails at the air/water interface and relatively lower surface tension.The fitted curves(the smooth lines in Fig.5)indicate that the diffusion process is not purely mono-exponential,except for small deviations at short and long adsorption times.
3.5Dilational viscoelasticity of CnmimBr solutions
The adsorption of CnmimBr molecules at the air/water interface forms a thin layer,which plays a key role in the formation or destruction of foam or emulsion41,42.The effect of oscillation frequency on the dilational viscoelasticity of CnmimBr at the air/ water interface has been studied and shown in Fig.6.The concentrations that are smaller than the cmcs of CnmimBr were used. Fig.6 shows that the dilational modulus,elastic modulus and viscous modulus increase with an increase in oscillating frequencies,while the phase angles show a reverse trend.The results are consistent with those reported43-45.Generally,the dependence of dilational modulus on frequency is caused by relaxation processes of the surfactants at the air/water interface46,47.There are two types of relaxation processes.One is the exchange of molecules between the bulk solution and the interface,and the other is the conformational change of molecules in the interfacial layer. During the compressing process,some molecules dissolve into the subsurface of the layer.When the oscillation frequency is low, there will be no resistance to the process of compression-expansion.At the time,the surface tension changes by a small extent, that is,the dilational modulus is small.When the oscillation frequency is high and the exchange rate is lower than the frequency of the disturbance,the layer behaves as an insoluble monolayer.The interface tension changes notably with the oscillation frequency,resulting in a high dilational modulus. Therefore,the modulus increases with the increase in oscillation frequency.At the same dilution multiple,the oscillating modulus of C14mimBr layer are slightly higher than those of C12mimBr layer.It is due to the longer hydrocarbon chain length of C14mimBr,the adsorption or desorption of C14mimBr molecules causes more notable variation of surface tension than that of C12mimBr molecules.
At a given oscillation frequency,the dilational modulus,elastic modulus and viscous modulus of CnmimBr increase at first,and then decrease with the increase of concentration,as shown in Fig.7.The maximum values appear at 10.3 mmol∙L-1(C12mimBr) and 2.55 mmol∙L-1(C14mimBr),respectively.It has been reported that the increase of concentration can affect the surface dilational modulus in two aspects.One is that the increase of concentration in bulk results in ascending surface concentration of surfactants, and the other is the increasing of diffusing ability for surfactants from bulk to interface for the increased concentration gradient between bulk solution and surface.The former can raise the surface tension gradient,resulting in the increase of dilational modulus,while the latter may exert a contrary effect.At lowconcentration,increasing surface concentration of surfactant plays a dominant role on the dilational modulus48.When the concentrations are up to 10.3 mmol∙L-1(for C12mimBr)and 2.55 mmol∙L-1(for C14mimBr),the effect of two parameters on the dilational modulus have achieved equilibrium,and the dilational modulus reaches the maximum values.The subsequent increase in concentration can only induce the CnmimBr molecules diffusing from the bulk to the interface,resulting in the obvious decrease of dilational modulus49.
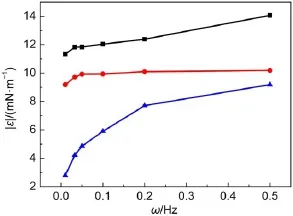
Fig.8 Effect of temperature on oscillating modulus of C12mimBr solutions with 1.03×10-3mol∙L-1The temperatures are 25°C(■),35°C(●),and 45°C(▲).
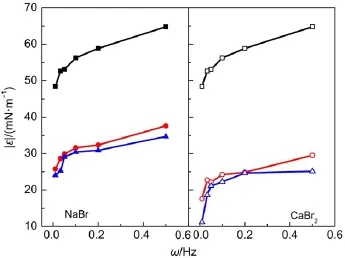
Fig.9 Effect of inorganic salts on the oscillating modulus of C12mimBr solutions with 1.03×10-3mol∙L-1The concentrations of inorganic salts are 0 mol∙L-1(■,□), 1.03×10-3mol∙L-1(●,○),and 1.03×10-2mol∙L-1(▲,△).
