Solubilization of potassium containing minerals by high temperature resistant Streptomyces sp.isolated
2016-08-26fromearthwormgut
from earthworm's gut
DianFeng Liu1,2,3·Bin Lian1,3·Bin Wang4
Solubilization of potassium containing minerals by high temperature resistant Streptomyces sp.isolated
from earthworm's gut
DianFeng Liu1,2,3·Bin Lian1,3·Bin Wang4
©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2016
A potassium solubilizing bacterial strain designated EGT,which is tolerant of high temperature,was isolated from an earthworm's gut to obtain a bacterium that can weather potassium-bearing rock effectively through solid-state fermentation.Molecular phylogeny and 16S rRNA gene sequence analysis demonstrated the bacterial strain was a member of the Streptomyces genus.To assess its potential to release potassium from silicate minerals,this strain was used to degrade potassium-bearing rock powder by solid-state fermentation.After fermentation,the amount of water-soluble Al,Fe and K of the substrate with active inoculum was higher than those of the control,which had autoclaved inoculum,and those of the fresh substrate.The result indicated that the strain had the ability to weather potassium-bearing rock and could be used as an inoculum in the production of potassium bio-fertilizer,due to its potassium release activity from rock and tolerance to high temperature.
Weathering·Potassium solubilizing bacteria· Streptomyces
1 Introduction
Potassium(K),an element essential for plant growth,plays an essential role for enzyme activation,protein synthesis and photosynthesis(Armengaud et al.2009;Basak and Biswas 2009;Lin 2010).It is one of the three main macronutrients,together with Nitrogen(N)and Phosphorus(P),which are needed for crop growth and crop productionincrease(AmtmannandArmengaud2007;Sugumaran and Janarthanam 2007;Chen et al.2008). Potassium in soil is typically divided into four forms:water-soluble(solution K),exchangeable,non-exchangeable and structural or mineral forms(Jalali 2006;Basak and Biswas 2009).Potassium from the water-soluble pool is directly available for plant uptake.Water-soluble K is readily replenished by the soil exchangeable K,which is held by negatively charged clay minerals and organic matter in soils.Some non-exchangeable K can become exchangeable when the water-soluble and exchangeable K are depleted by plant removal,leaching,or exchange reactions with other cations.Mineral K,which is the major proportion of total K in soils,can only become available very slowly through long-term soil weathering(Peterburgsky and Yanishevsky 1961;Basak and Biswas 2009;Ghiri et al.2010).Total reserves of K in soil are generally large,but more than 98%of them exists in the form of silicate minerals(microcline,muscovite,orthoclase biotite,feldspars,etc.)and can't be directly absorbed by plants (Sugumaran and Janarthanam 2007).However,crops need large amounts of potassium during growth.A large amount ofpotassiumfertilizermustbeaddedintosoiltoadequately supply crops with the potassium needed for sustaining crop production.
Many countries,such as China,India and Brazil,are important agricultural countries and need large amounts of fertilizers every year,yet they are deficient in potassium fertilizerresources(Sunetal.2009;BasakandBiswas2010). However,thesecountriesarefortunatetoberichinlow-grade potassium-bearing rock(PBR)(Chen 2000;Basak and Biswas2009).Ifthesematerialscanbeconvertedintopotassium fertilizer by some suitable chemical or biological means,the lack of potassium fertilizer resources would be greatly eased inthoseagriculturalcountries.Someresearchershaveproved thatcompostingtechnologycaneffectivelyconvert unavailable K into plant available form because of the acidic environment prevailing during composting,and the product of mineral K compost can increase crop yields(Nishanth and Biswas 2008;Biswas 2010).
The natural weathering rates of minerals are very low. Some microorganisms must be added into the compost to degrade potassium-bearing minerals and quickly release enough K.It has been found that many microorganisms can enhance the weathering of potassium-bearing minerals,however most of them cannot survive high temperature.The temperatureinsidecompostisgenerallyhigh,oftentimesmore than50°Cduringcomposting.Therefore,somemicrobesthat are tolerant to high temperatures are necessary if weathering potassium-bearing rocks through composting technology.
Earthworms have been described as ecosystem engineers due to their key role in altering the biological activity and physical structure of soils(Liu et al.2011;Zangerle´ et al.2011).The role of earthworms in promoting the weathering of potassium-bearing minerals have been previously reported,and earthworms'gut microbes may play an important role in increasing the rates of mineral weathering(Basker et al.1994;Carpenter et al.2007,2008;Liu et al.2011).Therefore,earthworms'guts are potential resources from which some potassium solubilizing microorganisms could be isolated.In this study,a potassium solubilizing bacterium,which tolerated high temperature,was isolated from the gut of the earthworms after they fed on potassium-bearing rock powder for 10 days.Subsequently,this strain was used to degrade potassium-bearing rock powder by solid-state fermentation to examine its weathering ability.
2 Materials and methods
2.1Preparation of K-bearing minerals,earthworms and other materials
Potassium-bearing rock resource is rich in Luoyang,Henan Province,China.To develop and utilize the resource,we collected some PBR from Luoyang.The rock was crushed and sieved to pass through a 0.074 mm mesh.The mineral composition and the elemental composition of the rock were determined using the K-value method of X-ray diffraction(Rigaku,D/MAx-2200)(Li et al.2003)and X-ray Fluorescence(Axios,PW4400),respectively,in the Institute of Geochemistry,Chinese Academy of Sciences. Therock'smineralcompositioncontainsfeldspar (70.99%),quartz(23.45%),hematite(3.62%),montmorillonite(1.66%),illite(0.15%)and hornblende(0.13%). The major element oxides of the PBR are K2O(6.36%),Al2O3(11.52%),SiO2(70.36%),Fe2O3(0.53%),CaO (0.38%),MgO(0.06%),Na2O(2.53%)and Loss on ignition(LOI,8.02%).
