钠与惰性气体及烷烃准分子对吸收系数的实验与理论评价
2016-09-05盖宝栋曹战利郭敬为中国科学院大连化学物理研究所化学激光重点实验室辽宁大连603四川大学原子与分子物理研究所成都60064
胡 墅 盖宝栋 曹战利 郭敬为,* 王 繁(中国科学院大连化学物理研究所化学激光重点实验室,辽宁大连603;四川大学原子与分子物理研究所,成都60064)
钠与惰性气体及烷烃准分子对吸收系数的实验与理论评价
胡墅1盖宝栋1曹战利2郭敬为1,*王繁2
(1中国科学院大连化学物理研究所化学激光重点实验室,辽宁大连116023;2四川大学原子与分子物理研究所,成都610064)
准分子泵浦钠金属激光器(XPNaL)在钠导星中有着极为重要的应用。但是,传统的准分子对,例如Na-He和Na-Ar等对相对于泵浦源的吸收系数很小。本文对Na-Ar、Na-Xe、Na-CH4、Na-C2H6四个体系进行了研究,从荧光实验和结合能的高精度量化理论计算两方面来探究比较好的准分子对。实验结果表明:这四个准分子对体系的荧光强度曲线峰面积比为1.0:6.4:4.9:10.4。同时,通过CCSD(T)手段和基组外推法对Na-Ar、Na-Xe、Na-CH4和Na-C2H6准分子对的结合能计算结果分别为52.8、124.5、117.7和150.0 cm-1。因此,可以推断量化计算与实验结果能够较好地符合。随后,Na-C2H6准分子对从实验和理论两方面被发现是效率最高的体系,更有希望被发展成为高能准分子宽带泵浦钠金属激光器。本工作还证明了采用大基组对结合能的高精度量化计算,对用于准分子宽带泵浦碱金属激光器的准分子对筛选是很好的评判标准。
准分子对;准分子宽带泵浦碱金属激光器;CCSD(T);基组;荧光;吸收系数
[Article]
www.whxb.pku.edu.cn
1 Introduction
Sodium guide star enables the adaptive optics(AO)systems on the ground to observe the target at high altitude with high resolution,and this is crucial for applications such as universe observation,laser telecommunications etc.The key prerequisite to realize sodium guide star is sodium beacon laser,which must be accurately tuned to the wavelength of the sodium D2line(32P3/2→32S1/2)at 589.16 nm(vacuum)in order to obtain resonant backscatter from the sodium layer1,2.All the prevalent sodium beacon lasers are not based on the stimulated emission of sodium atoms, such as sum-frequency generation(SFG)3,4,stimulated Raman scattering(SRS)5,and optical parametric amplifier(OPA)6.Sodium beacon laser requires very accurate wavelength and narrow linewidth(less than 3 GHz)to obtain resonance efficiently.This special requirement complicated all the prevalent sodium beacon laser system,and led to a higher price and liability of system malfunction.Furthermore,sodium beacon lasers based on the mechanism mentioned above have relatively poorer power scalability in comparison with gas lasers.
Excimer pumped sodium laser(XPNaL)is one kind of excimer pumped alkali lasers(XPAL)7-11,and it could overcome problems of the prevalent sodium beacon lasers.The mechanism of XPNaL is illustrated in Fig.1.Excimer pairs of sodium-rare gas system or sodium-alkane system are firstly formed by collision,and the excess energy are converted to the thermal energy of collision pairs or the third party.Excimer pairs are excited from X2Σ+1/2state to B2Σ+1/2state by the blue satellite pumping11.This blue satellite absorption was also explained as the free-free transitions of transient collision pairs in earlier literature12.Potential curve of thestate is repulsive and excimer pairs quickly dissociate to produce Na of excited state(32P3/2,32P1/2).Lasers of 589.16 and 589.76 nm can thus be observed from the stimulated emissions of 32P3/2→32S1/2(D2)and 32P1/2→32S1/2(D1).An etalon or a Volume Bragg Grating(VBG)could be used to select the output of D1or D2line.Although XPNaL has been fulfilled in Na-He system13,the output power is rather limited.The major reason is,similar to all XPALs,the pumping efficiency is too low.Therefore it is essential to explore Na-M(M=Ar,Xe,CH4,C2H6)excimer pair systems and find the optimal system with a large absorption coefficient.In this work,excimer systems of Na-Ar,Na-Xe,Na-CH4,and Na-C2H6were studied by fluorescence spectroscopy.Under the same pumping condition,the stronger intensity of fluorescence means larger absorption coefficient and higher pumping efficiency. Binding energies for these excimer systems,which are closely related to the absorption coefficients,were also investigated based on quantum chemistry calculations.Both experimental and computational results provided the same ordering among the four excimer pairs studied in terms of absorption coefficient;and showed that Na-C2H6was the optimal system for XPNaL.
