HCCI燃烧中NO与异辛烷相互作用简化动力学模型构建与分析
2016-09-06郑朝蕾吕祝梅
郑朝蕾 吕祝梅
(重庆大学,低品位能源利用技术及系统教育部重点实验室,重庆400044)
HCCI燃烧中NO与异辛烷相互作用简化动力学模型构建与分析
郑朝蕾*吕祝梅
(重庆大学,低品位能源利用技术及系统教育部重点实验室,重庆400044)
为了分析废气再循环中NO对HCCI燃烧的影响,本文构建了一个新的NO与异辛烷相互作用的化学动力学机理,包括167种组分和835个反应,其中异辛烷分支反应包括112种组分和467个反应。NO分支的子机理是在Anderlohr等人对NO与异辛烷详细机理研究的基础上根据路径分析而得到的。新IC8H18-NO机理的验证分为:IC8H18分支机理验证了在激波管中温度范围为855-1269 K,压力范围为2-6 MPa,化学计量比为0.5和1.0条件下的着火延迟时间;IC8H18-NO机理验证了在HCCI发动机中NO添加浓度为0-500×10-6(体积分数),同时也发现不同的NO添加浓度对IC8H18的HCCI燃烧的影响有所不同。因此,本文利用CHEMKIN PRO软件中的零维单区化学动力学模型,模拟了在不同NO浓度下NO对异辛烷燃烧影响。通过敏感性分析和产率分析,得出了NO添加后对异辛烷燃烧影响的关键性反应为R476。在IC8H18燃烧初期通过R476产生活性基OH,从而体现对燃烧的促进作用。但是在NO添加浓度较大时,由于NO浓度较大结合活性基(如OH)的能力增强,进而NO对燃烧的促进作用被削弱。
反应路径;化学动力学;敏感性分析;产率分析;关键反应
1 Introduction
Homogeneous charge compression ignition(HCCI)engine combines the advantages of a spark ignition engine with those of a compression ignition engine.However,ignition delay times and combustion rate are difficult to control.Studies have been conducted to overcome these difficulties,and advanced technologies have been applied to control HCCI combustion.For example, exhaust gas recirculation(EGR),including external EGR and internal EGR,has been extensively investigated as a means to modify inlet temperature and control ignition delay of gasolineand diesel-fueled HCCI combustion1-10.Moreover,internal EGR can be applied effectively to achieve HCCI combustion in a variable valve structure.
EGR has drawn attention to research HCCI.The effects of EGR on HCCI combustion can be divided into four parts:a dilution effect,a thermal effect,a heat capacity effect,and a chemical effect1-9.The dilution,thermal,and heat capacity effects can be analyzed by thermodynamics,whereas the chemical effect can be analyzed by chemical kinetics.With several studies on HCCI combustion,the chemical effect drew more attention because its chemical kinetic mechanism is the key in controlling HCCI combustion1-4.EGR mainly includes active gases,such as carbon monoxide(CO)and nitrogen oxide(NO),carrying out the chemical effect.The chemical kinetic mechanism can be changed by NO,which is also an important component of environmental pollution.The active chemical property of NO has a significant influence on HCCI combustion and the emission process.
Recently,NO has been proved a significant influence on hydrocarbon oxidation10-16.Moréac et al.10investigated the effects of NO on the oxidation of n-heptane(NC7H16),iso-octane(IC8H18), toluene,and methanol in a jet-stirred reactor.The NO effect was found related to temperature.Risberg et al.11carried out experiments on the effects of NO with concentrations from 0 to 476× 10-6(volume fraction)on an HCCI engine fueled with primary reference fuel(PRF)and toluene reference fuel(TRF)which have the same research octane number of 84.The NO concentration with maximum influence on ignition delay was obtained under two engine conditions,one at high intake pressure(0.2 MPa)and low intake temperature(40°C)and the other at high intake temperature(100°C)and atmospheric intake pressure.However, the effects of NO on HCCI combustion are too complicated to be understood only by engine experiments.Therefore,the effects of NO should be analyzed using the chemical kinetic model17-26.The interaction between NO and C1-C4at high temperature was analyzed by Frassoldati et al.17through the chemical kinetic model. Dayma et al.18investigated the interaction between NO and PRF using the models and experiments.PRF is a binary blend of IC8H18and NC7H16.The reaction paths of NO and IC8H18were used through the chemical kinetic models of NO and PRF,containing 1085 components and 4405 reactions.