The earthworms(Eisenia foetida)were purchased from Henan Academy of Agricultural Sciences.Oyster mushroom culture waste was collected from Henan Institute of Science and Technology.Wheat bran and sugar were purchased from the market.
2.2Isolation of K-solubilizing bacteria
Seven hundred grams of K-bearing rock powder,mixed with 200 mL of deionized water,was autoclaved at 121°C for 30 min.After cooling,35 earthworms were placed into the mineral powder and fed for 10 days.The feeding containers were covered to avoid light;the laboratory temperature was about 25°C during feeding.The earthworms were sedated,surface sterilized with ethanol(75%)and dissected under sterile conditions after feeding on mineral powder for 10 days.The whole gut content of each specimen without gut wall was extracted with a sterile spatula and transferred into sterile 2 mL tubes.The gut content was immersed with sterile distilled water,and shaken at 200 rpm for 10 min at 28°C to detach the bacteria from the mineral particles.The liquid mixture was allowed to stand for 20 min to obtain the microbe-containing supernatant to isolate the mineral-solubilizing bacteria.The medium for isolating mineral-solubilizing bacteriawasAleksandrov'smedium(sucrose5.0 g,MgSO40.5 g,CaCO30.1 g,Na2HPO4·12H2O2.0 g,FeCl3·6H2O 0.005 g,potassium-bearing rock powders 1.0 g,agar 18.0 g,H2O 1.0 L,pH 7.0-7.2)(Li et al. 2008),a common medium used to isolate silicate-weathering bacteria.The supernatant was serially diluted,and the aliquots of each dilution were spread on plates of Aleksanderov's medium and incubated at 28°C for 2-3 days. Selected colonies were further purified using the same medium.The colonies were stored at 4°C until further use.
The above microbial strains were inoculated to Aleksanderov's medium and incubated at 50°C for 2 days.The colonies,which grew well at 50°C,were selected and stored onslantsforstudyingthesolubilityofK-bearingrockpowder.
2.3Morphology and biochemical characterization of the strain
The mycelia of the isolates,identified as species belonging to the genus Streptomyces by analyzing their morphological characteristics and by biochemical tests,were observed by using the cover slip method,in which sterile square cover slips were inserted at an angle of 45°in sterile Aleksandrov's agar medium in petri dishes.Individual cultures would grow to the intersection of the medium and cover slips.The cover slips were removed after three days of incubation,air-dried and observed with a microscope. Morphological characters were photographed(Thakur et al. 2007).
Biochemical tests,including Gram staining,nitrate reduction,amylohydrolysis,Voges-Proskauer reaction,and catalase activities,were performed according to the method we used in a previous study(Liu et al.2012).
2.4DNA extraction,amplification and sequencing
Cultures for DNA extraction were prepared by inoculating 100 mL of yeast extract medium(YEM)with 100 μL of spore suspension.The YEM medium[sucrose 10.0 g,yeast extract 0.2 g,(NH4)2SO40.5 g,CaCO31.0 g,MgSO40.5122 g,KCl 0.1 g,Na2HPO4·12H2O 2.507 g,H2O 1.0 L,pH 7.0-7.2]was prepared as previously described (Zhou et al.2010).After incubating at 37°C for 48 h on a rotary shaker at the rate of 180 rpm,the culture was transferred to a centrifuge tube(2 mL),and centrifuged at 10,000×g for 10 min to concentrate cells into a pellet for extraction of bacterial genomic DNA.DNA was extracted using a Bacterial Genomic DNA Extraction Kit(Omega,US)according to the manufacturer's instructions.
The 16S rRNA gene was amplified by polymerase chain reaction(PCR)using bacterial universal primers 16S-fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′)and 16S-rD1(5′-ACGGTTACCTTGTTACGACTT-3′)(Weisburgetal. 1991).The reaction mixture for PCR amplification was prepared as follows:0.4 mM of deoxynucleoside triphosphates,0.4 μM of each primer,1×PCR buffer,2 mM magnesiumchloride,1UofTaqDNApolymerase,and1 μL (about 5-15 ng)of template DNA in a final volume of 30 μL.Amplification was made using a touchdown procedure,as follows:the annealing temperature was set at 65°C and decreased by 1°C every cycle until reaching a‘‘touchdown''at 55°C.The amplification program consisted of 5 min at 94°C,and 10 touchdown cycles of denaturation at 94°C for 1 min,annealing at 65°C(with the temperature decreasing 1°C each cycle)for 1 min,and extension at 72°C for 2 min,followed by 25 cycles of 94°C for 1 min,55°C for 1 min,and 72°C for 2 min.During the last cycle,the length of the extension step was increased to 10 min. After amplification,the PCR product was checked by electrophoresis in 1.5%(w/v)agarose gels.
