Effect of pH on binding of pyrene to hydrophobic fractions of dissolved organic matter(DOM)isolated from lake water
2016-08-26YiMeiYingchenBaiLiyingWang
Yi Mei·Yingchen Bai·Liying Wang
Effect of pH on binding of pyrene to hydrophobic fractions of dissolved organic matter(DOM)isolated from lake water
Yi Mei1·Yingchen Bai2·Liying Wang1
©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2016
In order to better understand the compositional and structural complexity of dissolved organic matter (DOM)macromolecules and provide mechanistic information on the binding of hydrophobic organic contaminants(HOCs)to DOM,we fractionated large amounts of lake water into three hydrophobic DOM-fractions.The variation of the partitioning coefficients(KDOC)of pyrene at different pH levels was examined by florescence quenching titration.Results show that,relative to the more polar acidic DOM-fractions,the hydrophobic neutral fraction exhibits a higher sorption ability to pyrene.Generally,the sorption of pyrene to the three hydrophobic fractions is strongly pH-dependent.The KDOCvalues of pyrene generally increase with decreasing pH levels,which is especially obvious in the sorption of pyrene to the fulvic acid fractions,suggesting that the binding is controlled by hydrophobic interactions.The mechanisms underlying the binding of pyrene to the hydrophobic fractions were also discussed.Our data are beneficial to further understanding the binding of HOCs to DOM and how it has been affected,which may result in more accurate predictions of KDOC.
1 Introduction
Dissolved organic matter(DOM)is ubiquitous in the environment and has been reported to influence the fate and behavior of hydrophobic organic contaminants(HOCs)such as polycyclic aromatic hydrocarbon(PAH)(Gauthier et al.1997;Cox et al.2007;Perre et al.2014).Since DOM is a complex mixture composed of aromatic aliphatic hydrocarbon structures,it is arduous to confirm its chemical structure and reactivity.On the other hand,DOM is commonly characterized by fractionating it into secondary categories based on hydrophobic-hydrophilic characteristics with resin sorbents(Leeheer 1981).According to Leenheer's fractionation protocol,DOM can be isolated into hydrophobic fractions and hydrophilic fractions;furthermore,these two fractions can be further fractionated into so-called‘‘acid'',‘‘base''and‘‘neutral''fractions.This fractionation procedure facilitates the understanding of these fractions with different physical-chemical characteristics and provides mechanistic information on the binding of HOCs to DOM (Mei et al.2009a;Maoz and Chefetz 2010).Ilani et al.(2005)studied the interaction of hydrophobic fractions fractionated from wastewater with triazine herbicide and three PAHs(phenanthrene,fluoranthene and pyrene),and stated that the content of the hydrophobic fractions is more important to the effect of the mobility and transport process of HOCs than to the total organic carbon(TOC)concentration.In our previous studies(Mei et al.2009a),we investigated the binding of three PAHs(anthracene,phenanthrene and perylene)to three hydrophobic and three hydrophilic fractions,determining the partitioning coefficients(KDOC)of the three PAHs between the DOM-fractions and water by fluorescence quenching titration.Our data revealed that the hydrophobic fractions with higher aromaticity and lowerpolarity possessed a higher binding ability than that of the hydrophilic fractions.Other investigators obtained similar observations when studying the interaction of HOCs with DOM fractions(Maoz and Chefetz 2010;Xi et al.2012). All above show the significance of the hydrophobic fractions in the sorption of HOCs.
Since different DOM fractions possess different polarity and acid-base properties,solution chemistry such as pH may have varying effects on the sorption of HOCs to the DOM-fractions.Previous studies showed that the changes of pH lead to DOM conformation change and consequently affected its interaction with hydrophobic organic pollutants (Jones and Tiller 1999;Marschner et al.2005;Pan et al. 2008;Mei et al.2009b).However,inconsistent results were reported.With regard to the relation of pH to the KDOCvalues for HOCs,both positive and negative relationships have been observed(Schlautman and Morgan 1993;Jones and Tiller 1999;Akkanen and Kukkonen 2001;Marschner et al.2005).In our previous work(Mei et al.2009b),we studied the effects of pH and ion strength on DOM conformation by 3D fluorescence spectroscopy and fluorescence polarization technique;in this study,we further investigated the effects of pH on the interaction between pyrene and hydrophobic fractions.The objectives of this study are to(1)study the influence of pH on the interactive ability of hydrophobic fractions with pyrene;and(2)correlate the pH and adsorptive properties of the DOM-fractions.
2 Materials and methods
2.1Regents and chemicals
Pyrene was purchased from Sigma-Aldrich(99.9%pure,St.Louis,USA)and used without further purification. Concentrated pyrene stock solution was obtained by dissolvingthechemicalinmethanol(HPLCgrade,Mallinckrodt,USA).All other chemicals and solvents used are better than analytical grade.Freeze-dried DOM-fractions were dissolved in Milli-Q water(18.2 MΩ cm,Millipore)as concentrated stock,and the solution pH was adjusted by using 0.1 mol/L HCl and 0.1 mol/L NaOH.