At the same frequency,the surface oscillating elasticity(εd)of CnmimBr is higher than the surface oscillating viscous(ηd),indicating that the elastic character is dominant for the CnmimBr layers at the air/water interface.Both the εdand ηdincrease up to the maximum values with increasing CnmimBr concentrations, which are consistent with that of dilational modulus.It is the increase of surface concentration at low CnmimBr concentration and the molecular exchange at high CnmimBr concentration that play a key role in determining both εdand ηd50.The elasticity modulus of C14mimBr is larger than that of C12mimBr.However,the viscous modulus of C14mimBr is almost the same with that of C12mimBr, indicating that the hydrocarbon chain has more effects on the elastic modulus.
3.6Effect of temperature and inorganic salts on dilational viscoelasticity
The effect of temperature on dilational viscoelasticity of C12mimBr is shown in Fig.8.The dilational modulus decreases with increasing temperature while the frequencies are constant.It might be caused by two reasons.First,with increasing temperature,the thermal motion rate of C12mimBr increases and the strength of the interfacial film decreases,resulting in the decrease of dilational modulus.Second,the thermal motion rate of the C12mimBr molecules is larger than the interfacial oscillating frequency,and the C12mimBr molecules have enough time to restore the balance after being disturbed.In a word,raising temperature decreases the strength of the interfacial film and favors the thermal motion of C12mimBr molecules to restore the balance of interfacial film.
The addition of inorganic salt(NaBr or CaBr2)decreases the dilational viscoelasticity of C12mimBr remarkably,as shown in Fig.9.The larger the inorganic salt concentration is,the smaller the dilational viscoelasticity is.When the changing rate of area is constant,the smaller surface tension will result in a smaller dilatational modulus31.The dynamic surface tension has approved that the inorganic salts decrease the surface tension.That is,the addition of inorganic salts affects not only the aggregation behavior of C12mimBr at the air/water interface,but also its dilatational modulus.The compressing ability of Ca2+on the double electric layer around C12mim+is stronger than that of Na+,and therefore,the effect of CaBr2is more notable than that of NaBr.
4 Conclusions
The aggregation behaviors of SAILs,C12mimBr and C14mimBr, at the air/water interface have been investigated by an oscillating bubble method.The results of dynamic surface tension reveal that with the increase in adsorption time,more CnmimBr molecules adsorb at the air/water interface,resulting in the decrease of the surface tension.The dynamic surface tension decreases with the increase of CnmimBr concentration.The adsorption mechanism transforms from the diffusion-controlled to adsorption-controlled with increasing adsorption time.At fixed experimental conditions, temperature,inorganic salt and the alkyl chain length of SAIL affect the surface activity and the adsorption of CnmimBr.
The dilational rheological properties reveal that both the oscillation frequency and the concentration of CnmimBr affect the dilational modulus.With the increase in CnmimBr concentration, the modulus passes through a maximum value.The dilational modulus decrease while increasing temperature or adding the inorganic salts.The dilational modulus of C14mimBr is larger than that of C12mimBr,which is in good agreement with the results of dynamic surface tension.
The results indicate that the long alkyl chain SAILs possess the properties of surfactant,having potential utilization in various fields.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
References
(1)Zhu,S.;Wu,Y.;Chen,Q.;Yu,Z.;Wang,C.;Jin,S.;Ding,Y.; Wu,G.Green Chem.2006,8,325.doi:10.1039/B601395C
(2)Welton,T.Chem.Rev.1999,99,2071.doi:10.1021/cr980032t
(3)George,A.;Brandt,A.;Tran,K.;Zahari,S.M.S.N.S.;Klein-Marcushamer,D.;Sun,N.;Sathitsuksanoh,N.;Shi,J.;Stavila, V.;Parthasarathi,R.;Singh,S.;Holmes,B.M.;Welton,T.; Simmons,B.A.;Hallett,J.P.Green Chem.2015,17,1728. doi:10.1039/C4GC01208A
(4)Redel,E.;Krämer,J.;Thomann,R.;Janiak,C.J.Organomet. Chem.2009,694,1069.doi:10.1016/j.jorganchem.2008.09.050
(5)Vollmer,C.;Redel,E.;Abu-Shandi,K.;Thomann,R.;Manyar, H.;Hardacre,C.;Janiak,C.Chem.-Eur.J.2010,16,3849. doi:10.1002/chem.200903214
(6)Ochędzan-Siodłak,W.;Dziubek,K.;Czaja,K.Polym.Bull. 2013,70,1.doi:10.1007/s00289-012-0809-8
(7)Li,R.X.Green Solvent——Synthesis and Utilization of Ionic Liquids;Chemical Industry Press:Beijing,2004.[李汝雄.绿色溶剂——离子液体的合成与应用.北京:化学工业出版社,2004.]