The PCR product was excised from 2%low melting point agarose(Sigma,St-Louis,MO)and the DNA was purified using a Gel Isolation Kit following the manufacturer's instructions(Promega,Madison,WI,USA).The purified PCR product was sequenced by Shanghai Sangon Biological Engineering Technology&Services Co.,Ltd.
2.5Phylogenetic analysis
The obtained 16S rRNA gene sequence was compared to those in the National Center for Biotechnology Information database using the BLAST procedure(Altschul et al. 1997).The reference sequences with the highest similarities to the query were retrieved from GenBank.The target 16S rRNA gene sequence and the retrieved reference sequences were aligned with the ClustalX program(Larkin et al.2007)with parameters set to its default values. Alignments were improved by comparison to the secondary structures and any regions of uncertain alignment were omitted from the subsequent analyses.
The best-fit model of nucleotide substitution for the data sets was selected using the program PAUP 4.0b10(Swofford 2002)and Modeltest 3.06(Posada and Crandall 1998). Base composition and sequence variability were examined using the software package MEGA4.0(Tamura et al. 2007).A minimum evolution(ME)phylogenetic tree with 1000 bootstrap was then constructed using the Tamura-Nei model,with the pairwise deletion of gaps by Mega 4.0 package.
The outgroup used for further phylogenetic analysis was based on the above result of ME.Further phylogenetic analysis,conducted using PAUP 4.0b10 with ME method,was performed using heuristic searches with the random stepwise addition of 10 replicates and tree bisection-reconnection(TBR)rearrangements in effect.The optimal model of nucleotide substitution for the ME analyses,HKY85,was from the result of the best-fit nucleotide substitution model selection.Bootstrap analyses were performed for 1000 replicates to evaluate the robustness of each clade.
2.6Mineral weathering by the bacterial isolate
The isolate was revitalized on plates of Aleksanderov's medium and incubated at 37°C for 3-4 days to obtain its spores.The spores and aerial mycelia were scraped off the agar surface,using a sterilized inoculation loop,when a mass of gray spores appeared on the plates.The spore suspension was then prepared in sterile H2O.
The solid-state medium for the assessment of the effect of bacteria on mineral weathering was prepared as follows:the waste of the oyster mushroom culture(66%)was grounded,dried and mixed with rock powder(16%),wheat bran(16%)and sugar cane dregs(2%).Water was added into the mixture;the ratio of water to mixture was 1.1:1(v/m).The mixed substrate(about 1.5 kg wet wt)was poured into heat resistant polypropylene bags and sterilized(substrate temperature 121°C)for 30 min.
After the substrate had cooled,it was inoculated with 500 mL of spore suspension(108spore mL-1).Then,the substrate was incubated at 37°C for 14 days.The control,whose substrate components were the same with the above treatment,was inoculated with autoclaved inoculum,and also incubated at 37°C for 14 days.Each treatment was carried out on three biological replicates.The substrates,incubated for 14 days,were sampled to determine their water-soluble elements'content.
2.7Determination of water-soluble K,Al,Fe and Ca
The samples for determining elemental concentration were prepared by drying them in an oven at 70°C.To assess the isolate's potential for enhancing mineral weathering,concentrations of water-soluble K,Al,Fe and Ca of the three samples,including the fresh solid-state medium,the substrate inoculated with autoclaved inoculum and the one inoculated with living bacterium,were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES,Optima 2100 DV,Perkin Elmer,USA).
Determination of the concentration of water-soluble K,Al,Fe and Ca was performed using the methods as previously described(Liu et al.2011)with the following modifications:five grams of a dried sample was mixed with 50 mL of ddH2O in a 250 mL flask followed by 30 min of vigorous shaking.After shaking,the samples were centrifuged at 8000 rpm for 10 min.The supernatants were filtered through a 0.45 μm filter paper to obtain a purified extract.The concentrations of water-soluble K,Al,Fe and Ca in the purified extract were determined by ICP-AES.
3 Results
3.1Isolation of mineral potassium-solubilizing bacteria tolerant to high temperature
Afterisolationandrepeatedpurification,sevenfast-growing microbial strains were obtained from the Alexander silicate medium at 28°C.These strains were inoculated into the Alexander silicate medium and cultivated in an incubator at 50°C.One strain was found growing in the medium and forming a strain colony;this strain was labeled‘EGT'. Strain EGT grew well in the solid Alexander silicate medium at 28 and 37°C.At 50°C,however,the rate of growth was reduced and the strain colonies were small.EGT's colonies appeared to be white,dry and intensely woolly. After a while,gray spores appeared on the surface of the colonies(see Fig.1).Furthermore,white mycelium pellets formed when EGT was put into a liquid medium at a constant temperature of 28/37°C for 28 h with agitation at 180 r/min.The Strain EGT was Gram-positive.
The results of the measurements,including nitrate reduction,amylohydrolysis and catalase activities,were all positive;The Voges-Proskauer reaction,however,produced a negative result.The results of morphological observations were given in Fig.1.After the strain grew on for 3 days,a mass of gray spores appeared on the aerial hyphae.Straight spore chains could be clearly observed with an optical microscope.