2.2Sample collection and fractionation
Lake water sample was collected from the Hongfeng Lake where is located in Guizhou Province,China.A total volume of 1000 L surface water was collected in August 2010,and the water samples were filtered through a pre-combusted(450°C for 5 h)Whatman GF/F glass fiber filters for further fractionation.DOM fractions were isolated, based on the literature(Leeheer 1981;Chefetz et al.1998),using XAD-8/4 resin(Supelco Bellefonte,PA,USA). Detailed fractionation procedures were reported in our previous work(Mei et al.2009a).Briefly,we adjusted the pH of the filtered original water to 2 with HCl,and it flowed through the XAD-8/XAD-4 resin columns at a rate of 4 L/h.The hydrophobic acids(HOAs),which were composed of humic acid(HA)and fulvic acid(FA),adsorbed onto XAD-8 resin were eluted with 0.1 mol/L NaOH.Then,the HOAs were further acidified to pH 1 with HCl and they settled for 24 h to precipitate HA.The suspension was recycled through the XAD-8 resin and then eluted with 0.1 mol/L NaOH.The elution is the FA fraction.Then the XAD-8 resin was air dried and Soxhletextracted with methanol.The fraction contained in the methanol solution is the hydrophobic neutral fraction (HON).All fractions were concentrated by rotary evaporation at 35°C,and then were purified with 100 Da membrane dialysis and freeze-dried to a low-ash solid form.
2.3Dissolved organic carbon and molecular weights mea suremen ts
The dissolved organic carbon(DOC)of the hydrophobic fractions were determined using a high temperature catalytic method with a TOC/N IIanalyzer(Elementar,Germany).The relative precision of the DOC analysis was <3%,as obtained by repeated measurement.Molecular weights were obtained using high performancesize exclusion chromatography(HPSEC,Agilent 1100,PE)with a UV detector at 254 nm and an YMC-60 column(Waters,Milford,MA).The flow rate of the mobile phase composed ofthephosphatebuffer(0.001 mol/LNa2HPO4,0.001 mol/L NaH2PO4and 0.03 mol/L NaCl)was set at 0.5 ml/min.The DOM-fractions were dissolved in the phosphate buffer with the same composition as the HPSEC mobile phase.The solutions were then filtered through a per-combusted Whatman GF/F glass fiber filter.The number(Mn),weight-averaged(Mw)molecular weights,and polydispersity(ρ)were determined based on the method proposed by Chin et al.(1994).
2.4Absorbance and fluorescence measurements
Absorbance was obtained using a Shimadzu UV-3000 double Beam spectrophotometer with 1 cm quartz cell to determine the molar absorptivity at 280 nm(ε280)and to correct the inner filter effects(IFE)when conducting a fluorescence quenching experiment.Fluorescence intensities were collected on a Hitachi F-4600 spectrofluorometer (Hitachi,Japan)with a cuvette magnetic stirring systemcontaining a Teflon micro stir bar.The photomultiplier voltage was set at 700 V;excitation and emission bandwidth were set at 5 and 10 nm,respectively.The florescenceintensitiesofpyrenewerecorrespondingly determined at the excitation/emission wavelengths of 271/374 nm.The KDOCvalues were determined by a fluorescence quenching titration based on the Stern-Volmer equation(Gauthier et al.1997).The fluorescence quenching of the PAHs by the DOM have proven to be static quenching mechanisms based on diffusion,temperature studies and the fluorescence efficiency experiment(Tranina et al.1990;Chen et al.1994).Five dilutions of the fractions were prepared,after adjustment,to the corresponding pH with 0.1 mol/L HCl and 0.1 mol/L NaOH;then,all samples were bubbled for 5 min with pure Argon in the dark to reach anoxic conditions.The Raman peak of distilled water was collected at 348/397 nm to check the instrument stability during the experiment.A 3-mL aliquot of each fraction of dilution was pipetted into the cuvette.Then,an absorption scan from 250 to 470 nm was conducted.The cuvette was then moved to the Hitachi F-4600 to collect the background fluorescence of the fractions,which was subtracted from the spectra of next solution.An aliquot of pyrene stock solution was spiked into the cuvette to a final concentration of 2 μg,according to its solubility and fluorescence intensity;then,the cuvette was stirred for 3 min using a Teflon micro stir bar and allowed to settle for another 2 min before the fluorescence measurement.After initial spiking,the fluorescence intensity of all samples was collected for three times at 5,7,and 9 min,and the final data was an average of the three measurements.
All the fluorescence intensity values were corrected for primary and secondary IFE based on the following equation(Gauthier et al.1997):
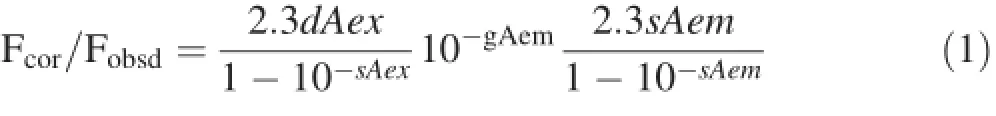
where Fcoris the corrected fluorescence intensity,Fobsdis the observed fluorescence intensity,Aexis the absorbance at the excitation wavelength,Aemis the absorbance at the emission wavelength,and d,g,and s are the fluorescence beam width(1 cm),distance from the edge of the sample beam to the edge of the cuvette(0.3 cm),and excitation beam thickness(0.2 cm),respectively.The binding of pyrene to the fractions was described by the Stern-Volmer equation(Gauthier et al.1997):where F0and F are the fluorescence intensity of pyrene in the absence and presence of DOM-fractions,respectively,and KDOCand[DOM]are the partitioning coefficients of pyrene and the concentration of the DOM-fractions,respectively.

