Effects of organic acids on dissolution of Fe and Mn from weathering coal gangue
2016-08-26ZuyongChenFangLiuTongdaBuYuanshengLiuJianZhu
Zuyong Chen·Fang Liu·Tongda Bu·Yuansheng Liu·Jian Zhu
Effects of organic acids on dissolution of Fe and Mn from weathering coal gangue
Zuyong Chen1·Fang Liu1·Tongda Bu2·Yuansheng Liu2·Jian Zhu1
©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2016
Understanding the effects of organic acids (OA)on the transformation of Fe and Mn to surface water from the weathering coal gangue is of great benefit to risk assessment and remediation strategies for contaminated water and soil.Based on the investigation on surface water in the central coal districts of the Guizhou Province,18 water samples were collected for heavy metal analysis.The results indicated that the pH value of surface water is low (3.11-4.92),and Fe concentration(1.31-5.55 mg L-1)and Mn concentration(1.90-5.71 mg L-1)were,on average,10.86 and 34.33 times the limit of Surface Water Quality Standards,respectively.In order to evaluate the effects of the OA on the dissolution of Fe and Mn from the weathering coal gangue,column elution and batch leaching experiments were conducted.The results show that the low molecular weight of organic acids(LMWOAs,i.e.,oxalic,tartaric,malic and citric acids)and fulvic acids significantly accelerated the dissolution of Fe and Mn;in addition,when the concentration of OA reached 25 mmol L-1,the concentrations of Fe,and Mn were 1.14-67.08 and 1.11-2.32 times as high as those in 0.5 mmol L-1OA,respectively.Furthermore,the migration of Fe and Mn was significantly influenced by the pH and Eh,especially for Fe;the ion Mn was dissolved from the gangue more easily than the ion Fe in the column leaching,which was contrary to the results of batch leaching.
Coal gangue·LMWOAs·Fulvic acids· Iron·Manganese·Leaching
1 Introduction
Wastewater effluents from coal refuses piles are considered as serious environmental pollutants,as they are acidic and contain several dissolved toxic metals.Under atmospheric conditions,the oxidation of sulfide minerals in ores might produce acid drainage waters with toxic heavy metals (Novikova and Gas'kova 2013).Studies have shown discharging pollutants from abandoned coalmines across the Guizhou Province(Changyuan et al.2009;Pan et al.2009). Coal mining has been forbidden,but there are still hundreds of abandoned mine sites near urban and agricultural areas.The mine waste and tailings have contaminated the soil,sediments and ground water(Navarro and Martı´nez 2010).
Natural organic matter(NOM),which is in dissolved form and solid form,plays a significant role in controlling the disposition of toxic heavy metal elements in the environment.Dissolved organic matter(DOM)bind metal ions in solution and change their speciation and mobility(Lee,et al.2011).The binding of metal ions is important for the transport and toxicity of many trace metals,but it has proven difficult to find the equations that describe this binding over a wide range of conditions(Kinniburgh et al. 1996).Root exudates have been shown to play a key role in Fe and Mn absorption by plants,due to their ability to enhance the metal solubility in soils(Jones and Darrah 1994;Rengel and Marschner 2005;Jiewen et al.2015). Fulvic acids(FA)were easily transported within the soil profile and predominately gave rise to the migration(eluviations)of Fe and Al compounds(Egli et al.2010).Highconcentrations of DOM enhance the release of structural iron in clay minerals.Repeated redox alternations lead to a translocation of iron in various directions(Ko¨gel-Knabner et al.2010).
Although extensive research has investigated the function of organic acids(OA)in different soils,a knowledge gap still exists on how the OA may affect the dissolution of harmful metal elements from a coal refuses pile site.This study was conducted to understand the leaching behavior of Fe and Mn in a weathering coal gangue when subjected to various OA,identify the impact of Fe and Mn mobility,and provide useful information for the optimization of abandoned mines vegetation restoration.
2 Materials and methods
2.1Research area
The studied region was located in the Huaxi coalfield (106°34′-106°37E,26°27′-26°31′N)of the Guizhou Province.The region has a subtropical continental monsoon climate and is warm and humid,with annual average precipitation of 1200 mm and a medial temperature of 14.9°C.The bedrock of the area is mainly composed of sedimentary carbonate rocks from the Permian-Triassic period.The coal-bearing strata are mainly hosted in the Permian Formation,and there are great differences both in the coal seam numbers(8-32)and thicknesses.There are several small streams in the study area and they receive the effluent from the mine drainage of the abandoned coalmines and discharge them into the Wu Jiang River.There are also innumerable abandoned coalmines upstream.
The Maiping coalmine is one of the abandoned mines and tailings dams where mining activities were forbidden by the local government,due to the chronic pollution of the municipal drinking water reservoir of Guiyang city. According to a field survey in 2009(Wei et al.2009),there are 50 families,34 genus and 50 species of flora in the study area,all of which have natural restoration around gangue dump.The Pinus massoniana and Betula luminifera are the largest plant community biomass and make the greatest contribution to the vegetation restoration in the gangue land.
2.2Surface water sampling around research area
Three small pits(50 cm diameter)were dug at the center of the coal refuses pile to receive approximately 10 m2rainfall.At the same time,three small pits were dug in a dredged channel around the coal refuses pile.The runoff water of the coal refuses pile was collected from the pits around the refuses pile on 21st May(rainfall intensity was 1.5 mm/h)and 12th August 2013(rainfall intensity was 2.0 mm/h).All the water samples were collected by polyethylene bottles,respectively.Subsequently,after being taken back to the laboratory,they were filtrated to a 50 mL plastic centrifuge tube with a 0.45 μm filter membrane. Each collected water sample was detected with pH,EC,and Eh within 24 h after they were taken.The latter was acidified with pure HNO3to adjust its pH value to below 2,to avoid Fe hydroxide precipitation.Then,all the samples were stored at 4°C in the refrigerator before any other determination.
2.3Preparation of materials for leaching
Research has shown that the organic acids in the forest litters were mainly oxalic,malic,citric and malonic acid,et al.(Jinfeng et al.2004).These low molecular weight organic acids(LMWOAs),as natural products,could be produced from root secretions,microbial metabolism,OA and the decomposition of plants,as well as animal residues (An et al.2011;Vesely´et al.2012;Almaroai et al.2013). As the largest plant community biomass in and around the research area,P.massoniana would increase the oxalic,tartaric,malic and citric acid exudation from their roots (Yuanchun et al.2007).The coal refuses are always piled up in the valleys with the P.massoniana forest on the opposite sides.On rainy days,the refuses will be scoured and leached by flowing water,which is full of DOM(i.e. FA)from the surrounding forest.In order to simulate the leaching behavior of metals in natural rainfall(light rain),we chose the oxalic,tartaric,malic,citric acids,FA,and the pine needle soak(PNS)as the rainwater in this leaching experiment.