3.2Identification and phylogeny of strain EGT
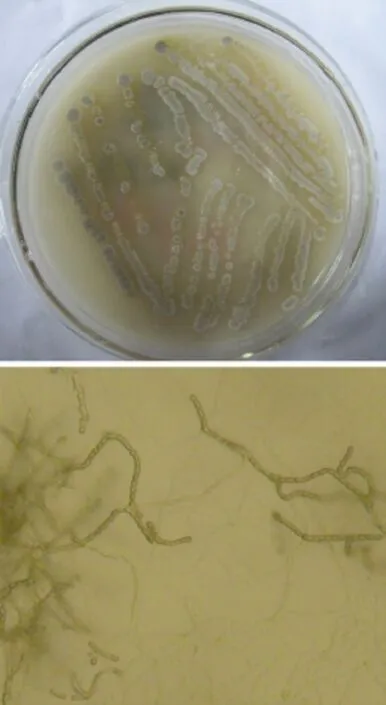
Fig.1 Colonies of the strain EGT and optical micrograph(×400)of its mycelium.The top figure showed the colonies of the strain EGT grown on solid Aleksanderov's medium at 28°C for three days.Gray spore had been formed.The bottom figure showed the morphological character of the strain EGT's mycelium observed with an optical microscope
The 16S rRNA gene sequence of Strain EGT derived from clones has been submitted to GenBank(accession number JF701918).Thegenesequencelengthof16rRNAis1381 bp,among which A occupies 22.3% (308 bp),T 18.0% (249 bp),G 33.8%(467 bp),C 25.9%(357 bp),A+T 40.3%(557 bp)and C+G 59.7%(824 bp).The four base group percentages are similar to those in the 16S rRNA gene sequences of other Streptomyces in the GenBank.
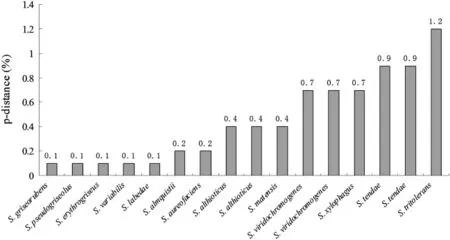
Fig.2 The divergences of 16S rRNA gene sequences between the strain EGT and some Streptomyces species
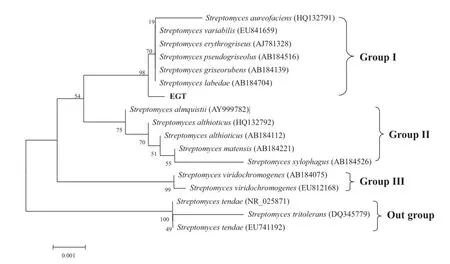
Fig.3 The minimum evolution tree for Tamura-Nei distance matrix of the nearly complete 16S rRNA gene sequences of the strain EGT and some Streptomyces species using the software package MEGA4.0.Numbers on nodes correspond to percentage bootstrap values for 1000 replicates
The 16S rRNAgenesequencewas analyzedby BLAST in thesequencebaseofGenBank.Theresultsshowthatthemost similar16S rRNAgenesequencesallbelongtoStreptomyces variants,indicating that EGT is one of the Streptomyces.The differences between the EGT and the thirteen 16S rRNAgenesequencesofStreptomycesareshowninFig.2.Itcanbe seen that the sequence difference is negligible.In particular,the divergence from S.griseorubens,S.labedae,S.erythrogriseus,S.variabilis and S.pseudogriseolus is only 0.1%,which is within the criterion 16S rRNA≤3%proposed for agreement(Embley and Stackebrandt 1994).
Multiple alignments using Clustal X,suggest that the strain's 16S rRNA gene sequence is 1384 bp(including gap),amongwhichA occupies 22.3%,T 18.2%,C 25.8%,G 33.7%,A+T 40.5%and C+G 56.5%.We also observethattheconcentrationofC+Gishigherthanthatof A+T,the average sequence divergence is 0.5%(p-distance),andtheaveragevalueofthetransformation/transversion ratio is 1.273.We note that the disparity between the base groups greatly affects the reconstruction of the phylogeny(Lockhart et al.1994).The results reveal that there are differences in the base groups;these differences must be considered when the phylogeny is analyzed.In addition,when the value of transformation/transversion is less than 2.0,the mutation of the gene sequence is in a saturation state and is easily affected by evolutionary noise. Consequently,errors may occur if extra weights are not added when reconstructing phylogeny(Knight and Mindell 1993;Liu and Jiang 2005).The average value of transformation/transversionintheexperimentis1.273andthishasto beaccountedforduringthephylogenyanalysis.Theresultof a hierarchical likelihood test performed using Model test shows that the best model in the phylogeny analysis is the Hasegawa-Kishino-Yano(HKY)model.This model considers both the differences in the base groups and the transformation/transversion ratio,and is thus suitable for phylogeny analysis of the current data group.
Figure 3 shows the ME phylogeny based on the Tamura-Nei model derived using MEGA 4.0 software. Four branches are given:the first branch includes S. aureofaciens,S.variabilis,S.erythrogriseus,S.pseudogriseolus,S.griseorubens,S.labedae and the strain EGT;the second branch includes S.xylophagus,S.althioticus,S. matensis and S.almquistii;the third includes S.viridochromogenes;and the fourth S.tendae and S.tritolerans. Figure 3 reveals that these latter,fourth-branch strains are the sister group of the other bacteria.Thus,these two strains have been used as outgroups when the phylogeny was reconstructed using PAUP 4.0 software.