(8)Prechtl,M.H.G.;Scholten,J.D.;Dupont,J.J.Mol.Catal.AChem.2009,313,74.doi:10.1016/j.molcata.2009.08.004
(9)Wang,H.Y.;Li,H.P.;Cui,G.K.;Li,Z.Y.;Wang,J.J.Acta Phys.-Chim Sin.2016,32,249.[王慧勇,李虹培,崔国凯,李志勇,王键吉.物理化学学报,2016,32,249.]doi:10.3866/ PKU.WHXB201512042
(10)dos Santos,M.R.;Diniz,J.R.;Arouca,A.M.;Gomes,A.F.; Gozzo,F.C.;Tamborim,S.M.;Parize,A.L.;Suarez,P.A.Z.; Neto,B.A.D.ChemSusChem 2012,5,716.doi:10.1002/ cssc.201100453
(11)Chatel,G.;Goux-Henry,C.;Mirabaud,A.;Rossi,T.;Kardos, N.;Andrioletti,B.;Draye,M.J.Catal.2012,291,127.doi: 10.1016/j.jcat.2012.04.016
(12)Shi,L.;Li,N.;Zheng,L.J.Phys.Chem.C 2011,115,18295. doi:10.1021/jp206325d
(13)Zhang,X.H.;Xu,H.Y.;Ge,L.L.;Guo,R.Acta Phys.-Chim. Sin.2016,32,356.[张晓红,许红岩,葛玲玲,郭荣.物理化学学报,2016,32,356.]doi:10.3866/PKU.WHXB201512012 (14)Li,X.W.;Gao,Y.A.;Liu,J.;Zheng,L.Q.;Chen,B.;Wu,L. Z.;Tung,C.H.J.Colloid Interface Sci.2010,343,94.doi: 10.1016/j.jcis.2009.11.010
(15)Liu,J.;Zhao,M.;Zhang,Q.;Sun,D.;Wei,X.;Zheng,L. Colloid Polym.Sci.2011,289,1711.doi:10.1007/s00396-011-2492-y
(16)Shi,L.;Li,N.;Yan,H.;Gao,Y.;Zheng,L.Langmuir 2011,27, 1618.doi:10.1021/la104719v
(17)Ao,M.;Huang,P.;Xu,G.;Yang,X.;Wang,Y.Colloid Polym. Sci.2008,287,395.doi:10.1007/s00396-008-1976-x
(18)Ao,M.;Xu,G.;Zhu,Y.;Bai,Y.J.Colloid Interface Sci.2008, 326,490.doi:10.1016/j.jcis.2008.06.048
(19)Liu,Y.;Yu,L.;Zhang,S.;Yuan,J.;Shi,L.;Zheng,L.Colloid Surf.A 2010,359,66.doi:10.1016/j.colsurfa.2010.01.065
(20)Qin,C.;Chai,J.;Chen,J.;Xia,Y.;Yu,X.;Liu,J.Colloid Polym.Sci.2008,286,579.doi:10.1007/s00396-007-1818-2
(21)Gochev,G.Curr.Opin.Colloid Interface Sci.2015,20,115.
doi:10.1016/j.cocis.2015.03.003
(22)Ravera,F.;Loglio,G.;Kovalchuk,V.I.Curr.Opin.Colloid Interface Sci.2010,15,217.doi:10.1016/j.cocis.2010.04.001
(23)Ravera,F.;Ferrari,M.;Santini,E.;Liggieri,L.Adv.Colloid Interface Sci.2005,117,75.doi:10.1016/j.cis.2005.06.002
(24)Dukhin,S.S.;Kretzhmar,G.;Miller,B.Dynamics of Adsorption at Liquid Interfaces——Theory,Experiment, Application;Elsevier:Amsterdam,1995.