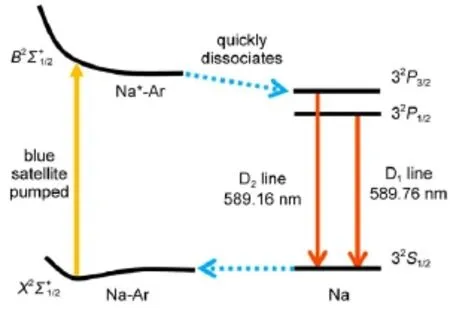
Fig.1 Schematic diagram of XPNaLoperation mechanism The excimer pair is Na-Ar.
2 Experimental set-up
The schematic diagram of the experimental set-up is shown in Fig.2.An Nd:YAG(Spectral Physics,6350,USA)pumped dye laser(Radiant Dyes,NarrowScanK,Germany)was utilized to provide the tunable laser with wavelength over the range of 544-572 nm,the pulse duration of dye laser was~6 ns(FWHM:full width at half maximum)and the repetition frequency was 30 Hz. The pumping laser was focused into the center of the sodium vapor cell.The cell was made of glass and was 20 cm long by 2.5 cm in diameter.An oven and a thermal controller were employed to precisely control the temperature of the sodium vapor cell;the side of the oven had a small opening for the perpendicular fluorescence detection.The fluorescence was coupled into a spectrometer (HOIBA JOBIN YVON,FHR1000,France)by an optical fiber. The spectrometer has very high scatter light annihilation ratio,the pump light had absolutely no effect on the fluorescence detection. The spectrometer has an intensified charge-coupled device(ICCD) detector with maximum gain of 4000.The exposure time of ICCD was set as 1 μs,to efficiently collect the fluorescence and reduce the dark noise as well.The synchronization of experiment was controlled by a time delay generator(Stanford Research System, DG645,USA).The relative positions of oven,vapor cells,optical fiber,and laser focus point were fixed to eliminate discrepancy caused by the fluorescence collection efficiency.
There were four kinds of buffer gases(Ar,Xe,CH4,and C2H6) used in the sodium vapor cells.All the pressure of buffer gases is 600 torr(1 torr=133 Pa)at room temperature,and the sodium vapor cell was heated to 280°C.Na atom vapor density is 1.24× 1014cm-3at 280°C.

Fig.2 Experiment scheme of XPNaLfluorescence spectrum
3 Experiment results and discussion
The absorption of XPAL is generally very weak at blue satel-lites;it is difficult to obtain the absolute absorption coefficients of different excimer pairs.Fortunately,the fluorescence intensity reflects the absorption condition,and it could be detected easily. Fluorescence intensities of D1and D2lines were recorded while scanning the wavelength of pump laser,and profiles of blue satellites were obtained and shown in Fig.3.The blue satellite spectrum indicated that the wavelength range of absorption was broad,meanwhile,the wavelength corresponding to the maximum fluorescence(named as center wavelength)could be obtained for Na-Ar,Na-Xe,Na-CH4,and Na-C2H6systems(listed in Table 1). Center wavelengths of Na-Ar and Na-Xe systems were 555 and 560 nm,respectively,and they were quite consistent with the results of literature14.Therefore,this demonstrated that our experimental results were reliable.The blue satellite center wavelengths of Na-CH4and Na-C2H6systems were obtained to be 555 and 553 nm,respectively.