Concentrated stock solutions of OA were prepared by dissolving each organic acid in its acid form(oxalic,tartaric,malic,citric acids and FA)or soaks form(PNS)in deionized water.Despite the fact that the LMWOAs concentrations were variable in the rhizosphere(various plant species etc.),we selected the values of 25,5,and 0.5 mmol L-1to simulate the high,middle,and low concentration ranges observed in soil solutions(Jones 1998;Jinfeng et al.2011;Vaneˇk et al.2012;Huan et al.2015). The LMWOAs and FA solutions were prepared from chemicals of analytical grade,and the PNS were prepared at 2 levels(PNS a and b).The half-decomposed withered leaves and the non-decomposed withered leaves of pine tree were soaked with deionized water according to the solid:liquid ratio of 1:1(w/v).After 24 h,the two mixtures were filtered with quick filter paper,respectively,and we named them as PNS a and b.The measured values of the organic substances used for leaching are shown in Table 1. The weathering coal gangue samples for leaching were collected by the coal refuse piles from the research areaand sifted through a 5 mm sieve,and then well-mixed.The parameters for the main chemical variables of the weathering coal gangue are presented in Table 2.
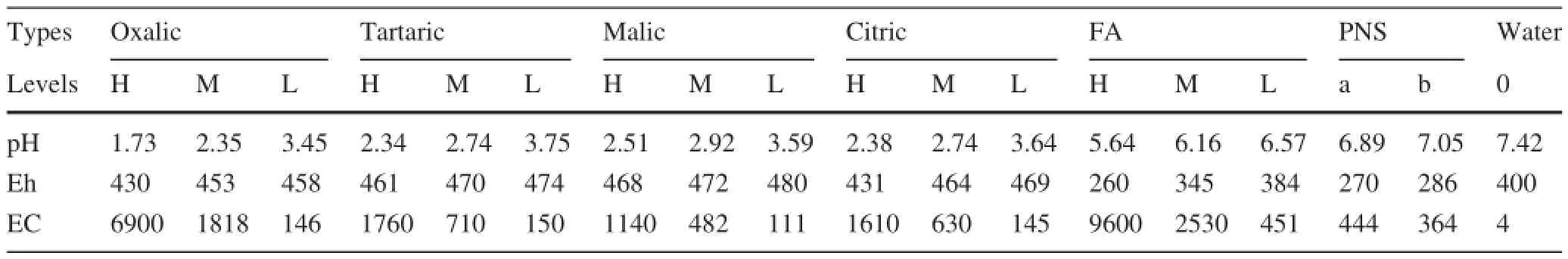
Table 1 The pH,Eh,and EC values of the organic leaching solution
2.4Organic acids column experiment
2.4.1Equipment
The schematic diagram of the column experiments is shown in Fig.1.All column leaching tests have been conducted under laboratory conditions(20°C)using PVC columns with an internal diameter of 7.5 cm and a length of 50 cm.The packing of air-dried weathering coal gangue took place in 15-20 increments,and each increment was packed slightly before the next one was placed on top,until the column was completely filled with 4 kg weathering coal gangue.After coal gangue is filled uniformly,a filter paper must be laid on the top of the gangue.We made sure the filter paper was level,so the liquid drip onto the filter paper was uniformly distributed on the surface of the coal gangue.The leaching of the solution was horizontally placed in a high position as far as possible,minimizing the hydraulic change during the leaching time.
2.4.2Procedure
The concentrated stock solutions of OA is composed of 5 kinds of OA with 3 concentrations and 2 kinds PNS. Deionized water was used as a control treatment.The experiments were performed twice for all treatments. Continuously leaching the coal gangue for 24 h with the leaching solution was taken up to simulate the light rain (12.7 mm 24 h-1,i.e.,1 drop min-1)leaching on the coal gangue.Prior to e,samples were filtrated to a 50 mL plastic centrifuge tube with a 0.45 μm filter membrane.The remaining collected liquid solution in the collector must be poured out entirely.The next leaching was carried every 7 days,and the whole leaching experiment last for 91 days (13 times).
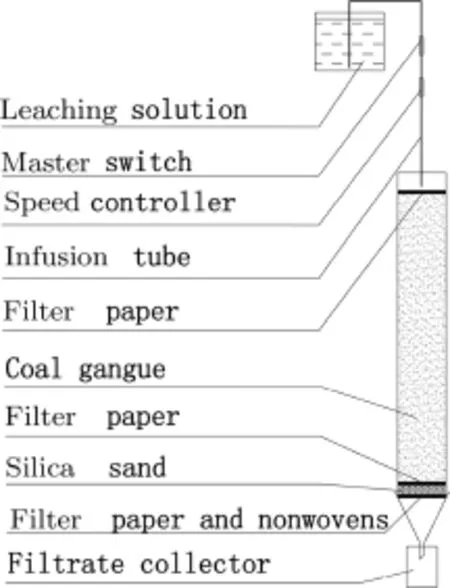
Fig.1 Scheme of the experimental setup used in the column elution experiments
Each collected water sample was detected for pH,EC,and Eh within 24 h after the sample was taken.The latter was acidified with pure HNO3to adjust its pH value to below 2,to avoid Fe hydroxide precipitation.All the samples were stored at 4°C in the refrigerator before any other determination.

Table 2 The total contents of ions in weathering coal gangue for leaching./(mg kg-1)
2.5Organic acids batch experiments
After a long rain(3-5 days)in the research area,many small pits were filled with water;then the natural leaching procedure started.In order to simulate this leaching behavior,we also used the deionized water as rainwater in our batch leaching experiments.The same concentrated stock solutions of OA as column leaching experiments were used in this leaching test.Twenty batches were runwith a solid:liquid ratio of 1:2(w/v),using 20 g of airdried weathering coal gangue and 40 mL of synthetic water.The leaching was conducted at a constant temperature of 20±3°C in acid-cleaned centrifugation tubes (PP).The leachates were sampled after 24,72,120,168 and 216 h.Deionized water was used as a control treatment.The experiments were performed twice for all treatments.Batches were sealed and placed on a rotary shaker for half an hour to achieve equilibrium,and prior to collecting samples(5 mL each)they were filtered using a 0.45 μm polypropylene filter.The same treatments were conducted as those from the column leaching after the sample was collected.
2.6Analytical methodology and statistical analysis
The pH,EC(electrical conductivity)and Eh(redox potential) ofthesamplesweremeasuredwith portable equipment(Shanghai Leici Instrument Co.,Ltd). The fraction of dissolved heavy metals was attained by analyzing the content of the filtered samples.The used filter was a 0.45 μm syringe filter.The major elements(Fe,Mn,Cu and Zn)in solutions were determined with Atomic Absorption Spectroscopy(AAS PE-800,USA),and the trace elements(Pb,Cr,and Cd)were analyzed by ICP-MS (Platform ICP).Blanks and appropriate certified standard reference materials were analyzed as unknowns with every batch of samples.All the related glassware was soaked in 10%HNO3for 24 h and then thoroughly rinsed with the deionized water before being used.
The analysis of statistical data was conducted by using the Statistical Product and Service Solutions(SPSS 22). The graphs were drawn with Origin Pro 9.1 or AutoCAD.