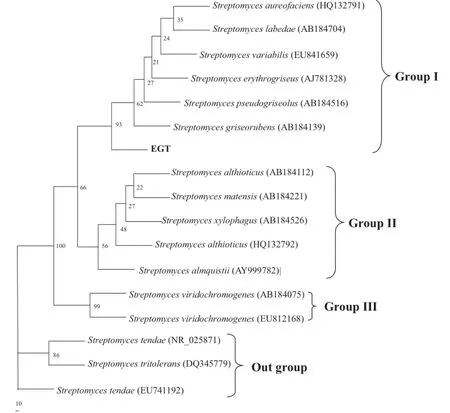
Fig.4 The minimum evolution tree for HKY85 distance matrix based on nearly complete 16S rRNA gene sequences of the strain EGT and some Streptomyces species using the program PAUP 4.0b10. Numbers on nodes correspond to percentage bootstrap values for 1000 replicates
The ME phylogeny based on the HKY85 model was established using PAUP 4.0 software(seen in Fig.4).Thetopological features of the phylogeny in this figure are similar to those in Fig.3.In addition to the outgroups,the ingroups of the phylogenetic tree also have three branches that are similar to those in Fig.3.Furthermore,the strain EGT also belongs to the first branch here.These two phylogenetictreesverifythatEGTisoneofthe Streptomyces.
3.3Effects of strain EGT on weathering of PBR
After the PBR powders were degraded by EGT through solid-state fermentation,the changes in the aqueous concentrations of major elements were tested to assess the isolate's potential for enhancing mineral weathering.The results are shown in Fig.5 and indicate that the proportions ofwater-solubleAl,FeandKinthesubstrateincubatedwith live inoculum for 14 days were higher than those obtained from the control with autoclaved inoculum and the fresh substrate.Theamounts ofaqueousAl,FeandKintheactive bacterial culture were 23.08%,123.19%and 30.99%,respectively,higher than that of the fresh substrate and were 45.45%,52.48%and9.13%,respectively,higherthanthat of the abiotic control incubated with autoclaved inoculum. The results reveal that EGT has a significant effect on the weathering of potassium-bearing mineral powders.
4 Discussion
4.1Phylogeny and molecular identification of strain EGT
Streptomyces is the main genus of the actinomycete microorganism.It is the most prevalent among the actinomycetes in soil,and contributes to the soil ecology and circulation of materials(Oskay 2009;Srivibool et al. 2010).Streptomyces have a diverse range of species and metabolism,and are important microbial resource for humans.At present,the number of Streptomyces granted legal names exceeds 600(Ka¨mpfer et al.2008).Since the 1940s,some 55%of the physiologically active substances from 12,000 microorganisms have been produced by Streptomyces(Xu et al.2001).
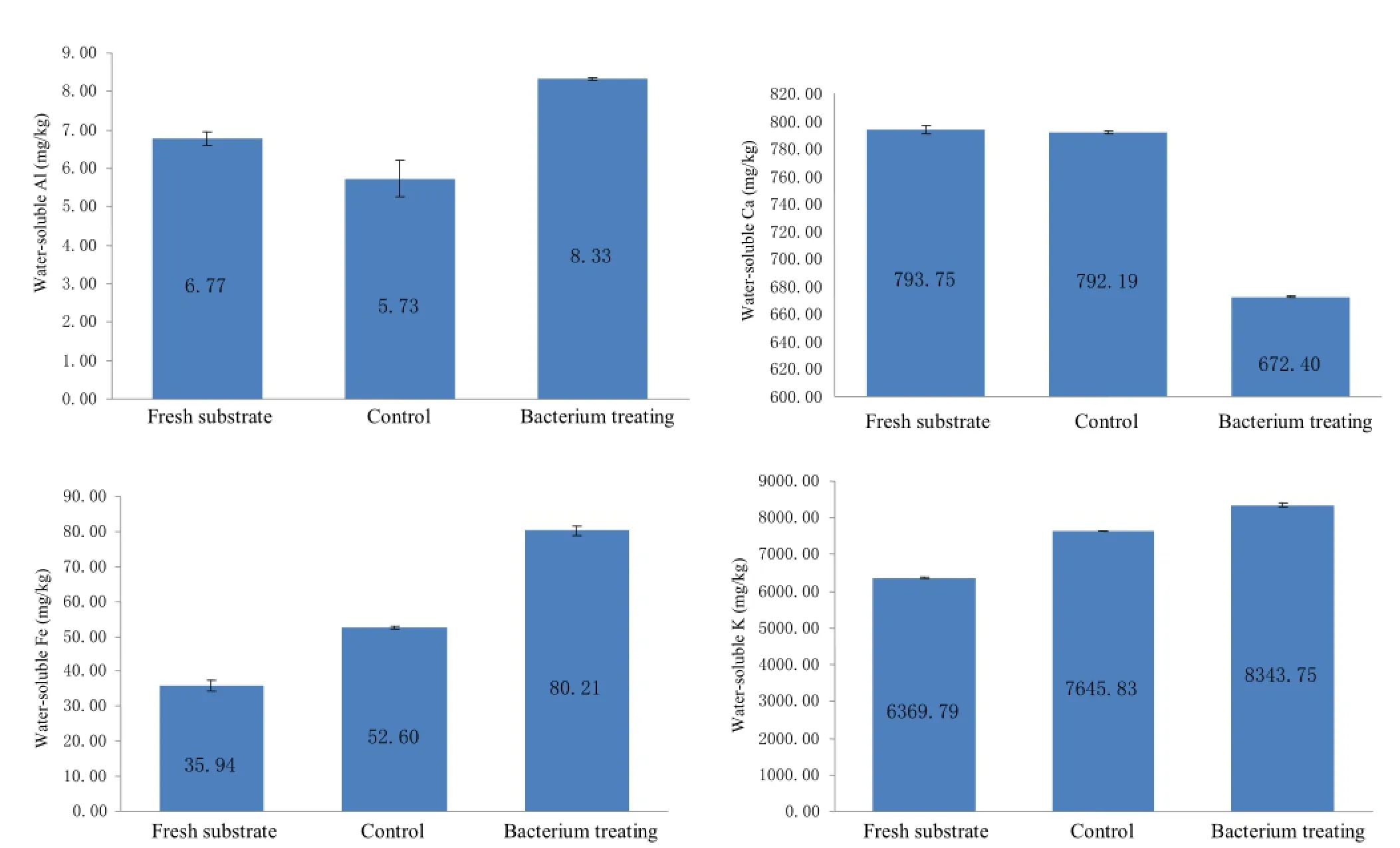
Fig.5 Amount of water-soluble elements released by the strain EGT after incubating for 14 days through solid-state fermentation(Each value represented the average of three biological replicates).Fresh substrates were the solid-state medium before composting.Controls were incubated for fourteen days by autoclaved inoculum.Error bars are±SD(n=3)
Considering its 16S rRNA gene sequence and molecular phylogeny,the strain EGT obtained from the earthworm intestinaltractcanbeidentifiedasaspeciesofStreptomyces.The identification and classification of the microbial strain underliesitsapplication.However,allofthesystemsusedto identify bacteria,regardless of whether they are phenotypic or genotypic,have their limitations,because no single test methodologyprovidesresultsthatare100%accurate(Janda and Abbott 2002).The main approach used for the identificationandclassificationofprokaryoticspeciesisbasedonits 16S rRNA gene sequence and phylogenetic analysis.Bergey's classification system,widely accepted by microbiologists and currently considered as the best approximation to an official classification,is based on the homologous and phylogenetic analysis of 16S rRNA genes and the classical microscopic and biochemical observation of the relatedness of the organisms(Clarridge 2004).Strains whose similarity to the 16S rRNA gene sequence are more than 95%can be classified into a genus(Clarridge 2004),and the strains whose similarity are more than 97%can be classified into a species(Embley and Stackebrandt 1994).