The above models17-19can describe the interaction between NO and hydrocarbons.However,the NO sub-mechanisms only describes the interaction between NO and small molecule hydrocarbons.
Researches proved that the interaction between NO and large molecule hydrocarbons should not be neglected20-26.The detailed mechanism of NO and C4H10/C5H12was successfully developed up to five carbon atoms by Glaude et al.24.Anderlohr et al.15proposed a detailed mechanism of PRF-NO,which was validated on ignition delay and concentration of the intermediate components in a jet-stirred reactor and HCCI engine.It shows that the same trend can be obtained by the computational results of this detailed mechanism and the experiment under HCCI engine conditions. Meanwhile,the detailed mechanism of PRF-NO proposed by Anderlohr et al.15was developed up to eight carbon atoms.The interaction between NO and large molecule hydrocarbon should be considered to improve the effects of NO on the oxidation of large molecule hydrocarbons.Some detailed and skeletal mechanisms of hydrocarbons are shown in Table 1.
The simplest and most essential surrogate fuel of gasoline is IC8H18,which is an alkane with similar ignition characteristics to gasoline.To investigate the effects of NO on gasoline-fueled HCCI combustion,the effects of NO on IC8H18oxidation should be analyzed.Recently,the effect of NO on IC8H18oxidation was investigated by Contino et al.19with the detailed mechanism of NO and PRF,containing 1062 components and 4494 reactions.Although validations of the detailed mechanism are in good agreement with experimental data,a defect exists in that the detailed mechanism do not contain the interaction between NO and large molecule hydrocarbons.In order to apply computational fluid dynamics,a smaller mechanism with less species and re-actions is needed.

Table 1 Overview of NO mechanism
In this study,a new IC8H18-NO mechanism is presented under extensive HCCI conditions to analyze the effects of NO on IC8H18in HCCI combustion,therefore understanding the chemical effect of EGR on IC Hin HCCI combustion.
2 validation of the new IC8H18-NO model
In this study,the IC8H18-NO mechanism consists of IC8H18and NO sub-mechanisms.
2.1IC8H18sub-mechanism
In this study,the IC8H18sub-mechanism is the mechanism of Kelley et al.27,consisting of 112 species and 467 reactions validated on laminar flame speeds and ignition delay time at different equivalence ratios and pressures.Curran28and Chaos29et al.obtained the mechanism by time-scale analysis with minimal loss of accuracy.The mechanism of Curran et al.28consisting of 860 species and 3600 reactions was not validated on high laminar flame speeds.Chaos et al.29proposed a PRF mechanism consisting of 106 species and 723 reactions.Comparison between the results of the above mechanisms and experiments is presented in Ref.29. Furthermore,the mechanism of Kelley et al.27can better describe IC8H18oxidation in HCCI combustion.Hence,the IC8H18mechanism of Kelley et al.27was chosen as the IC8H18sub-mechanism in this study.
All validations presented in this study were carried out using a zero-dimensional model in CHEMKIN PRO software.When the simulations were carried out in a shock tube,autoignition delay time is defined as the time needed for the mixture to reach a temperature of 400 K above the initial temperature.For the simulations in HCCI engine,the ignition delay time is defined as the time needed for the temperature to reach the inflection point, which is the maximum value of the temperature slope20.
Ignition delay times were also measured by Davidson et al.30in a shock tube with IC8H18.The temperature range was from 855 to 1269 K,and the equivalence ratios(φ)were 0.5 and 1.0.Computational results are in good agreement with experimental data at 2 and 6 MPa,as shown in Fig.1.Results show that the mechanism used in this study is closer to the experiment than the mechanism of Contino et al.19at both 2 and 6 MPa,as shown in Fig.1(a)and(b),respectively.These results indicate that the mechanism used in this study is more suitable for IC8H18oxidation than the mechanism of Contino et al.19.