3 Results and discussion
3.1Concentrations of heavy metals in surface water
around research areaThe concentrations of the dissolved metals in the surface water are presented in Table 3.The analytical results show that the pH values ranges from 3.11,with the low tide (affected water),to 6.99,with the high tide(unaffected water).The low pH values(3.11-4.92)of these affected waters are probably responsible for the metal mobilization. The concentrations of Fe,Mn and Cd are 1.31-5.55,1.90-5.71 and 0.005-0.015 mg L-1,respectively;and they are on average 10.86,34.33 and 2.00 times the limit of the Surface Water Quality Standards(China's Water Quality Standards GB3838-2002),respectively.The ion concentration decreases as quickly as the distance increases from the center of the pile.The concentrations of Fe,Mn,andCd in the water obtained from edge of the pile decreased 61.38%,56.08%and 57.14%of those in the water obtained from center of the pile,respectively.
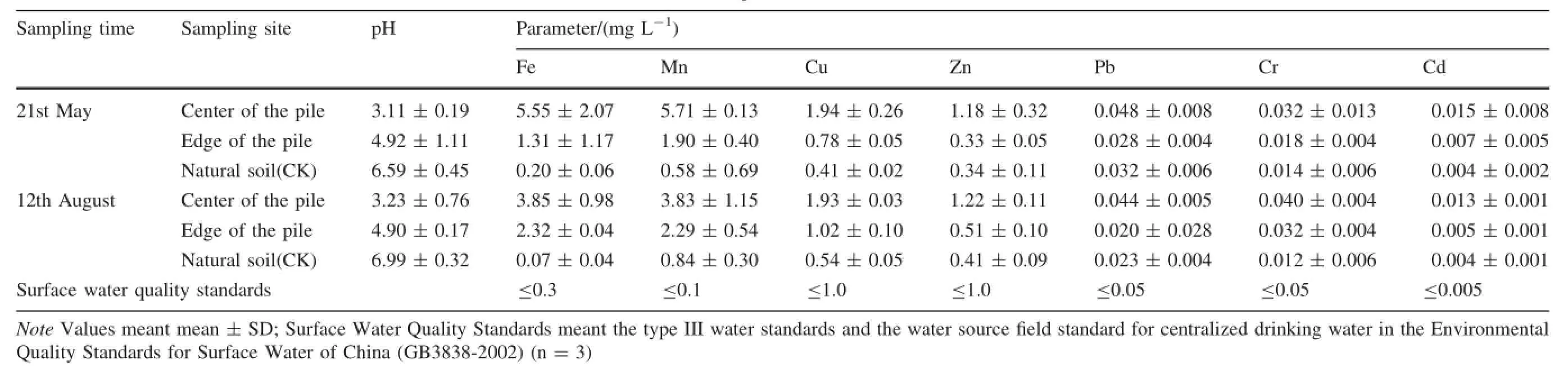
Table 3 The concentrations of dissolved metals in the surface water around coal refuse pile
High concentrations of Fe and Mn were found in the samples and reflected the contribution of pyrite oxidation. The oxidation of the sulfide minerals(pyrite)induced the hydrogen ion to be constantly released into the surface water in the presence of oxygen(Backes et al.1985)and the oxidation of metal sulfides containing heavy metals gives rise to the release of heavy metals into the surrounding environment(Zhi et al.2001).These contaminated surface waters transfer the metallic pollutants to stream waters and other ecosystems situated in the vicinity. The chemical composition of the affected water is similar to that observed in previous studies(Changyuan et al. 2009;Pan et al.2009;Heikkinen et al.2009;Smuda et al. 2014).
3.2The concentrations of Fe and Mn in column leaching
Based on the above analysis,we found that Fe and Mn were the main water pollution elements in the research area.In the following sections,we mainly discuss the effects on Fe and Mn.
The result of column elution leaching is shown in Table 4.Compared to the surface waters collected around the research area(Table 3),the concentration of Mn in the column leaching solutions are very close to the level of affected water at the center of the pile,while the concentration of Fe in all treatments are far lower than that in the surface water.When the concentration of OA rises to 25 mmol L-1(High level),the concentrations of SO42-,Fe and Mn are 1.04-1.13,1.14-5.68 and 1.11-1.51 times as highasthoseinthelowconcentrationofOA (0.5 mmol L-1),respectively.
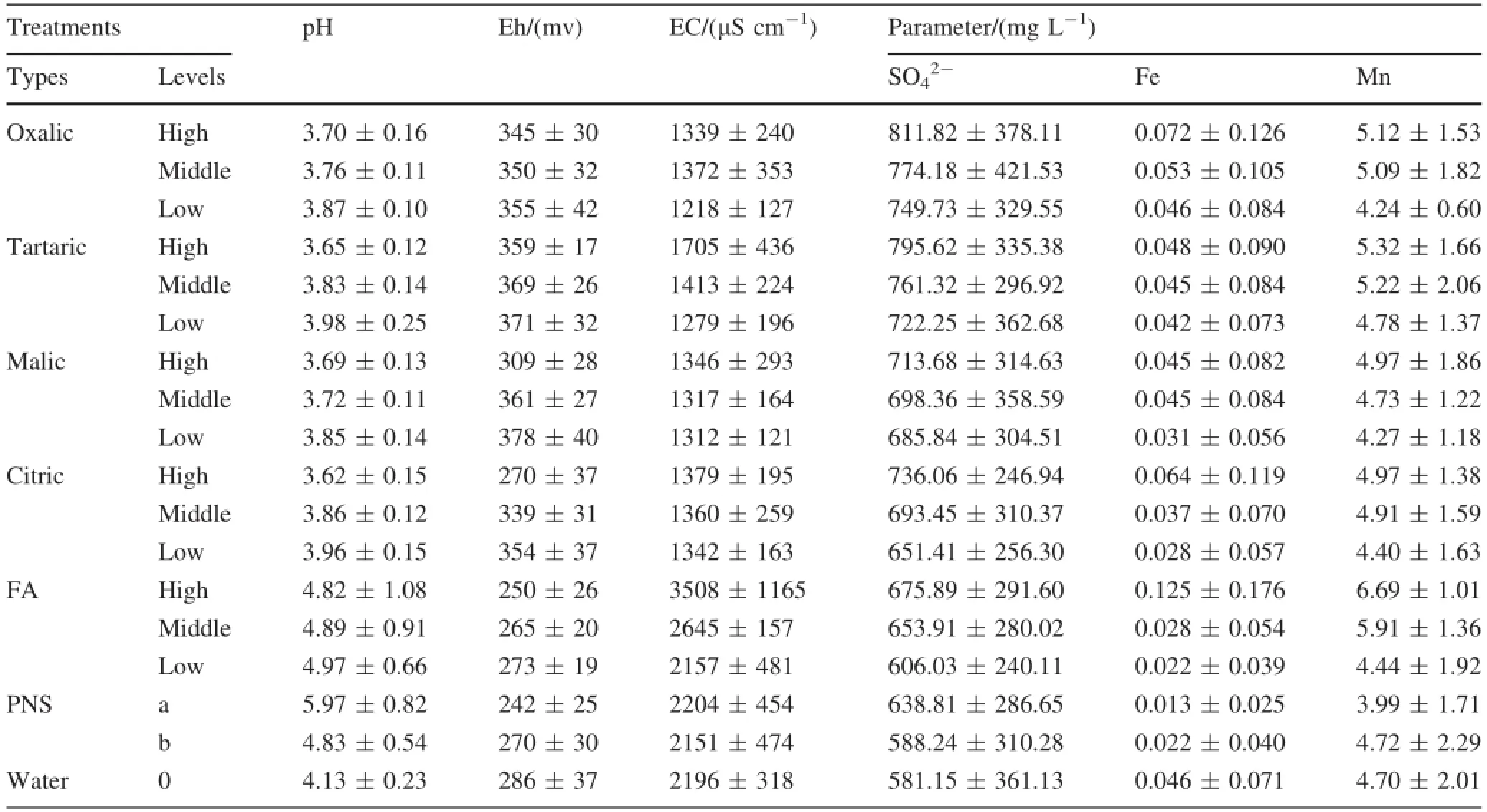
Table 4 The concentrations of dissolved ions in column leaching solutions
3.2.1Column leaching characteristics of Fe
The concentration curves of Fe in different concentrations of OA are in Fig.2.It is clear from the figures that almost all the concentration of total Fe gradually decreased nearly to their quantification limits on the 50th day,except for the high concentration of FA leachates.Compared to water,the higher concentration of LMWOAs is where the most dissolution of Fe would be,while PNS and FA were both lower than water in the first 63 days.As mentioned before,high concentration of DOM enhances the changes and accelerates the release of structural iron,and supports the formation of ferrihydrite.Repeated redox alternations lead to a translocation of iron in various directions,andparticularly increase the crystallinity of iron oxides(Ko¨gel-Knabner et al.2010).But if the rocks exhaust their neutralizing potential,the formation of metal organic complexes inhibits the surface oxidation of the sulfide minerals and reduces the removal of heavy metals into the solution (Novikova and Gas'kova 2013).