Despite the EGT being confirmed as one of the Streptomyces by its phylogenetic position and similarity to the 16S rRNA gene sequence with other Streptomyces,the species name of this strain cannot currently be ascertained.The maximum sequence divergence of the 16S rRNA gene between EGT and the thirteen Streptomyces downloaded from GenBank is just 1.2%,among which the sequence divergence between EGT and the five bacteria,including S. griseorubens,S.pseudogriseolus.S.erythrogriseus,S.variabilis and S.labedae,is only 0.1%.This suggests a general lack of suitability for Streptomyces.The strains can however be classified into a species when their 16S rRNA gene has a similarity greater than 97%.This is generally used as a criterion for most prokaryotic species.It seems the 16S rRNA gene does not have sufficient resolution to differentiate the genus and the species(Ka¨mpfer et al.2008).Due to the complexity of the Streptomyces classification and a lack of acknowledged standard,the conception of species for this genus in the systematics has always been a difficult problem (Syed et al.2007).To unequivocally identify the EGT strain,extensive phenotypic and genotypic testing is required.
4.2Effect of EGT on the weathering of PBR
Microorganisms play an important role in the process of mineral weathering.They promote the formation of soil and they offer plants nutrients such as phosphorus,potassium and silicon(Banfield et al.1999;Kim et al.2004;Hall et al.2005).For instance,potassium-solubilizing bacteria can release potassium from minerals,promote the growth of plants,improve harvests,strengthen the anti-virus ability of plants and improve soil quality(Sheng 2005a;Basak and Biswas 2009,2010;Youssef et al.2010).It has a wide application in agriculture.
Many microorganisms have been found to possess this potassium-releasing ability,e.g.Pseudomonas,Burkholderia,Acidithiobacillus ferrooxidans,Bacillus mucilaginosus,B.edaphicus and B.circulans,etc.(Lian et al.2002;Sheng 2005b;Zhaoetal.2008).However,therearestill limitations to the use of these bacteria as microbial fertilizers.First,microbial fertilizers are usually manufactured using composting at a high temperature,often above 50°C.As these bacteria'snormallivingtemperatureisfarlowerthan50°C,they may die at the high temperatures used.In addition to this,althoughsomebacteria,suchasBacillusmucilaginosus,have good potassium-releasing capability,and they also release excessive amounts of polysaccharide during the fermentation,whichmakesthefermentationbrothverythick and viscid.This makes it difficult to produce the required liquid inoculum for microbiological fertilizers that use the liquid state deep-seated fermentation method.
In contrast,the strain EGT has many advantages.Firstly,it can successfully weather potassium-bearing mineral powders and it can resist high temperatures and survive at 50°C.Secondly,procedures for seed preparation are simple due to the large number of spores yielded by the EGT after the desired incubating time.Therefore,EGT is a good choice for use in microorganism fertilizers.
AcknowledgmentsThisworkwassupportedbytheNationalNatural Science Foundation of China(41173091,U1204405),Aid Project for theLeadingYoungTeachersinHenanProvincialInstitutionsofHigher Education of China(2012GGJS-284),and Natural Science Foundation of Henan Educational Committee,China(12B180027,14B180010).