2.2NO sub-mechanism
The interaction between IC8H18and NO not only concentrates on small molecules but also exists in large molecules20-26.Based on the research of Glaude et al.24on the interaction between NO and large molecule hydrocarbons,Anderlohr et al.21analyzed the interaction between NO and gasoline surrogate fuel systematically. Furthermore,they proposed a detailed mechanism of TRF-NO with the results of GRI-MECH 3.031,consisting of 536 species and 3000 reactions.The TRF-NO mechanism was validated on ignition delay in a rapid compression machine,a shock tube,a jetstirred reactor,a perfectly stirred reactor,and an HCCI engine. Computational results show that the same trend can be obtained by this detailed mechanism under HCCI engine conditions as shown in the experiments of Dubreuil4,Moréac10,and Risberg11et al.Based on the TRF-NO mechanism of Anderlohr et al.21, analysis of the productivity rate of nitride was carried out.The productivity rate of nitride,which mostly participates in the transformation of the IC8H18branch,is shown in Fig.2.As shown in Fig.2(a,b),the main reaction of the consumption of NO and production of NO2is R476,whereas the main reaction of the production of NO and transformation of HONO to NO and OH is R609.As shown in Fig.2(c,d),the main reaction of the consumption of HNO and production of HONO is R619,which also shows the transformation of HNO and NO2to HONO and NO. Therefore,through a similar analysis of the productivity rate of nitride,the reaction paths of NO sub-mechanism were proposed according to the detailed mechanism of TRF-NO21as shown in Fig.3.

Fig.1 Comparison between experimental and calculated results in a shock tube(a)φ=0.5;(b)φ=1.0
The reaction paths of NO sub-mechanism,with transformation of the IC8H18branch,contain the following parts:
(1)The transformation of NO and NO2.RO and NO2were generated by the interaction between NO and hydroperoxyalkyl (RO2).The interaction between NO and oxohydroperoxy radical (HOOQOOH)occurred along with the formation of small molecules,including OH,NO2,aldehyde,and olefin.RO2and NO were generated by the interaction between NO2and alkane(RH).
(2)The decomposition and formation of RNO2.The transfor-mation between the reactant RNO2and reactants RO and NO2occurred.
(3)The transformation of NO to HNO.HNO and R were generated by the interaction between NO and alkane(RH).
(4)The transformation of HONO and NO2.The transformation between the reactants RH and NO2and reactants R and HONO occurred.
Considering the interaction between NO and all range of carbon atoms in IC8H18,the NO sub-mechanism in this study contains the following parts:

Fig.2 Reaction rate of main nitrides(a)NO;(b)NO2;(c)HONO;(d)HNOR476:NO+HO2=NO2+OH,R477:NO+O+M=NO2+M,R478:NO2+O=NO+O2,R479:NO2+H=NO+OH,R502:NO+HO2=NO2+OH,R505:HNO+ OH=NO+H2O,R598:C2H5O+NO=CH3CHO+HNO,R563:HCCO+NO=HCNO+NO,R600:CO+NO2=CO2+NO,R604:C3H5-A+NO2=C2H3+CH2O, R606:NO2+HO2=HONO+O2,R609:NO+OH(+M)=HONO(+M),R610:CH3+NO2=CH3O+NO,R660:IC4H7+NO2=CH3+C2H3CHO+NO,R662:XC7H13+ NO2=IC4H9+C2H3CHO+NO,R619:HONO+NO=NO2+HNO,R729:NO+HCO=HNO+CO,R740:C2H3+NO=C2H2+HNO

Fig.3 Reaction paths of NO
(1)The interaction between NO and C0-C1.This part consists of the NO sub-mechanism in PRF-NO by Contino et al.19.Reactions R468-R488,with the results of GRI-MECH2.11,were updated with the results of GRI-MECH3.031.Based on the research of Anderlohr21and Wang22et al.,NO and CH2O can be generated by combining CH3NO2with active radicals such as OH.Therefore, when the NO sub-mechanism in this study was built,reactions R574-R577 were considered.