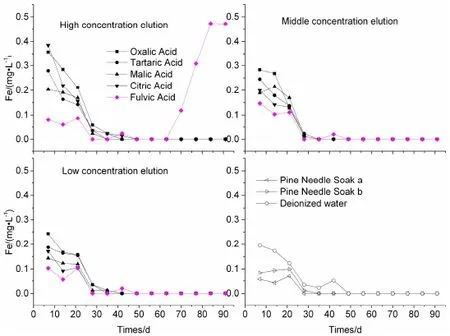
Fig.2 Time-dependent patterns of concentration curves of Fe in OA elution leachates from weathering coal gangue;depicted values are means of 2 replicates with RSD lower than 10%(not shown)
3.2.2Column leaching characteristics of Mn
The concentration curves of Mn in different concentrations of OA are in Fig.3.Except for the high concentration of FA leachates,the concentration of total Mn in all leachates gradually decreased and was stuck in a range between 3 and 9 mg L-1.In particular,the concentration of Mn decreased more in the first 28 days.Similar to Fe,the dissolution of Mn in the FA leachates(high concentration elution)showed an upward trend after a decline of 49 days. Compared to LMWOAs,it seemed that the FA had a stronger ability to enhance the release of Mn(high and middle elution).But in the low concentration of OA (0.5 mmol L-1),therewasnosignificantdifference between the FA and LMWOAs,and the dissolution of Mn in FA was even lower than those in LMWOAs in the last 21 days.
In conclusion,the high and the middle concentrations of OA dissolve more of the ions Fe and Mn than the low concentrations of OA.When the concentration of OA rise to 25 mmol L-1,the concentrations of Fe and Mn are on average 2.43 and 1.22 times as high as that in the 0.5 mmol L-1OA,respectively.The ion Mn can be dissolved from the gangue more easily than ion Fe in the column leaching.
3.3The concentrations of Fe and Mn in batch leaching
The result of batch leaching is shown in Table 5.We found that the concentrations of Fe and Mn in the batch leaching solutions with low concentration treatments(0.5 mmol L-1)and deionized water were lower than those in the surface waters(Table 3),but the concentration of Fe in the high and middle concentration treatments(25,and 5 mmol L-1)were far higher than those in surface water.The concentration of Mn was also very close to the level of affected waters in the pile.When the concentration of OA rose to 25 mmol L-1,the concentrations of SO42-,Fe and Mn were 1.07-1.31,9.82-67.08 and 1.92-2.32 times as high as that in the 0.5 mmol L-1OA,respectively.
3.3.1Batch leaching characteristics of Fe

Fig.3 Time-dependent patterns of concentration curves of Mn in OA elution leachates from weathering coal gangue;depicted values are means of 2 replicates with RSD lower than 10%(not shown)
Table 5 The concentrations of dissolved ions in batch leaching solutions
Different OA were also used to soak the weathering coal gangue,and the results of Fe are shown in Fig.4.From the figure we can see that the dissolution of Fe in the low concentrations of OA obviously decrease in comparison to that in the high and middle concentrations of OA.The lower concentrations of OA of the soak are the solutions wherefewerdissolutionsofFewouldbe.The concentration curves of Fe imply that their release is strongly affected by citric and oxalic(Vaneˇk et al.2012),especially in the high and middle concentrations of OA;the highest concentration of Fe is found in high concentrations of citric leachates(up to 20.31 mg L-1),followed by oxalic(up to 20.15 mg L-1).It must be highlighted that,considering the Fe trends,a gradual increase is observed in the FA solutions,especially in the low and middleconcentrations of OA.In contrast,the efficiency of PNS in extracting Fe is negligible(1.85 mg L-1in maximum)and leads to the lowest leachability compared to water(Fig.4). These findings can be interpreted by the formation of Fe complexes with citric and oxalic(log KFe(III)-citric=13.1;log KFe(III)-oxalic=7.5,Vaneˇk et al.2012),which enhances Fe solubilization.
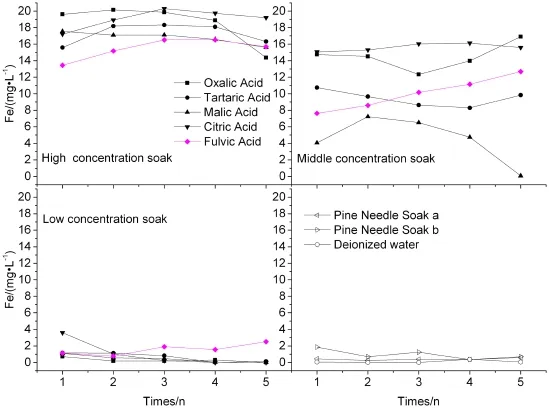
Fig.4 Time-dependent patterns of concentration curves of Fe in OA soak leachates from weathering coal gangue;depicted values are means of 2 replicates with RSD lower than 10%(not shown)
3.3.2Batch leaching characteristics of Mn
The concentration curves of Mn in different concentrations of batch leachates are shown in Fig.5.The dissolution of Mn in the 3 concentration levels shows the same trends as Fe.The lower concentration group of the soak solution is where fewer dissolutions of Mn would be.But the gap between the 3 concentrations in the dissolution of Mn is smaller than those of Fe.The maximum concentration of Mn is detected in FA leachates,reaching 10.30 mg L-1. In general,the reducing order of Mn is FA(6.61 mg L-1),to tartaric(5.03 mg L-1),citric(4.88 mg L-1),oxalic (4.62 mg L-1),malic acid(4.53 mg L-1),PNS(3.62 mg L-1)to deionized water(2.58 mg L-1).The FA leachates imply that the Mn concentration in the LMWOAs is higher on average in the 3 concentration levels,as compared to Fe. In conclusion,the higher concentrations of OA are the where more dissolution of Fe and Mn would be.When the concentration of OA rises to 25 mmol L-1,the concentrations of Fe,and Mn are on average 32.35 and 2.16 times as high as they are in the 0.5 mmol L-1OA,respectively. Contrary to column leaching,it is clear that the ion Fe can be dissolved from the gangue more easily than ion Mn in the batch leaching.