References
Altschul SF,Madden TL,Scha¨ffer AA,Zhang J,Zhang Z,Miller W,Lipman DJ(1997)Gapped BLAST and PSI-BLAST:a new generation of protein database search programs.Nucleic Acids Res 25:3389-3402
Amtmann A,Armengaud P(2007)The role of calcium sensorinteracting protein kinases in plant adaptation to potassiumdeficiency:new answers to old questions.Cell Res 17:483-485
Armengaud P,Sulpice R,Miller AJ,Stitt M,Amtmann A,Gibon Y (2009)Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogenassimilationinarabidopsisroots.PlantPhysiol 150:772-785.doi:10.1104/pp.108.133629
Banfield JF,Barker WW,Welch SA,Taunton A(1999)Biological impact on mineral dissolution:application of the lichen model to understanding mineral weathering in the rhizosphere.Proc Natl Acad Sci USA 96:3404-3411
Basak BB,Biswas DR(2009)Influence of potassium solubilizing microorganism (Bacillus mucilaginosus)and waste mica on potassium uptake dynamics by sudan grass(Sorghum vulgare Pers.)grown under two Alfisols.Plant Soil 317:235-255
Basak BB,Biswas DR(2010)Co-inoculation of potassium solubilizing and nitrogen fixing bacteria on solubilization of waste mica and their effect on growth promotion and nutrient acquisition by a forage crop.Biol Fert Soils 46:641-648. doi:10.1007/s00374-010-0456-x
Basker A,Kirkman JH,Macgregor AN(1994)Changes in potassium availability and other soil properties due to soil ingestion by earthworms.Biol Fert Soils 17:154-158
Biswas DR(2010)Nutrient recycling potential of rock phosphate and waste mica enriched compost on crop productivity and changes in soil fertility under potato-soybean cropping sequence in an Inceptisol of Indo-Gangetic Plains of India.Nutr Cycl Agroecosyst,pp.1-16
Carpenter D,Hodson ME,Eggleton P,Kirk C(2007)Earthworm induced mineral weathering:preliminary results.Eur J Soil Biol 43:S176-S183
Carpenter D,Hodson ME,Eggleton P,Kirk C(2008)The role of earthworm communities in soil mineral weathering:a field experiment.Mineral Mag 72:33-36
Chen J(2000)Development and utilization of potassium-bearing rock resource and their prospects.Geol Chem Miner 22:58-64
Chen HC,Wang SY,Chen MJ(2008)Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and cultureindependent methods.Food Microbiol 25:492-501
Clarridge JE(2004)Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases.Clin Microbiol Rev 17(4):840-862
Embley TM,Stackebrandt E(1994)The molecular phylogeny and systematicsoftheActinomycetes.AnnuRevMicrobiol 48:257-289
Ghiri MN,Abtahi A,Jaberian F,Owliaie HR(2010)Relationship between soil potassium forms and mineralogy in highly calcareous soils of southern Iran.Aust J Basic Appl Sci 4:434-441
Hall K,Arocena JM,Boelhouwers J,Liping Z(2005)The influence of aspect on the biological weathering of granites:observations fromtheKunlunMountains, China.Geomorphology 67:171-188.doi:10.1016/j.geomorph.2004.09.027
Jalali M(2006)Kinetics of non-exchangeable potassium release and availability in some calcareous soils of western Iran.Geoderma 135:63-71.doi:10.1016/j.geoderma.2005.11.006
Janda JM,Abbott SL(2002)Bacterial identification for publication:when is enough enough?J Clin Microbiol 40:1887-1891.doi:10. 1128/jcm.40.6.1887-1891.2002
Ka¨mpfer P,Huber B,Buczolits S,Thummes K,Gru¨n-Wollny I,Busse HJ(2008)Streptomyces specialis sp.nov.Int J Syst Evol Microbiol 58(11):2602-2606
Kim J,Dong H,Seabaugh J,Newell SW,Eberl DD(2004)Role of microbes in the smectite-to-illite reaction.Science 303:830-832. doi:10.1126/science.1093245
Knight A,Mindell DP(1993)Substitution bias,weighting of DNA sequence evolution,and the phylogenetic position of Fea's viper. Syst Biol 42(1):18-31
Larkin MA et al(2007)Clustal W and Clustal X version 2.0. Bioinformatics23:2947-2948.doi:10.1093/bioinformatics/ btm404
Li YH,Xu FG,Wang J,Song ZK(2003)Quantitative analysis is of ettringite in cement hydration products by‘‘value K''method of XRD.Chin J Spectrosc Lab 20:334-337
Li ZG,Luo YM,Teng Y(2008)Research method of soil and environmental microbiology.Science Press,Beijing
Lian B,Fu PQ,Mo DM,Liu CQ(2002)A comprehensive review of the mechanism of potassium releasing by silicate bacteria.Acta Mineral Sin 22:179-183
Lin YH(2010)Effects of potassium behaviour in soils on crop absorption.Afr J Biotechnol 9:4638-4643
Liu DF,Jiang GF(2005)Molecular phylogenetic analysis of Acridoidea based on 18S rDNA with a discussion on its taxonomic system.Acta Entomol Sin 48(2):232-241
Liu D,Lian B,Wang B,Jiang G(2011)Degradation of potassium rock by earthworms and responses of bacterial communities in its gut and surrounding substrates after being fed with mineral. PLoS One 6:e28803.doi:10.1371/journal.pone.0028803