CH3NO2+OH=CH2O+NO+H2O(R574)
CH3NO2+O=CH2O+NO+OH(R575)
CH3NO2+H=CH2O+NO+H2(R576)
CH3NO2+CH3=CH2O+NO+CH4(R577)
(2)The interaction between NO and C2-C8.This part consists of the NO sub-mechanism in TRF-NO by Anderlohr et al.21.Research demonstrated that NO initially reacts with large molecule hydrocarbons upon the addition of NO.Based on the analysis of Dayma et al.18on the interaction between NO and PRF,the effect of NO on PRF depends on reactions RO2+ NO=RO+NO2and NO+OH=HONO.When the NO submechanism in this study was built,reactions R595,R596,and R620-R626 were considered.The new NO sub-mechanism consists of 55 species and 368 reactions.
CH3OO+NO=CH3O+NO2(R595)
C2H5OO+NO=C2H5O+NO2(R596)
IC3H7O2+NO=IC3H7O+NO2(R620)
TC4H9O2+NO=TC4H9O+NO2(R621)
IC4H9O2+NO=IC4H9O+NO2(R622)
NEOC5H11O2+NO=NEOC5H11O+NO2(R623)
AC8H17O2+NO=AC8H17O+NO2(R624)
BC8H17O2+NO=BC8H17O+NO2(R625)
CC8H17O2+NO=CC8H17O+NO2(R626)
Combined with the results of Section 2.1,the new IC8H18-NO mechanism consists of 167 species and 835 reactions,as shown in the supplementary material.

Fig.4 Comparison between experimental and calculated results in HCCI engine(a)φ=0.22;(b)φ=0.26.CAD:crank angle degree

Table 2 Character of tested engine23

Fig.5 Effect on main components with the different NO concentrations(a)IC8H18;(b)NO;(c)OH
The comparison between the experimental and computational results is shown in Fig.4.The characteristics of the tested engine are listed in Table 2.Experiments on the effect of NO on IC8H18in HCCI engine were performed at 420 K and 0.14 MPa at the speed of 1000 r∙min-1with equivalence ratios of 0.22 and 0.2623.The computational results are in good agreement with the experimental results on ignition delay times in HCCI engine,as shown in Fig.4.The computational results in this study can more successfully reproduce the trend of the experimental results than the computational results of Contino et al.19,especially at low concentrations of NO.The results of whether these reactions between NO and large molecules is included in the NO submechanism are also shown in Fig.4.The results of the mechanism with these reactions compared with those of mechanisms without such reactions are improved at all NO concentrations.

Fig.6 Reaction rate of IC8H18at the NO concentrations of 50×10-6(a)and 500×10-6(b)R346:IC8H18=IC4H9+TC4H9,R348:IC8H18+H=AC8H17+H2,R350:IC8H18+H=CC8H17+H2,R351:IC8H18+H=DC8H17+H2,R354:IC8H18+O=CC8H17+OH, R356:IC8H18+OH=AC8H17+H2O,R357:IC8H18+OH=BC8H17+H2O,R358:IC8H18+OH=CC8H17+H2O,R359:IC8H18+OH=DC8H17+H2O

Fig.7 Reaction rate of OH at the NO concentrations of 50×10-6(a)and 500×10-6(b)R7:CO+OH=CO2+H,R8:H+O2=O+OH,R10:O+H2O=OH+OH,R32:CH2O+OH=HCO+H2O,R42:HO2+O=OH+O2, R49:HO2+OH=H2O+O2,R51:OH+OH(+M)=H2O2(+M),R476:NO+HO2=NO2+OH
From the above validations,the present IC8H18-NO mechanism is concluded suitable for studying the effects of NO on IC8H18in HCCI combustion.Though there are only 167 species and 835 reactions included in this model,it can provide excellent computational results similar to experimental results.
3 Analysis of productivity rate
The effects of NO on IC8H18in HCCI combustion differ at various NO concentrations.In order to explain this phenomenon, analysis of the productivity rate at different NO concentrations was proposed.