3.4Influence factors of the migration of Fe and Mn
The inter-individual correlations between the measured values of leachates are summarized in Table 6,based on the data from Tables 4 and 5.The correlation analysis demonstratesthathighpositive/negativecorrelations between the majorities of the measured values.For the following pairs of values:Fe-EC,Fe-SO42-,Mn-EC and Mn-SO42-,the correlation coefficients are positive correlations.The surface oxidation of the sulfide minerals(pyrite)in the coal refuse pile is the main reason for the ion exchange from the solid matrix(Changyuan et al.2009;Pan et al.2009;Mastrocicco et al.2011).That might be the reason that Fe and Mn strongly correlated to SO42-and EC.
3.4.1Influence of pH on Fe and Mn
A significant negative correlation between Fe and pH (r=-0.562,P<0.001)is found in Table 6,while a significant negative correlation between Mn and pH (r=-0.173,P=0.314)is also found in that table.From previous analysis,we found that the concentrations of Fe and Mn increase with the increase of concentration of OA. The stronger the acid concentration is,the higher the acidity and the more functional groups there are(i.e.-COOH and-OH).The acidic functional group quantities of FA are larger than that of humic acids(HA);the acidity is higher than that of HA too.The FA is transported more easily within the soil profile than HA and predominately gives rise to the migration(eluviations)of compounds(i.e. Fe)due to their-COOH and-OH functional groups(Egli et al.2010).
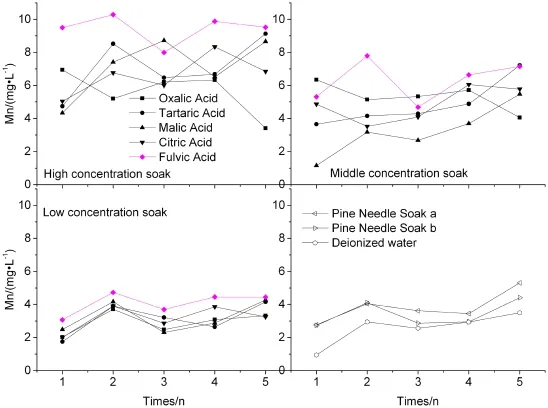
Fig.5 Time-dependent patterns of concentration curves of Mn in OA soak leachates from weathering coal gangue;depicted values are mean of 2 replicates with RSD lower than 10%(not shown)
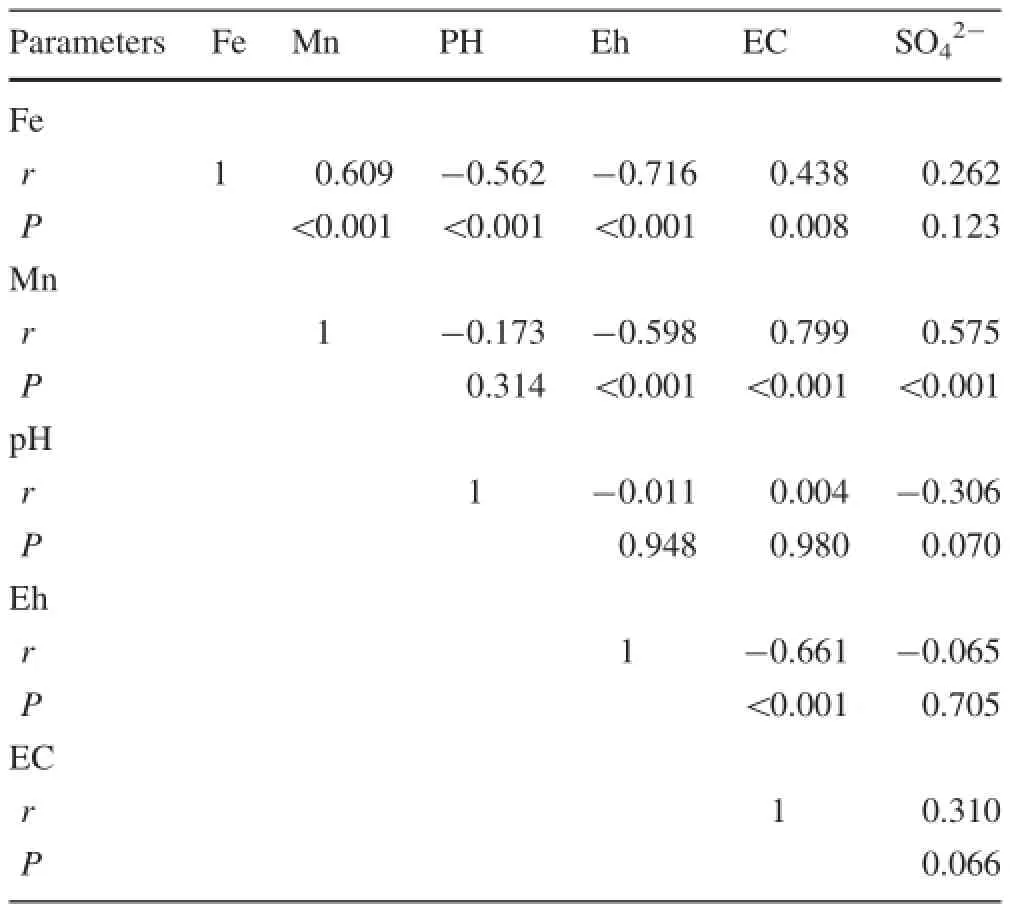
Table 6 Pearson Product Moment Correlation Coefficients for leachates in column and batch leaching(n=36)
The ion Fe always exists in two common oxidation states(Fe2+and Fe3+).The ion Mn exists in the form of a bivalent in water solution,while trivalent and quadrivalent Mn exist in the forms of oxide and hydroxide in the solid phase.The pH ranges of precipitating bivalent Fe and Mn are 6.5-9.7 and 7.8-8.8,respectively,and the pH range of precipitating the trivalent Fe is 1.5-4.1(Xiangfei 2015). The pH ranges from the column and batch leaching results are 3.62-5.97 and 2.54-5.21,respectively(Tables 4 and 5),so it seems that the influence of the 3 concentrations of OA (Relate to pH)on Fe is stronger than that of Mn,which leads to the fact that the concentration of Fe is lower in FA than that in LMWOAs generally.But it is contrary to the fact that the Mn for the pH value of FA(5.64-6.57)before leaching is higher than that of LMWOAs(1.73-3.64)(Table 1).It can be concluded that the influence of pH on Fe is stronger than that on Mn.