Fig.3 Plots of fluorescence intensity in relationship with wavelength of pump laser for sodium based excimer systems at 280°CThese plots are equivalent to blue satellite absorption spectra of excimer pairs.

Table 1 Comparison of the four excimer pairs
After the range of blue satellites was obtained,pumping laser was tuned to the wavelengths that produced the strongest fluorescence(center wavelength)to pump the sodium vapor cell, while fluorescence intensities of D1and D2lines were recorded. The energy of dye laser was kept the same value throughout the fluorescence detection.Fluorescence of Na-Ar,Na-Xe,Na-CH4, and Na-C2H6systems were compared at 280°C and are shown in Fig.4.The fluorescence intensity follows the order of Na-C2H6> Na-Xe>Na-CH4>Na-Ar.In Fig.4,fluorescence linewidths for excimer systems of Na-C2H6and Na-CH4were significantly broader than that of Na-Xe system,and this is mainly due to the collision broadening mechanism.Collision broadening is proportional to collision cross section,and the square root of the reciprocal of reduced mass of collision pair.The square roots of the reciprocal of reduced mass are 0.23,0.32,and 0.28 for collision pairs of Na-Xe,Na-CH4,and Na-C2H6;the collision crosssections are 26´10-16cm2and 36´10-16cm2for collision pairs of Na-Xe and Na-CH4,this gave the linewidth ratio of 0.52 between collision pairs of Na-Xe and Na-CH4.The collision cross section for collision pairs of Na-C2H6is more complicated to calculate.But considering the overall effects of collision cross section and reduced mass,fluorescence linewidths for collision pairs of Na-C2H6and Na-CH4should be similar.The peak area of fluorescence intensity curves should be a better indication of the order of the absorption coefficients(A)of different excimer systems,and it was calculated as A(Na-Ar):A(Na-Xe):A(Na-CH4):A(Na-C2H6)=1.0:6.4:4.9:10.4.

Fig.4 Comparison of fluorescence intensities of D1and D2lines of sodium based excimer systems at 280°C
4 Computational method and results
There are many possible excimer pairs for potential XPNaL applications and it would be really costly to explore every possible excimer pair.Quantum chemistry calculation was thus a very good alternative option to explore optimal excimer pair for XPNaL. Absorption coefficient normally required the calculation of Frank-Condon factor;thus,the full potential curves for the ground state and excited states,as well as transition dipole moments were necessary15.It is important to note that binding energy of the Na-M complex is very small(in a magnitude of 100 cm-1or even smaller)with a long Na-M distance.Also the excited states are normally pre-dissociative states.Methods with high accuracy together with very large basis set augmented with diffused functions is required to obtain reliable potential curves of ground and excited pre-dissociation states.This would be computationally very demanding.On the other hand,larger binding energy will lead to shorter bond length,thus more compact nuclear wavefunction,and bigger transition moment and absorption coefficient was expected.Transition probabilities from loose bond ground state to unbounded state should be closely related to the binding energy of the ground state.Therefore the binding energies of Na-M complexes were calculated,and were employed to relate to the strengths of absorption of the involved complexes.Comparison between fluorescence experiment results and binding energy calculations would provide a good evaluation for this relationship.
The Na-M systems investigated in the present work are van der Waals complexes and their binding energies originate from London dispersion force,i.e.,interaction between the induced dipoles of Na and M.According to the London formula,the interaction energy can be estimated based on the following equation16:
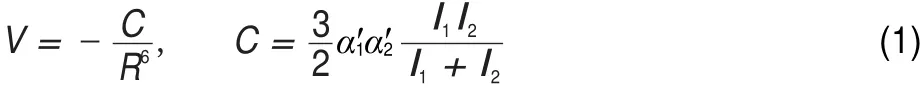
whereα1′andα2′are the polarizability volumes of Na and M,I1and I2are corresponding ionization potentials,and R is the distance between Na and M.Experimental ionization potentials17as well as polarizability volumes18,19for the involved atoms and molecules are listed in Table 2 together with the calculated C term in Eq.(1). Ionization potential of Na is smaller than those of the others to a large extent,which means that differences in ionization energies of M only has a minor effect on C.On the other hand,polarizabilities of the rare gas atoms or the alkyls have a larger effect on magnitude of C.One can see from this table that C increases in the same order as polarizabilities,i.e.,in the order of Ar,CH4,Xe, C2H6.However,the interaction energy is proportional to the inverse sixth power of R according to Eq.(1)and the distance between Na and M will thus play an important role in the binding energy.