(25)Zhai,X.R.;Liu,T.;Xu,G.Y.;Tan,G.R.;Lü,X.;Zhang,J. Acta Phys.-Chim.Sin.2013,29,1253.[翟雪如,刘腾,徐桂英,檀国荣,吕鑫,张健.物理化学学报,2013,29,1253.] doi:10.3866/PKU.WHXB201303251
(26)Yuan,Z.W.;Yuan,J.;Lü,X.;Zhang,J.;Xu,G.Y.Acta Phys.-Chim.Sin.2013,29,449.[苑再武,苑敬,吕鑫,张健,徐桂英.物理化学学报,2013,29,449.] doi:10.3866/PKU.WHXB201212283
(27)Cao,C.;Lei,J.;Zhang,L.;Du,F.P.Langmuir 2014,30, 13744.doi:10.1021/la502890w
(28)Cao,C.;Lei,J.;Huang,T.;Du,F.P.Soft Matter 2014,10, 8896.doi:10.1039/C4SM01666A
(29)Huang,T.;Cao,C.;Liu,Z.L.;Li,Y.;Du,F.P.Soft Matter 2014,10,6810.doi:10.1039/C4SM00950A
(30)Inoue,T.;Ebina,H.;Dong,B.;Zheng,L.J.Colloid Interface Sci.2007,314,236.doi:10.1016/j.jcis.2007.05.052
(31)Deng,Q.;Li,H.;Li,C.;Lv,W.;Li,Y.RSC Adv.2015,5, 61868.doi:10.1039/C5RA09120A
(32)Dong,B.;Zhao,X.;Zheng,L.;Zhang,J.;Li,N.;Inoue,T. Colloid Surf.A 2008,317,666.doi:10.1016/j. colsurfa.2007.12.001
(33)Rosen,M.J.Surfactants and Interfacial Phenomena;Wiley: New York,2004.
(34)Jaycock,M.J.;Parfitt,G.D.Chemistry of Interfaces;John Wiley and Sons:New York,1981.
(35)Polavarapu,P.;Krafft,M.P.J.Fluorine Chem.2015,171,12. doi:10.1016/j.jfluchem.2014.10.020
(36)Krafft,M.P.;Fainerman,V.B.;Miller,R.Colloid Polym.Sci. 2015,293,3091.doi:10.1007/s00396-015-3622-8
(37)Jeribi,M.;Almir-Assad,B.;Langevin,D.;Hénaut,I.;Argillier, J.F.J.Colloid Interface Sci.2002,256,268.doi:10.1006/ jcis.2002.8660
(38)Li,J.B.;Zhang,Y.;Yan,L.L.Angew.Chem.Int.Edit.2001, 40,891.doi:10.1002/1521-3773(20010302)40:5<891::AIDANIE891>3.0.CO;2-K
(39)Serrien,G.;Geeraerts,G.;Ghosh,L.;Joos,P.Colloid Surface1992,68,219.doi:10.1016/0166-6622(92)80208-J
(40)Miller,R.;Aksenenko,E.V.;Liggieri,L.;Ravera,F.;Ferrari, M.;Fainerman,V.B.Langmuir 1999,15,1328.doi:10.1021/ la980956b
(41)Klitzing,R.V.;Müller,H.J.Curr.Opin.Colloid Interface Sci. 2002,7,42.doi:10.1016/S1359-0294(02)00005-5
(42)Lv,Q.;Li,Z.;Li,B.;Li,S.;Sun,Q.Ind.Eng.Chem.Res. 2015,54,9468.doi:10.1021/acs.iecr.5b02197
(43)Li,Y.M.;Xu,G.Y.;Xin,X.;Cao,X.R.;Wu,D.Carbohydr. Polym.2008,72,211.doi:10.1016/j.carbpol.2007.08.008
(44)Xin,X.;Xu,G.;Wu,D.;Gong,H.;Zhang,H.;Wang,Y. Colloid.Surf.A 2008,322,54.doi:10.1016/j. colsurfa.2008.02.025
(45)Zhu,Y.;Xu,G.;Xin,X.;Zhang,H.;Shi,X.J.Chem.Eng. Data 2009,54,989.doi:10.1021/je800788f
(46)Ivanov,I.B.;Danov,K.D.;Ananthapadmanabhan,K.P.;Lips, A.Adv.Colloid Interface Sci.2005,114-115,61.doi:10.1016/ j.cis.2004.11.001
(47)Stubenrauch,C.;Fainerman,V.B.;Aksenenko,E.V.;Miller, R.J.Phys.Chem.B 2005,109,1505.doi:10.1021/jp046525l
(48)Wu,D.;Xu,G.;Feng,Y.;Li,Y.Int.J.Biol.Macromol.2007, 40,345.doi:10.1016/j.ijbiomac.2006.09.004
(49)He,F.;Xu,G.;Pang,J.;Ao,M.;Han,T.;Gong,H.Langmuir 2011,27,538.doi:10.1021/la103478c