Citric is a tribasic acid,but oxalic,tartaric and malic acid are all dicarboxylic acids.The pH range of oxalic before leaching is 1.73-3.45(Table 1),while the pH ranges of citric,tartaric,and malic acid is 2.34-3.75.In different concentrations of OA and treatments(elution or soak),the dissolving ability of OA are different,and it is difficult to give an exact sequence of Fe and Mn released by the OA. The previous studies have shown that the trends of metals released by the OA differ greatly in different situations (Alin et al.1997;Caiying et al.2004;Yunlong et al.2008;Hailin and Bohan 2010;Guoyong et al.2014).When the concentration of OA rise from 0.5 to 25 mmol L-1,theconcentration of Fe and Mn increase about 1.11-67.08 times in both leachings(Tables 4 and 5).Accordingly,it can be concluded that the strongest effect of OA on Fe is the oxalic acid,followed by citric,tartaric,and malic acid,and the weakest effect of OA on Fe is the FA;on the contrary,the strongest effect of OA on Mn is the FA,followed by the 4 LMWOAs.
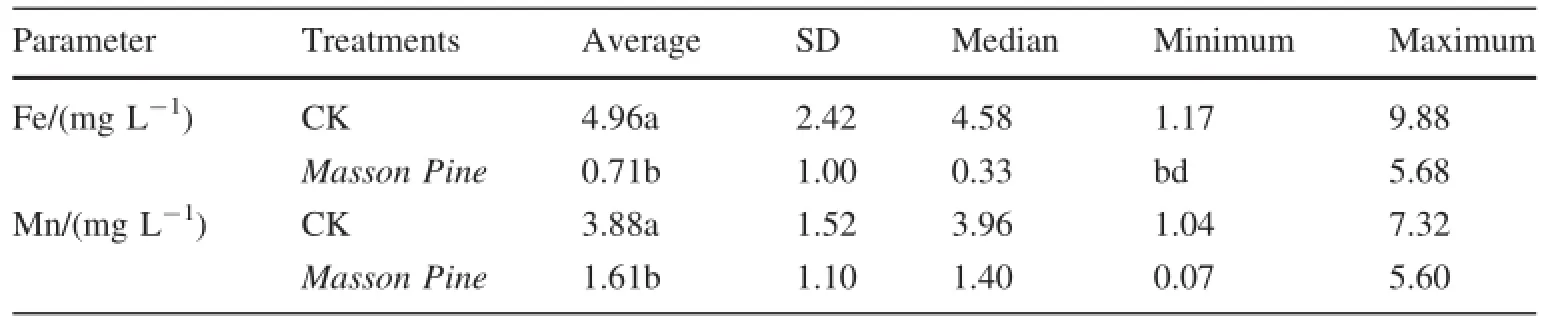
Table 7 Concentrations of Fe and Mn in leachates form weathering coal gangue with and without P.massoniana
3.4.2Influence of Eh on Fe and Mn
Just as we have shown in Table 6,two significant negative correlations of Eh with both Fe and Mn(r=-0.716,P<0.001;r=-0.598,P<0.001,respectively)are observed.Previous research has shown that the Fe2+is precipitated by DOM under anaerobic conditions(Ko¨gel-Knabner et al.2010).The amorphous Fe and Mn oxides dissolve during flooding and anaerobiosis,and chemical reduction removed with(NH4)2C2O4effectively removed about 88%and 93%of Fe and Mn,respectively.But,oxidation of the sample with H2O2removed considerably less of the metallic oxides(Ololade et al.2015).It was clear that Fe and Mn were both closely related to Eh.
But on average,the Eh of leachates in batch leaching (255mv)is lower than that in column leaching(319mv).In batch leaching,the concentration of Fe released by the OA average ranges from 0.28 to 18.58 mg L-1(Table 5),which reduces from 0.013 to 0.072 mg L-1in all the 3 concentrations of OA(Table 4)in column leaching;this phenomenon can also be found in Mn,but the decreased range(2.50%on average)is much smaller than Fe (99.50%on average).It can be suggested that the released Fe and Mn in low valence transform to a higher oxidation state in column leaching experiments,leading to the formationofhydroxideprecipitationmoreeasily,and absorption on the solid phase.To conclude,the influence of Eh on Fe is stronger than that on Mn.
4 Discussions
As previously mentioned,the concentrations of Fe and Mn released by deionized water are far lower than that in the surface water collected from the field.But via elution and soak,the concentrations of Fe and Mn released by OA are so high that many of them are far higher than that in surface water,especially in the high and middle concentration of OA.That is to say the water with high concentration of OA will significantly increase the risk of releasing Fe and Mn.But Fe and Mn are essential plant nutrients,and previous studies have concluded that they can be absorbed by and enriched in P.massoniana abundantly,particularly Mn (Xi et al.2004).In order to confirm that vegetative plants (i.e.P.massoniana)in natural vegetation restoration will enhance the release of Fe and Mn from the weathering coal gangue,we performed a seepage water pot experiment(not shown)using the P.massoniana(80-150 cm high),and the results(Table 7)indicated that the dissolutions of Fe and Mn in seepage water decrease significantly with the existence of P.massoniana.
In general,soil within the field environment can be viewed as a complex of aerobic and anaerobic microenvironments.Some studies have shown that Fe and Mn were significantly extracted by acidic solution(Yingqun et al. 2013;Zhuhong et al.2008;Ololade et al.2015).Organic substances significantly affect the behaviors of some pollutants in natural environments,such as trace metal speciation and toxicity,mineral growth and dissolution,the solubilization and adsorption of hydrophobic pollutants,as well as redox behavior in soils(Kang et al.2002;Atalay et al.2009;Debela et al.2010;Yanyu et al.2011).But depending on the dissociation properties and number of the carboxylic groups,LMWOAs carry varying negative charges,thereby allowing the complexation of metal cations(Fe,Ca,K etc.)in soil solution,as well as their release from soil(Jones and Darrah 1994).After root exudation,LMWOAsentertherhizospherethrough chelation and H+-promoted reactions,promoting the dissolution of soil phases and facilitating the uptake of dissolved elements into plants(Ettler et al.2009;Ko¨gel-Knabner et al.2010;Haibo et al.2012;Lin and Teresa et al.2015).But there were also some other research reporting that citric acids were able to induce the removal of heavy metals from soil without increasing the leaching risk(Clı´stenes Williams et al.2006).
There are 50 families,34 genus and 50 species ecesis in the study area.As reported previously,plants would release OA(e.g.citric acids),increasing the concentration of soluble iron from oxides in soil and overcoming the status ofdissolution iron deficiency(Lo´pez-Bucio et al.2000;Jinfeng et al.2011).On a rainy day,flowing water full of DOM will form in the upper-stream,and will then run through the whole coal refuses pile.There are also many low concentration OAs dissolving from the rhizosphere of the pile internal.This will increase the risk of releasing Fe and Mn,too.
Since the plants will absorb the dissolved elements,and the concentration of OA in the rhizosphere was low enough (i.e.0.025-1 mmol L-1,Fox et al.1990;Tani et al.1995;Alin et al.1997;Yongzhen et al.2005),it can be inferred that the root exudates(i.e.LMWOAs)of vegetation restoration will not increase the risk of releasing Fe and Mn from the weathering coal gangue.But more research is needed to gain more insight in the composition,concentration and behavior of OA under the condition of the plant growth,in order to improve the prediction of metal leaching in the field.