Alternatively,binding energies between Na and M are calculated with quantum chemistry approach.Electron correlation must be treated reliably to achieve a reasonable description on these systems and the CCSD(T)approach,the so called“gold standard”of quantum chemistry,is employed in the present work.Moreover, Na only has one valence electron and its correlation energy will be zero if core electrons are not considered in calculations of correlation energies.This indicates that contribution of core electrons to correlation energy is important for these systems.The correlation-consistent basis set with core-valence correlation effects augmented with a set of diffuse basis functions,i.e.,aug-ccpCVQZ20-22is employed in calculations for all the involved atoms except for Xe.The ECP28MDF23pseudopotential developed by the Stuttgart/Cologne groups is used for Xe together with the corresponding aug-cc-pWCVQZ24basis set.All electrons that are not treated via pseudopotentials are correlated in calculations and all calculations are carried out using MOLPRO25on clusters of National Supercomputing Center in Shenzhen.It is worth noting that equation-of-motion coupled-cluster approach may also be adopted for such systems26,27.

Table 2 Experimental ionization potentials(IPs)17and polarizability volumes(α′)18,19for the involved atoms and molecules together with the value of C term in Eq.(1)
Optimized distance between Na and Ar or Xe can be obtained with ease.On the other hand,structures of CH4and C2H6in Na-CH4and Na-C2H6systems are taken from experimental data of isolated CH428and C2H629molecules and kept frozen in geometry optimization to facilitate calculations.Interaction between Na and CH4or C2H6is rather weak and its effect on structure of CH4or C2H6is neglected.Pilot calculations with a small basis set indeed show that effect of using experimental structure for CH4or C2H6in Na-CH4or Na-C2H6system on binding energy is negligible. Optimized structures for these systems are obtained based on numerical gradients as implemented in MOLPRO.The optimized structures for Na-CH4and Na-C2H6are illustrated in Fig.5.One can see from this figure that both these two systems have a C3vsymmetry.
Calculated distances between Na and M as well as their binding energies are listed in Table 3.Experimental results30,31for Na-Ar and Na-Xe are also listed for comparison.It should be noted that due to their weak interaction,interaction energies are in fact insensitive to the obtained distances when they are not far from those in the optimal structures.One can see from Table 3 that the calculated bond lengths for Na-Ar and Na-Xe are in good agreement with experimental values.Furthermore,binding energies between Na and M are in consistent with polarizabilities of M.To further investigate effects of basis set on the calculated results, interaction energies with the aug-cc-pCV5Z basis set at the optimized structures obtained with the aug-cc-pCVQZ basis set are also calculated for these systems.Unfortunately,the number of basis functions for the Na-C2H6system is too large and we are not able to carry out calculation for this system due to limited computational resources.The calculated results are also listed in Table 3 and result for Na-C2H6is estimated based on difference in binding energy of Na-CH4with these two basis sets.One can see from this table that binding energies with the aug-cc-pCV5Z basis set are consistent with those using the aug-cc-pCVQZ basis set. Effect of basis set superposition error(BSSE)32is usually considered to correct binding energies of weak interaction systems. However,BSSE correction will decrease the obtained interaction energies.According to results in Table 3,we can see that the binding energies actually increase with the size of the basis set. BSSE correction thus should not be used to improve results for the investigated systems.On the other hand,basis set extrapolation is employed to estimate binding energies at the basis set limit with the following equations33:

The Hartree-Fock energy used in Eq.(2)is taken from result with aug-cc-pCV5Z basis set.is the CCSD(T)correlation energy obtained with the aug-cc-pCVXZ basis set.The parameters a and the extrapolated complete basis set limit CCSD(T)correlation energyin Eq.(3)are determined from correlation energies with the aug-cc-pCVQZ and aug-cc-pCV5Z basis sets. The obtained results are listed in Table 3 and once again,the binding energies are consistent with those using the aug-ccpCVQZ basis set.In comparison with experimental results,the achieved binding energies for Na-Ar and Na-Xe are about 10 cm-1larger.