The productive rates of the main components,including IC8H18, NO,and OH,at different mole fractions of NO are shown in Fig.5. As shown in Fig.5(a),the beginning and ending consumption times of IC8H18advanced with the appearance of NO,indicating an advance in the ignition delay time.The consumption and production of NO at different concentrations of NO shown in Fig.5 (b),indicates that NO is consumed at the beginning with the appearance of NO.The consumption and production of OH,which is the most important active radical,are shown in Fig.5(c).The higher the concentration of NO,the faster OH accumulates.
The seven main reactions rates of IC8H18at NO concentrations of 50×10-6and 500×10-6were measured using the chemical kinetic model,as shown in Fig.6.The consumption of IC8H18is depicted in R356,which is the dehydrogenation reaction.IC8H18consumption time at 500×10-6is enhanced compared with that at 50×10-6.In addition,the main reaction rates of IC8H18remained constant at 500×10-6.It is seen from Fig.6 that the duration of IC8H18consumption is shortened because of the NO addition, respectively 17.5°CAand 13.5°CAfor the NO concentrations of 50×10-6and 500×10-6.
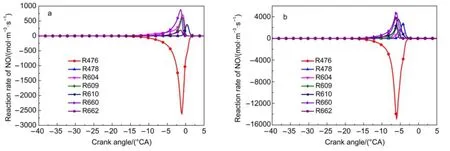
Fig.8 Reaction rate of NO at the NO concentrations of 50×10-6(a)and 500×10-6(b)R476:NO+HO2=NO2+OH,R478:NO2+O=NO+O2,R604:C3H5-A+NO2=C2H3+CH2O+NO,R609:NO+OH(+M)=HONO(+M), R610:CH3+NO2=CH3O+NO,R660:IC4H7+NO2=CH3+C2H3CHO+NO,R662:XC7H13+NO2=IC4H9+C2H3CHO+NO
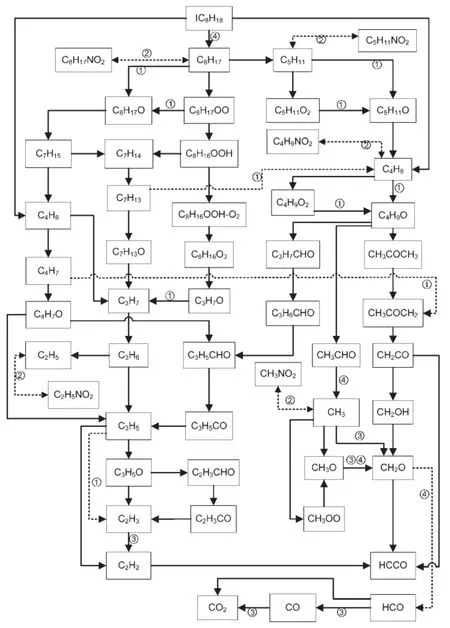
Fig.9 Interaction between NO and IC8H18①the conversion between NO and NO2;②the decomposition of RNO2;③the conversion between NO and HNO;④the conversion between NO2and HONO
The seven main reaction rates of OH at NO concentrations of 50×10-6and 500×10-6are shown in Fig.7.Reaction R476 contributes to the reaction rate of OH at 500×10-6but not at 50×10-6.The response time of R476 is earliest among the seven main reaction rates of OH at 500×10-6,indicating that the resource of OH at the initial IC8H18consumption is mainly from R476,which is the result of the promoting effect of NO on IC8H18consumption.

Fig.10 Sensitivity coefficients at the NO concentrations of 0 and 50×10-6

Fig.11 Sensitivity coefficients at the NO concentrations of 50×10-6and 500×10-6
The seven main reaction rates of NO at NO concentrations of 50×10-6and 500×10-6are shown in Fig.8.The reaction rates of these main reactions are magnified by 5 to 6 times at 500×10-6compared with those at 50×10-6.Most reaction rates of NO consumption are from R476 regardless of the concentration of NO (Fig.8).The main production reactions at 50×10-6are R604, R609,and R660,whereas those at 500×10-6are R609,R610,and R660.The above reactions are generated by the addition of NO, which indicates that NO participates in the transformation of the IC8H18branch.