5 Conclusions
The purpose of this study is to estimate the effects of organic acids on the dissolution of Fe and Mn from the weathering coal gangue.The conclusions are as follows:
(1) The research results of the surface water around the research area show that the pH values range from 3.11,with the low tide,to 6.99,with the high tide. The concentrations of Fe and Mn in the water obtained from edge of the pile are 61.38%and 56.08%of those in the water obtained from center of the pile,respectively.High concentrations of Fe and Mn are on average 10.86 and 34.33 times the limit of the Surface Water Quality Standards,respectively.
(2) The low molecular weight organic acids(LMWOAs)and fulvic acids significantly increase the dissolution of Fe and Mn from the weathering coal gangue via the strong complexation of organic acids(OA). When the OA concentration rise to 25 mmol L-1,the concentrations of Fe and Mn are 1.14-67.08,and 1.11-2.32timesashighasthatofinthe 0.5 mmol L-1OA,respectively.The strongest effect of OA on Fe is oxalic acid,followed by citric,tartaric,and malic acid,and the weakest effect is the FA;conversely,the strongest effect of OA for Mn is the FA,followed by the 4 LMWOAs.The role of the pine needle soaks in the total process of Fe and Mn mobilization seems to be of less importance.
(3) The migration of Fe and Mn,especially Fe,is significantly influenced by pH and Eh.The ion Mn can be dissolved from the gangue more easily than ion Fe in column leaching,which is contrary to the batch leaching;the concentration of Fe in column leaching decreases on average 99.50%of that in batch leaching,while the concentration of Mn in column leaching decreases 2.50%of that in batch leaching.
(4) Since the plants will uptake the dissolved elements,and the concentration of OA in the rhizosphere was low enough(i.e.0.025-1 mmol L-1),it can be inferred that the root exudates(i.e.LMWOAs)of vegetation restoration will not increase the risk of Fe and Mn releasing from the weathering coal gangue.
AcknowledgmentsThis project was sponsored by The Innovative Talent Team Construction Project for Science and Technology of Guizhou Province(Project Number[2012]4005).
References
Alin S,Xueyuan L,Shongrong W(1997)The composition characteristics of low molecular weight organic acids in soil and their roles on soil material cycling.Plant Nutr Fertil Sci 3(4):363-371 (in Chinese with English abstract)
Almaroai YA,Usman ARA,Ahmad M,Kimd K,Vithanageae M,Oka YS(2013)Role of chelating agents on release kinetics of metals and their uptake by maize from chromated copper arsenate-contaminated soil.Environ Technol 34:747-755
An CJ,Huang GH,Wei J,Yu H(2011)Effect of short-chain organic acids on the enhanced desorption of phenanthrene by rhamnolipidbiosurfactantinsoil-waterenvironment.WaterRes 45:5501-5510
Atalay YB,Carbonaro RF,Di Toro DM(2009)Distribution of proton dissociationconstantsformodelhumicandfulvicacid molecules.Environ Sci Technol 43(10):3626-3631
Backes CA,Pulford ID,Duncan HJ(1985)Neutralization of acidity in colliery spoil possessing pH-dependent change.Reclam Reveg Res 4:145-153
Caiying N,Guangming T,Yongming L,Yingxu C(2004)Influences of organic compounds and nitric acid solutions on release of copper,zinc and lead from a mixed metal-polluted agricultural soil.Acta Pedol Sin 41(2):237-244(in Chinese with English abstract)
Changyuan T,Pan W,Xiuzhen T(2009)The basin acidification affected by AMD:a case study in Xingren county,Guizhou,China.Carsol Sin 28(2):135-143
Clı´stenes Williams AN,Dula A,Xing BS(2006)Comparison of natural organic acids and synthetic chelates at enhancing phytoextraction of metals from a multi-metal contaminated soil. Environ Pollut 140:114-123
Debela F,Arocena JM,Thring RW,Whitcombe T(2010)Organic acid-induced release of lead from pyromorphite and its relevance toreclamationofPb-contaminatedsoils.Chemosphere 80(4):450-456
Egli M,Sartori G,Mirabella A,Giaccai D(2010)The effects of exposure and climate on the weathering of late Pleistocene and Holocene Alpine soils.Geomorphology 114(3):466-482
Ettler V,Vrtisˇkova´R,MihaljevicˇM,Sˇebekb O,Grygarc T,Drahota P (2009)Cadmium,lead and zinc leaching from smelter fly ash in simple organic acids-simulators of rhizospheric soil solutions. J Hazard Mater 170:1264-1268
Fox TR,Comerford NB,McFee WW (1990)Phosphorus and aluminum release from a spodic horizon mediated by organic acids.Soil Sci Soc Am J 54:1763-1769
Guoyong H,Qingling F,Jun Z,Tianying W,Hongqing H(2014)Effects of low molecular weight organic acids on speciation of exogenous Cu in an acid soil.Environ Sci 35(8):3091-3095(in Chinese with English abstract)
Haibo L,Fang L,Jian Z,Xiaoyan Z,Silin C(2012)Available heavy metal concentrations in dominant species rhizospheres of natural vegetations in coal gangue dumps.Chin J Ecol 31(12):3207 -3212(in Chinese with English abstract)
Hailin Y,Bohan L(2010)Extraction condition for heavy metals from contaminated soil by using low molecular organic acids.J Agro-Environ Sci 29(12):2330-2337(in Chinese with English abstract)
Heikkinen PM,Ra¨isa¨nen ML,Johnson RH (2009)Geochemical characterisation of seepage and drainage water quality from two sulphide mine tailings impoundments:acid mine drainage versus neutral mine drainage.Mine Water Environ 28:30-49
Huan L,Yunguo L,Guangming Z,Jieli X,Bohong Z,Xiaofei T,Dafei W,Zhichao S,Jian N,Zhengjiang J,Chao G,Wei L,Shufan W (2015)Mitigation mechanism of Cd-contaminated soils by different levels of exogenous low-molecular-weight organicacidsandPhytolaccaamericana.RSCAdv 5(56):45502-45509
Jinfeng S,Xiaoyang C,Yong L(2004)Determination of organic acids in forest litters by a capillary gas chromatography.Sci Silvae Sini 40(4):185-188(in Chinese with English abstract)
Jinfeng S,Xiaoyang C,Zhengquan W (2011)Effects of low molecular-weight organic acids P,Fe and K availablity of dark brown forest soils and absorption of forest seedlings.J Soil Water Conserv 25(1):123-127
Jiewen Y,Laiyuan Z,Liming L(2015)Influence of aqueous Fe(III)on release of Mn(II)from low molecular weight organic acid promoted dissolution of an Oxisol and γ-MnO2.Environ Earth Sci 74(2):1625-1632