Fig.5 Optimized structures of Na-CH4and Na-C2H6systems

Table 3 Optimized distance(R)between Na and M and binding energies(De)of Na-M(M=Ar,Xe,CH4,C2H6)
Binding energies of the Na-M complexes follow the order of Na-C2H6>Na-Xe>Na-CH4>Na-Ar.According to the analysis above,the absorption coefficients were expected to follow the same order.Fluorescence experimental results from Fig.4 and Table 1 showed exactly same order and even with similar pattern. Both binding energy and fluorescence intensity of Na-C2H6are significant larger than those of Na-Xe and Na-CH4,and are much larger than those of Na-Ar.This shows that theoretical and experimental results are consistent with each other.This result indicates that binding energy calculated by high precision quantum chemistry method in combination with large basis set is a very good approximation for the ordering of the absorption coefficients and fluorescence intensities of Na-M complexes.This result pavedthe road to the optimal Na-M excimer system by exploring Nacomplexes in a very large scale.The fluorescence experiment results also showed that Na-Xe and Na-C2H6are optimal systems for possible XPNaL system,although lasering experiment is still required to support this conclusion.
5 Conclusions
In this work,we measured fluorescence intensity for excimer systems of Na-Ar,Na-Xe,Na-CH4,and Na-C2H6to investigate absorption coefficients of these systems.Under the condition of same pump energy,the bigger the absorption coefficient was,the intenser fluorescence was expected.The peak area ratio of fluorescence intensity curves for these systems was given as 1.0:6.4: 4.9:10.4 by experiment.On the other hand,Binding energies for excimer pairs of Na-Ar,Na-Xe,Na-CH4,and Na-C2H6with the CCSD(T)approach and basis set extrapolation were calculated as 52.8,124.5,117.7,and 150.0 cm-1,respectively.Therefore predication by quantum chemistry calculation was in good consistent with experimental results.This demonstrated that quantum chemistry calculations could play an important role in the selection of excimer pairs of XPNaL,and it was expected to be applicable for all XPAL as well.Experimental results showed that for XPNaL laser operation,excimer pair of Na-C2H6was the best choice among the four systems studied in this work,and it was 10 times more efficient than excimer pairs of Na-Ar.For the application for sodium beacon light purpose,both excimer pairs of Na-C2H6and Na-Xe were good choice and they were approximately 6 times more efficient than Na-Ar system.The fluorescence experimental results were crucial for the development of high power XPNaL,because to achieve the same amount of output energy,Na-C2H6system was expected to only require one tenth of pumping energy of Na-Ar system.