The reaction paths of the IC8H18branch were obtained by analyzing the reaction paths.The new reaction paths in the IC8H18branch were found to have resulted from the addition of NO,as shown in Fig.9 as dashed line.Meanwhile,the original reaction paths in the IC8H18branch(without the addition of NO)are shown as solid line in Fig.9.The transformation of the IC8H18branch was accelerated by the new reaction paths(with the addition of NO), whereas the original reactions in the IC8H18branch weakened.This result also explains why the reaction rates of OH in the IC8H18branch are reduced with increasing NO concentration,as shownin Fig.7.
4 Sensitivity analysis
Sensitivity analysis at different NO concentrations was conducted,with temperature at 420 K,pressure at 0.14 MPa,and equivalence ratio of 0.22.
As shown in Fig.10,the temperature sensitivity coefficients of the key reactions of IC8H18-NO mechanism without the addition of NO in HCCI engine include those in dehydrogenation reactions R364-R367,R389,and R390,showing the promoting effect on IC8H18combustion.On the other hand,the temperature sensitivity coefficients of those reactions at NO concentration of 50×10-6are lower,indicating that the original reactions in the IC8H18branch are weakened by the addition of NO,which in turn is in accordance with the conclusion from Section 3.
As shown in Fig.11,the temperature sensitivity coefficients of the key reactions of IC8H18-NO mechanism at NO concentration of 500×10-6in HCCI engine consist of R476,R606,R610,R675, R683,and R798,showing the promoting effect on IC8H18combustion.On the other hand,the temperature sensitivity coefficient of R683 at NO concentration of 500×10-6is lower than that at 50×10-6,which is attributed to the enhanced ability of NO to combine with active radicals with increased NO concentration (R476).
5 Conclusions
(1)A new chemical kinetic model of IC8H18-NO,consisting of 167 species and 835 reactions,was developed by considering the effects of NO on large molecule hydrocarbons.The reaction paths of NO in IC8H18-NO mechanism were carried out.
(2)The IC8H18sub-mechanism of IC8H18-NO mechanism was validated on ignition delay times in shock tubes.The experimental and computational results are in good agreement with ignition delay times at temperature range of 855 to 1269 K at pressures of 2 and 6 MPa with equivalence ratios of 0.5 and 1.0.The new IC8H18-NO mechanism was also validated in HCCI engine.The computational results are in good agreement with the experimental data on ignition delay time over an NO concentration range of 0-500×10-6.
(3)Generally,the addition of NO in HCCI engine changes the reaction paths of the IC8H18branch.The transformation of the IC8H18branch is accelerated by the new reaction paths after the addition of NO.Meanwhile,the original reactions in the IC8H18branch are weakened by the addition of NO,which is in accordance with the results of the productivity rate analysis.Through the analysis of productivity rate,the response time of R476 was found to be earliest among the seven main reaction rates of OH at NO concentration of 500×10-6.This phenomenon indicates that the main resource of OH at the beginning of IC8H18consumption is R476,which is the result of the promoting effect of NO on IC8H18consumption.
(4)The temperature sensitivity coefficients of the 20 key reactions in IC8H18-NO mechanism at NO concentration of 500× 10-6consist of R476,R606,R610,R675,R683,and R798,which show the promoting effect of NO on IC8H18combustion.The temperature sensitivity coefficient of R683 at NO concentration of 500×10-6is lower than that at NO concentration of 50×10-6, which is attributed to the enhanced ability of NO to combine with active radicals with increase in NO concentration,such as R476.