(50)Stubenrauch,C.;Miller,R.J.Phys.Chem.B 2004,108,6412. doi:10.1021/jp049694e
doi:10.3866/PKU.WHXB201602251
Dilational Viscoelasticity of Imidazole-Based Surface Active Ionic Liquids at the Air/Water Interface
LI YanCHAI Jin-Ling*
(Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong,College of Chemistry,Chemical Engineering and Materials Science,Shandong Normal University,Jinan 250014,P.R.China)
Two imidazole-based surface active ionic liquids(CnmimBr)were synthesized,and their aggregation behavior at the air/water interface was studied via an oscillating bubble method.The effects of the CnmimBr concentration,inorganic salts(NaBr and CaBr2),and temperature on the aggregation behavior were investigated. The results of the adsorption dynamics showed that the adsorption-controlled process dominated,but the relaxation process was not purely mono-exponential.The addition of inorganic salt or increase in temperature improved the surface activity of the CnmimBr and lowered the dynamic surface tension.The dilational rheological results revealed that the dilational modulus,elastic modules,and viscous modules increased with increasing oscillating frequencies,and the modulus reached a maximum value with increasing CnmimBr concentration. Increasing the temperature or adding inorganic salts(NaBr or CaBr2)decreased the dilational modulus.The elastic modulus was dominant for the CnmimBr layer,and the elastic modulus of C14mimBr was larger than that of C12mimBr.
Adsorption dynamics;Dilational modulus;Air/water interface; Imidazole-based surface active ionic liquid
1 Introduction
Ionic liquids(ILs),composed of organic cation and inorganic (or organic)anion,possess many excellent properties,such as designability,negligible vapor pressure,low melting point,wideliquid range,strong electrostatic field,wide electrochemical window,good conductivity and high thermal conductivity1-3.It has attracted much attention and been used widely in many fields including polymerization,selective alkylation,amination,acylation,esterification,rearrangement reactions of the chemical bonds,the catalytic hydrogenation reaction,the epoxidation of olefins,electrochemical synthesis and the preparation of branchedchain fatty acids4-12.
December 7,2015;Revised:February 19,2016;Published on Web:February 22,2016.
O647
10.3866/PKU.WHXB201602223
*Corresponding author.Email:jlchai@sdnu.edu.cn;Tel:+86-531-86180743.
The project was supported by the National Natural Science Foundation of China(21306092,21476133)and Science and Technology Plan Project of Shandong Provincial University,China(J12LA02).
国家自然科学基金(21306092,21476133)和山东省高校科技计划(J12LA02)资助项目