References
(1)Max,C.E.;Olivier,S.S.;Friedman,H.W.;An,J.;Avicola, K.;Beeman,B.V.;Bissinger,H.D.;Brase,J.M.;Erbert,G. V.;Gavel,D.T.;Kanz,K.;Liu,M.C.;Macintosh,B.;Neeb, K.P.;Patience,J.;Waltjen,K.E.Science 1997,277,1649.doi: 10.1126/science.277.5332.1649
(2)Rochester,S.M.;Otarola,A.;Boyer,C.;budker,D.; Ellerbroek,B.;Holzlöhner,R.;Wang,L.J.Opt.Soc.Am.B 2012,29(8),2176.doi:10.1364/JOSAB.29.002176
(3)Lee,I.;Jalali,M.;Vanasse,N.;Prezkuta,Z.;Groff,K.;Roush, J.;Rogers,N.;Andrews,E.;Moule,G.;Tiemann,B.;Hankla, A.K.;Adkins,S.M.;d'Orgeville.C.Proc.SPIE Adaptive Optics Systems 2008,7015,70150N.doi:10.1117/12.790534
(4)Wang,P.;Xie,S.;Bo,Y.;Wang,B.;Zuo,J.;Wang,Z.;Shen, Y.;Zhang,F.;Wei,K.;Jin,K.;Xu,Y.;Xu,J.;Peng,Q.;Zhang, J.;Lei,W.;Cui,D.;Zhang,Y.;Xu,Z.Chin.Phys.B 2014,23 (11),094208.doi:10.1088/1674-1056/23/9/094208
(5)Cong,Z.;Zhang,X.;Wang,Q.;Chen,X.;Fan,S.;Liu,Z.; Zhang,H.;Tao,X.;Wang,J.;Zhao,H.;Li,S.Laser Phys.Lett. 2010,7(12),862.doi:10.1002/lapl.201010076
(6)Duering,M.;Kolev,V.;Luther-Davies,B.Opt.Express 2009, 17(2),437.doi:10.1364/OE.17.000437
(7)Dhiflaoui,J.;Berriche,H.;Heaven,M.C.AIP Conf.Proc. 2011,1370,234.doi:10.1063/1.3638107
(8)Merritt,J.M.;Han,J.;Chang,T.;Heaven,M.C.Proc.SPIE 2009,7196,71960H.doi:10.1117/12.815155
(9)Readle,J.D.;Verdeyen,J.T.;Eden,J.G.;Davis,S.J.; Galbally-Kinney,K.L.;Rawlins,W.T.;Kessler,W.J.Opt. Lett.2009,34(23),3638.doi:10.1364/OL.34.003638
(10)Hewitt,J.D.;Houlahan,T.J.,Jr.;Gallagher,J.E.;Carroll,D. L.;Palla,A.D.;Verdeyen,J.T.;Perram,G.P.;Eden,J.G. Appl.Phys.Lett.2013,102,111104.doi:10.1063/1.4796040
(11)Palla,A.D.;Carroll,D.L.;Verdeyen,J.T.;Heaven,M.C. J.Phys.B:At.Mol.Opt.Phys.2011,44,135402.doi:10.1088/ 0953-4075/44/13/135402
(12)Szudy,J.;Baylis,W.E.J.Quantum Spectrosc.Ra.1975,15 (7-8),641.doi:10.1016/0022-4073(75)90032-1
(13)Markov,R.V.;Plekhanov,A.I.;Shalagin,A.M.Phys.Rev. Lett.2002,88(21),213601.doi:10.1103/ PhysRevLett.88.213601
(14)Chung,H.K.;Shurgalin,M.;Babb,J.F.AIP Conf.Proc.2002, 645,211.doi:10.1063/1.1525457
(15)Alioua,K.;Bouledroua,M.;Allouche,A.R.;Aubert-Frecon, M.J.Phys.B:At.Mol.Opt.Phys.2008,41(17),175102.doi: 10.1088/0953-4075/41/17/175102
(16)Atkins,P.;De Paula,J.Physical Chemistry,8th ed.;Oxford University Press:Oxford,UK,2006;p 634.
(17)Martin,W.C.;Musgrove,A.;Kotochigova,S.;Sansonetti,J. E.2011,Ground Levels and Ionization Energies for the NeutralAtoms(version 1.3).National Institute of Standards and Technology,Gaithersburg,MD.[Online]Available:http:// physics.nist.gov/IonEnergy[Wednesday,22-Apr-2015,21: 45:55 EDT].