References
(1)Machrafi,H.Energ.Convers.Manage.2008,49(11),2956.
doi:10.1016/j.enconman.2008.06.016
(2)Machrafi,H.Energ.Convers.Manage.2010,51(10),2025.
doi:10.1016/j.enconman.2010.02.036
(3)Machrafi,H.;Guibert,P.;Cavadias,S.Combust.Sci.Technol. 2008,180(7),1245.doi:10.1080/00102200802049380
(4)Dubreuil,A.;Foucher,F;Mounaïm-Rousselle,C.;Dayma,G.; Dagaut,P.Proc.Combust.Inst.2007,No.31,2879. doi:10.1016/j.proci.2006.07.168
(5)Fathi,M.;Saray,R.K.;Checkel,M.D.Appl.Energ.2011,88 (12),4719.doi:10.1016/j.apenergy.2011.06.017
(6)Lijima,A.;Yoshida,K.;Shoji,H.;Lee,J.T.Int.J.Automot. Techn.2007,8(2),137.
(7)Piperel,A.;Montagne,X.;Dagaut,P.SAE Tech.Pap.Ser. 2007,2007-24-0087.doi:10.4271/2007-24-0087
(8)Fu,J.Q.;Deng,B.L.;Wang,Y.;Yang,J.;Zhang,D.M.;Xu, Z.X.;Liu,J.P.Fuel 2014,No.124,102.doi:10.1016/j. fuel.2014.01.092
(9)Kozarac,D.;Vuilleumier,D.;Saxena,S.;Dibble,R.W.Energ. Convers.Manage.2014,No.87,1186.doi:10.1016/j. enconman.2014.04.085
(10)Moréac,G.;Dagaut,P.;Roesler,J.F.Combust.Flame 2006, 145(3),512.doi:10.1016/j.combustflame.2006.01.002
(11)Risberg,P.;Johansson,D.;Andrae,J.;Kalghatgi,G.; Björnbom,P.;Ångström,H.E.SAE Tech.Pap.Ser.2006, 2006-01-0416.doi:10.4271/2006-01-0416
(12)Dagaut,P.;Dayma,G.Combust.Flame 2005,143(1-2),135. doi:10.1016/j.combustflame.2005.06.006
(13)Bendtsen,A,B.;Glarborg,P.;Dam-Johansen,K.Combust.Sci. Technol.2000,No.151,31.doi:10.1080/00102200008924214
(14)Masurier,J.B.;Foucher,F.;Dayma,G.;Dagaut,P.Proc. Combust.Inst.2015,No.35,3125.doi:10.1016/j. proci.2006.07.168
(15)Anderlohr,J.;Cruz,A.P.D.;Bounaceur,R.;Leclerc,F.B. Modeling of NO Sensitization of IC Engines Surrogate Fuels Auto-Ignition and Combustion.21st Int.Colloquium on the Dynamics of Explosions and Reactive Systems,Poitiers France,Jul 23-27,2007.
(16)Piperel,A.;Dagaut,P.;Montagne,X.Proc.Combust.Inst. 2009,No.32,2861.doi:10.1016/j.proci.2008.08.004
(17)Frassoldati,A.;Faravelli,T.;Ranzi,E.Combust.Flame 2003, 135(1-2),97.doi:10.1016/S0010-2180(03)00152-4
(18)Dayma,G.;Ali,K.H.;Dagaut,P.Proc.Combust.Inst.2007,No.31,411.doi:10.1016/j.proci.2006.07.143
(19)Contino,F.;Foucher,F.;Dagaut,P.;Lucchini,T.;D′Errico,G.;Mounaim-Rousselle,C.Combust.Flame 2013,160(8),1476.
doi:10.1016/j.combustflame.2013.02.028
(20)Faravelli,T.;Frassoldati,A.;Ranzi,E.Combust.Flame 2003, 132(1-2),188.doi:10.1016/S0010-2180(02)00437-6
(21)Anderlohr,J.M.;Bounaceur,R.;Cruz,A.P.D.;Leclerc,F.B. Combust.Flame 2009,156(2),505.doi:10.1016/j. combustflame.2008.09.009
(22)Wang,Y.;Zheng,Z.L.;He,Z.W.;Zhang,Q.F.;Wang,F. Energy Sources 2015,37(9),997.doi:10.1080/ 15567036.2011.601789
(23)Wang,Y.An Experimental Investigation and Numerical Simulation about Effect of Nitric Oxide on HCCI Combustion. Ph.D.Dissertation,Chongqing University,Chongqing,2012.[王迎.一氧化氮对均质压燃燃烧影响的试验研究与数值模拟[D].重庆:重庆大学,2012.]