Liu D,Lian B,Dong H(2012)Isolation of Paenibacillus sp.and assessment of its potential for enhancing mineral weathering. GeomicrobiolJ29:413-421.doi:10.1080/01490451.2011. 576602
Nishanth D,Biswas DR(2008)Kinetics of phosphorus and potassium release from rock phosphate and waste mica enriched compost and their effect on yield and nutrient uptake by wheat(Triticum aestivum).Bioresour Technol 99:3342-3353
Oskay M (2009)Comparison of Streptomyces diversity between agricultural and non-agricultural soils by using various culture media.Sci Res Essay 4:997-1005
Peterburgsky AV,Yanishevsky FV(1961)Transformation of forms of potassium in soil during long-term potassium fertilization. Plant Soil 15:199-210.doi:10.1007/bf01400454
Posada D,Crandall KA(1998)MODELTEST:testing the model of DNA substitution.Bioinformatics 14:817-818
Sheng XF(2005)Growth promotion and increased potassium uptake of cotton and rape by a potassium releasing strain of Bacillus edaphicus.Soil Biol Biochem 37:1918-1922
Srivibool R,Jaidee K,Sukchotiratana M,Tokuyama S,Pathom-aree W (2010)Taxonomic characterization of Streptomyces strain CH54-4 isolated from mangrove sediment.Ann Microbiol 60:299-305.doi:10.1007/s13213-010-0041-4
Sugumaran P,Janarthanam B(2007)Solubilization of potassium containing minerals by bacteria and their effect on plant growth. World J Agric Sci 3:350-355
Sun AW,Zhang WF,Du F,Gao LW,Zhang FS,Chen XP(2009)China's development strategy on potash resources and fertilizer. Mod Chem Ind 29(9):10-16
Swofford DL(2002)PAUP*phylogenetic analysis using parsimony (*and other methods),Version 410b10.Sinauer Associates,Sunderland
Syed DG,Agasar D,Kim CJ,Li WJ,Lee JC,Park DJ,Xu LH,Tian XP,Jiang CL(2007)Streptomyces tritolerans sp.nov.,a novel actinomycete isolated from soil in Karnataka,India.Antonie van Leeuwenhoek 92(4):391-397
Tamura K,Dudley J,Nei M,Kumar S(2007)MEGA4:molecular evolutionary genetics analysis(MEGA)software version 4.0. Mol Biol Evol 24:1596-1599
Thakur D,Yadav A,Gogoi BK,Bora TC(2007)Isolation and screening of Streptomyces in soil of protected forest areas from the states of Assam and Tripura,India,for antimicrobial metabolites.J Mycol Me´d 17:242-249
Weisburg WG,Barns SM,Pelletier DA,Lane DJ(1991)16S Ribosomal DNA amplification for phylogenetic study.J Bacteriol 173:697-703
Xu P,Zhang LP,Yu LY(2001)Classification of Streptomyces with theV-2variableregionin16SrDNA.BiodiversSci 9(2):129-137
Youssef GH,Seddik WMA,Osman MA(2010)Efficiency of natural minerals in presence of different nitrogen forms and potassium dissolving bacteria on peanut and sesame yields.J Am Sci 6:647-660
Zangerle´A,Pando A,Lavelle P(2011)Do earthworms and roots cooperate to build soil macroaggregates?A microcosm experiment.Geoderma 167-168:303-309.doi:10.1016/j.geoderma. 2011.09.004
Zhao F,Sheng XF,Huang Z,He LY(2008)Isolation of mineral potassium-solubilizing bacterial strains from agricultural soils in Shandong Province.Biodiver Sci 16:593-600
Zhou XY,Du Y,Lian B(2010)Effect of different culture conditions on carbonic anhydrase from Bacillus mucilaginosus inducing calciumcarbonatecrystalformation.ActaMicrobiolSin 50:956-962
13 November 2015/Revised:15 January 2016/Accepted:29 March 2016/Published online:9 April 2016
✉ Bin Lian bin2368@vip.163.com;hn_ldf@126.com
1Jiangsu Key Laboratory for Microbes and Functional Genomics,Jiangsu Engineering and Technology Research Center for Microbiology,College of Life Sciences,Nanjing Normal University,No.1,Wenyuan Road,Nanjing 210023,People's Republic of China
2Department of Bioengineering,Puyang Vocational& Technical Institute,Puyang 457000,People's Republic of China
3State Key Laboratory of Environmental Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences,Guiyang 550081,People's Republic of China
4Department of Bio-Engineering,Henan Institute of Science and Technology,Xinxiang 453003,People's Republic of China
杂志排行
Acta Geochimica的其它文章
- Effects of organic acids on dissolution of Fe and Mn from weathering coal gangue
- Photosynthetic capability and Fe,Mn,Cu,and Zn contents in two Moraceae species under different phosphorus levels
- Geochemistry of Tertiary sandstones from southwest Sarawak,Malaysia:implications for provenance and tectonic setting
- Effect of pH on binding of pyrene to hydrophobic fractions of dissolved organic matter(DOM)isolated from lake water
- A hydrochemical study of the Hammam Righa geothermal waters in north-central Algeria
- The effects of soil sand contents on characteristics of humic acids along soil profiles