(18)Olney,T.N.;Cann,N.M.;Cooper,G.;Brion,C.E.Chem. Phys.1997,223(1),59.doi:10.1016/S0301-0104(97)00145-6
(19)Langhoff,P.W.;Karplus,M.J.Opt.Soc.Am.1969,59(7), 863.doi:10.1364/JOSA.59.000863
(20)Dunning,T.H.,Jr.J.Chem.Phys.1989,90(2),1007.doi: 10.1063/1.456153
(21)Woon,D.E.;Dunning,T.H.,Jr.J.Chem.Phys.1994,100(4), 2975.doi:10.1063/1.466439
(22)Woon,D.E.;Dunning,T.H.,Jr.J.Chem.Phys.1993,98(2), 1358.doi:10.1063/1.464303
(23)Peterson,K.A.;Figgen,D.;Goll,E.;Stoll,H.;Dolg,M. J.Chem.Phys.2003,119(21),11113.doi:10.1063/1.1622924
(24)Peterson,K.A.;Yousaf,K.E.J.Chem.Phys.2010,133(17), 174116.doi:10.1063/1.3503659
(25)Werner,H.J.;Knowles,P.J.;Knizia,G.;Manby,F.R.;Schütz, M.Wires Comput.Mol.Sci.2012,2,242;MOLPRO,version 2012.1,http://www.molpro.net.doi:10.1002/wcms.82
(26)Liang,Y.N.;Wang,F.Acta Phys.-Chim.Sin.2014,30(8), 1447.[梁艳妮,王繁.物理化学学报,2014,30(8),1447.] doi:10.3866/PKU.WHXB201405302
(27)Cao,Z.L.;Wang,Z.F.;Yang,M.L.;Wang,F.Acta Phys.-Chim.Sin.2014,30(3),431.[曹战利,王治钒,杨明理,王繁.物理化学学报,2014,30(3),431.]doi:10.3866/PKU. WHXB201401023
(28)Sverdlov,L.M.;Kovner,M.A.;Krainov,E.P.Vibrational Spectra of Polyatomic Molecule;Wiley:New York,1974.
(29)Benran,K.Bond Lengths and Angles in Gas-Phase Molecules, 3rd ed.II;Maruzen Company,LTD.:Tokyo,Japan,1984;p 649.
(30)Baumann,P.;Zimmermann,D.;Brühl,R.J.Mol.Spec.1992, 155(2),277.doi:10.1016/0022-2852(92)90517-R
(31)Schwarzhans,D.;Zimmermann,D.Eur.Phys.J.D 2003,22 (2),193.doi:10.1140/epjd/e2002-00242-8
(32)Boys,S.F.;Bernardi,F.Mol.Phys.1970,19(4),553.doi: 10.1080/00268977000101561
(33)Pahl,E.;Figgen,D.;Thierfelder,C.;Peterson,K.A.;Calvo,F.; Schwerdtfeger,P.J.Chem.Phys.2010,132(11),114301.doi: 10.1063/1.3354976
Experimental and Theoretical Evaluation of the Absorption Coefficients of Excimer Pairs of Sodium with Noble Gases and Alkanes
HU Shu1GAI Bao-Dong1CAO Zhan-Li2GUO Jing-Wei1,*WANG Fan2
(1Key Laboratory of Chemical Lasers,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023, Liaoning Province,P.R.China;2Institute of Atomic and Molecular Physics,Sichuan University,Chengdu 610064,P.R.China)
The excimer-pumped sodium laser(XPNaL)is very important for its application in sodium guide star.However,the absorption coefficients(for the pumping source)of traditional excimer pairs,such as Na-He and Na-Ar,are very small.In this work,four systems(Na-Ar,Na-Xe,Na-CH4,and Na-C2H6)are investigated based on both fluorescence experiment and theoretical binding energies obtained from highly accurate quantum chemistry calculations to determine better excimer pairs.The experiment results show that the peak area ratio of fluorescence intensity curves for the excimer pairs of Na-Ar,Na-Xe,Na-CH4,and Na-C2H6was 1.0:6.4:4.9: 10.4.Meanwhile,using the CCSD(T)approach and basis set extrapolation,binding energies for these four systems were calculated as 52.8,124.5,117.7,and 150.0 cm-1,respectively.Therefore,predication by quantum chemistry calculation was consistent with experimental results.The Na-C2H6system was found to be the most efficient system both experimentally and theoretically,and has the potential to be used in the development of a high power XPNaL.This work also demonstrates that the binding energy from highly accurate quantum chemistry calculations with a large basis set is a very good criterion for the selection of excimer pairs for the excimer-pumped alkali laser(XPAL).
Excimer pair;XPAL;CCSD(T);Basis set;Fluorescence;Absorption coefficient
October 28,2015;Revised:January 13,2016;Published on Web:January 15,2016.*Corresponding author.Email:jingweiguo@dicp.ac.cn;Tel:+86-411-84379715. The project was supported by the National Natural Science Foundation of China(11475177,11304311,61505210,61405197).
O641
10.3866/PKU.WHXB201601151
国家自然科学基金(11475177,11304311,61505210,61405197)资助项目