(24)Glaude,P.A.;Marinov,N.;Matsungaga,N.;Hori,M.Energy Fuels 2005,No.19,1839.doi:10.1021/ef050047b
(25)Andrae,J.C.Energy Fuels 2013,27(11),7098.doi:10.1021/ ef401666c
(26)Dayma,G.;Dagaut,P.Experimental and Kinetic Modeling Study of the Impact of NO and NO2on the Oxidation of a Primary Reference Fuels Mixture.Proceedings of the European Combustion Meeting,ViennaAustria,Apr 14-17, 2009.
(27)Kelley,A.P.;Liu,W.;Xin,Y.X.;Smallbone,A.J.;Law,C.K. Proc.Combust.Inst.2011,33(1),501.doi:10.1016/j. proci.2010.05.058
(28)Curran,H.J.;Gaffuri,P.;Pitz,W.J.;Westbrook,C.K. Combust.Flame 2002,129(3),253.doi:10.1016/S0010-2180 (01)00373-X
(29)Chaos,M.;Kazakov,A.;Zhao,Z.;Dryer,F.L.Int.J.Chem. Kinet.2007,39(7),399.doi:10.1002/kin.v39:7
(30)Davidson,D.F.;Gauthier,B.M.;Hanson,R.K.Proc. Combust.Inst.2005,30(1),1175.doi:10.1016/j. proci.2004.08.004
(31)http://www.me.berkeley.edu/gri_mech/(accessed Feb 25, 2014).
Generation and Analysis for a Skeletal Chemical Kinetic Model of IC8H18with Nitric Oxide in HCCI Combustion
ZHENG Zhao-Lei*LÜ Zhu-Mei
(Key Laboratory of Low-grade Energy Utilization Technologies and Systems,Ministry of Education, Chongqing University,Chongqing 400044,P.R.China)
A new mechanism for IC8H18with nitric oxide(IC8H18-NO)in homogeneous charge compression ignition(HCCI)combustion is presented to investigate the effects of NO in exhaust gas recirculation(EGR)on combustion.The IC8H18sub-mechanism consists of 112 species and 467 reactions.A NO sub-mechanism is developed through reaction path analysis.The reaction paths of NO are summarized on the basis of the detailed NO mechanism reported byAnderlohr to describe the effects of NO on IC8H18.Anew IC8H18-NO mechanism with 167 species and 835 reactions is described.The IC8H18sub-mechanism of IC8H18-NO mechanism was validated by the ignition delay times in a shock tube.Experimental and computational results are in good agreement with those of ignition delay times at 855 to 1269 K and at 2 and 6 MPa with equivalence ratios of 0.5 and 1.0.The new IC8H18-NO mechanism is also validated in an HCCI engine.Computational results are consistent with experimental data of ignition delay times at a NO concentration range of 0 to 500×10-6(volume fraction).The effects of NO on IC8H18differ as the NO concentration increases.Therefore,the effects of NO on IC8H18are simulated using a zero-dimensional model using the CHEMKIN PRO software.Key reactions at different NO concentrations are proposed by analyzing the sensitivity and productivity rates.The resource of OH for initial IC8H18consumption is mainly generated through R476,which occurs as a result of the promoting effect of NOon IC8H18consumption.The ability of NO to combine with active radicals,such as those in R476,is enhanced as the NO concentration is increased.
November 20,2015;Revised:January 29,2016;Published on Web:February 17,2016.
Reaction path;Chemical kinetics;Sensitivity analysis;Analysis of productivity rate; Key reaction
O643
10.3866/PKU.WHXB201602174
*Corresponding author.Email:zhengzhaolei@cqu.edu.cn;Tel:+86-23-65102473.
The project was supported by the Fundamental Research Funds for the Central Universities,China(CDJZR13 14 55 01).中央高校基本科研业务费(CDJZR13 14 55 01)资助