Jones DL(1998)Organic acids in the rhizosphere-a critical review. Plant Soil 205:25-44
Jones DL,Darrah PR(1994)Role of root derived organic acids in the mobilization of nutrients from the rhizosphere.Plant Soil 66:247-257
Kang K,Shin HS,Park H(2002)Characterization of humic substances present in landfill leachates with different landfill ages and its implications.Water Res 36(16):4023-4032
Kinniburgh DG,Milne CJ,Benedetti MF,Pinheiro JP,Filius J,Koopal LK,Van Riemsdijk WH(1996)Metal ion binding by humic acid:application of the NICA-Donnan Model.Environ Sci Technol 30(5):1687-1698
Ko¨gel-Knabner I,Amelung W,Cao Z,Fiedler S,Frenzel P,Jahn R,Kalbitz K,Ko¨lbl A,Schloter M (2010)Biogeochemistry of paddy soils.Geoderma 157(1-2):1-14
Lee SS,Nagy KL,Park C,Fenter P(2011)Heavy metal sorption at the muscovite(001)-fulvic acid interface.Environ Sci Technol 45(22):9574-9581
Lin G,Teresa JC(2015)Remediation of AMD contaminated soil by two types of reeds.Int J Phytorem 17(4):391-403
Lo´pez-Bucio J,Nieto-Jacobo MF,Ramı´rez-Rodrı´guez V (2000)Organic acid metabolism in plants:from adaptive physiology to tansgenic varieties for cultivation in extreme soils.Plant Sci 160:1-13
Mastrocicco M,Prommer H,Pasti L,Palpacelli S,Colombani N (2011)Evaluation of saline tracer performance during electrical conductivitygroundwatermonitoring.JContamHydrol 123(3-4):157-166
Navarro Flores A,Martı´nez Sola F(2010)Evaluation of metal attenuation from mine tailings in SE Spain(Sierra Almagrera):a soil-leaching column study.Mine Water Environ 29:53-67
Novikova SP,Gas'kova OL(2013)Influence of natural fulvic acids on the solubility of sulfide ores(experimental study).Russ Geol Geophys 54(5):509-517
Ololade IA,Oladoja NA,Alomaja F,Ololade OO,Olaseni EO,Oloye FF,Adelagun ROA(2015)Influence of organic carbon and metal oxide phases on sorption of 2,4,6-trichlorobenzoic acid under oxic and anoxic conditions.Environ Monit Assess 187(1):4170
Pan W,Changyuan T,Chongqiang L,Lijun Z,Tingquan P,Lijuan F (2009)Geochemical distribution and removal of As,Fe,Mn and Al in a surface water system affected by acid mine drainage at a coalfield in Southwestern China.Environ Geol 57:1457-1467
Rengel Z,Marschner P(2005)Nutrient availability and management in the rhizosphere:exploiting genotypic differences.New Phytol 168:305-312
Smuda J,Dold B,Spangenberg JE,Friese K,Kobek MR,Bustos CA,Pfeifer H(2014)Element cycling during the transition from alkaline to acidic environment in an active porphyry copper tailings impoundment,Chuquicamata,Chile.J Geochem Explor 140:23-40
Tani M,Higashi T,Nageztsuka S(1995)Low molecular weight aliphatic acids in some andisols of Japan.In:Huang PM,Berthelin J,Bollag JM,McGill WB,Page AL(eds)Envionmental impact of soil componnent interaction.Lewis,Chelsea
Vaneˇk A,Koma´rek M,Chrastny´V,Galusˇkova´I,MihaljevicˇM,Sˇebek O,Drahota P,Tejnecky´V,Vokurkova´P(2012)Effect of low-molecular-weight organic acids on the leaching of thallium and accompanying cations from soil-A model rhizosphere solution approach.J Geochem Explor 112:212-217
Vesely´T,TlustosˇP,Sza´kova´J(2012)Organic acid enhanced soil risk element(Cd,Pb and Zn)leaching and secondary bioconcentration in water lettuce(Pistia Stratiotes L.)in the rhizofiltration process.Int J Phytorem 14(4):335-349
Wei W,Fang L,Yangzhou X(2009)The vegetation investigation and analysis of coal mine abandons in maiping of Huaxi District. J Guizhou Univ 26(2):132-135(in Chinese with English abstract)
Xi F,Dalun T,Wenhua X,Baoyu C(2004)Absorption,accumulation and dynamic of heavy metal elements in Pinus massoniana plantation in guangxi.Guihaia 24(5):437-442(in Chinese with English abstract)
Xiangfei M (2015)Study on variation of Fe and Mn content in fluctuation zone of groundwater level:An example in Shenyang Huangjia water source.Jilin University,Jilin
Yanyu W,Shaoqi Z,Xiuya Y,Dongyu C,Ke Z,Fanghui Q(2011)Transformation of pollutants in landfill leachate treated by a combined sequence batch reactor,coagulation,Fenton oxidation and biological aerated filter technology.Process Saf Environ Prot 89(2):112-120
Yingqun M,Quan W,Yanwen Q,Binhui Z,Lei Z,Yanming Z(2013)Migration chracteristic of heavy metals in soil around Hongtoushan copper mining area in Liaoning Province.Environ Pollut Control 09:1-8(in Chinese with English abstract)
Yongzhen D,Zhi'an L,Bi Z(2005)Low molecular weight organic acids and their ecological roles in soil.Soils 37(3):243-250(in Chinese with English abstract)
Yuanchun Y,Jian Y,Li F,Defeng J(2007)Organic Acids Exudation from the Roots of Cunninghamia lanceolata and Pinus massoniana Seedlings Under Low Phosphorus Stress.J Nanjing For Univ(Natural Sciences Edition)31(2):9-12(in Chinese with English abstract)Yunlong M,Qingru Z,Hao H,Jie P,Xiaoyan L,Bohan L(2008)Effect of low molecular weight organic acids on desorptions of Pb,Cd,Cu and Zn from soils.Chin J Soil Sci 39(6):1419-1423 (in Chinese with English abstract)
Zhi D,Chongqiang L,Zhong L(2001)Experimental simulation of chemical activity of heavy metals in coal gangue.J South China Univ Technol(Natural Science Edition).29(12):1-5(in Chinese with English abstract)
Zhuhong D,Daqiang Y,Xin H,Xi W,Liangyan X(2008)Extraction of heavymetalsandmineralelementsinagriculturalsoilsaroundmine area using biodegradable and non-biodegradable chelator.J Agro-EnvironSci27(5):1774-1778(inChinese withEnglish abstract)
19 October 2015/Revised:27 November 2015/Accepted:25 February 2016/Published online:26 March 2016
✉ Fang Liu lfang6435@163.com
1College of Resources and Environmental Engineering,Guizhou University,Guiyang 550025,China
2College of Agriculture,Guizhou University,Guiyang 550025,China
杂志排行
Acta Geochimica的其它文章
- Initiation and evolution of the South China Sea:an overview
- Nuclear field shift effects on stable isotope fractionation:a review
- Characteristics and distributions of atmospheric mercury emitted from anthropogenic sources in Guiyang,southwestern China
- The effects of soil sand contents on characteristics of humic acids along soil profiles
- Solubilization of potassium containing minerals by high temperature resistant Streptomyces sp.isolated
- A hydrochemical study of the Hammam Righa geothermal waters in north-central Algeria