3 Results and discussion
3.1DOM-fractions characterization
DOM is composed of a mixture of low-molecular weight compounds and chemically heterogeneous macromolecules (Leenheer and Croue´2003).Fractionation of the DOM is necessary to better understand the contribution of the individual fraction to its total sorption properties.
Table 1 shows the results of elemental analysis of the hydrophobic fractions.The lake-derived water in this study contained 77%hydrophobic fractions(based on DOC analysis),which is consistent with the results obtained by other investigators(Polubesova et al.2007).Elemental analysis exhibited that the hydrophobic fractions possess a lower C/H ratio,suggesting their distinct aliphatic property.Relative to the HA and FA fraction,the HON fraction contains a high content of carbon and lower content of oxygen.With respect to atomic ratios,HON demonstrates a lower C/H ratio and a high C/O ratio,indicating its lower polarity and higher hydrophobicity relative to the acidic fractions(i.e.,HA and FA).Since the HA and FA fraction are characterized by a higher oxygen content and lower C/O ratio,and the carboxyl group may be the dominant polar functional group in the acidic fractions.
3.2Partitioning coefficients of the hydrophobic fractions
The Stern-Volmer plots for the sorption of pyrene to FA,HA and HON were curved towards the y-axis and not all plots were linear(Fig.1).Nonlinear sorption isotherms of pyrene binding to the DOM have been reported previously (Laor and Rebhun 2002;Borisover et al.2006;Polubesova et al.2007).It is well known that the binding of HOC to aquatic DOM is usually ascribed to a non-specific partitioning mechanism,and the sorption isotherm is linear in this case.Laor and Rebhun(2002)argued that the nonlinearity of pyrene isotherms resulted from the combination of non-specific(partitioning)and specific(adsorption)binding mechanisms.Since the three fractions contain both polar and non-polar functional groups,the polar interactions,such as the π-π electron donor-acceptor interaction (EDA)and H-bonding,may greatly contribute to the nonlinearity of sorption isotherms.
Table 2 shows the logKDOCvalues of pyrene binding to the three hydrophobic fractions at pH 7.Our data are well consistent with the reported results(Wijnja et al.2004;Marschner et al.2005;Polubesova et al.2007).Increasingly,the sorption ability of HON for pyrene was higher than that of HA and FA,with logKDOCvalues of 4.61,4.49,and 4.25 for HON,HA and FA,respectively.It is wellknown that the binding of neutral PAHs molecules to DOM is governed by hydrophobic interactions,which is the combination of dipole-induced dipole interactions(i.e.,dispersion forces)and the thermodynamic gradient driving force.The magnitude of inducing a dipole moment is related to the polarizability of the molecules,which can be estimated by the sum of their bond polarizability.Therefore,an increase in the number of conjugated double bonds (-HC=CH-)will increase the molecular polarizability and thereby increase the magnitude of the dispersion forces.As a result,an increase in the aromaticity of DOM will increase the polarizability of the molecules,and accordingly increase its binding ability to the PAHs(Gauthier et al.1997;Peuravuori 2001).The atomic C/H ratios,molecular weights of DOM and UV absorbance at 280 nm (ε280),were thought to be surrogate for the aromaticity of DOM(Gauthier et al.1997;Tanaka et al.1997;Peuravuori 2001).However,our data was somewhat inconsistent with the aforementioned conclusions.Although the HON fraction possesses a lower atomic C/H ratio,its molecular weight and ε280are lower than those of the HA fraction. Besides higher aromaticity(lower C/H ratio),the higher sorption ability of HON to pyrene may result from its lower polarity.Xing et al.(1994)proposed a polarity index(PI)to represent the magnitude of DOM polarity,and defined FI as the atomic(O+N)/C ratio.Although the HA fraction possesses a higher molecular weight and ε280value,a higher content of polar compounds in HA impede the sorption more pyrene.Our data was well corroborated by the reported observations(Salloum et al.2002;Simpson et al.2003;Gunasekara et al.2003).Salloum et al.(2002)reported an increase in KDOCvalues after removing the polysaccharides of soil organic matter,and speculated that aromatic structures might be physically constrained by polarstructures.Likewise,whencarbohydrateswere selectively removed from humic acids,Simpson et al. (2003)discovered an increase in phenanthrene binding ability.After the similar observations were obtained,Gunasekara et al.(2003)proposed that the mobile sorption domains were free and would become more accessible to HOCs with the removal of rigid structures.Another possible contribution to the higher binding ability of HON may be the EDA interaction.The HON fraction was reported to be rich in methyl and carbonyl groups,which could accept electrons from electron-donor pyrene.
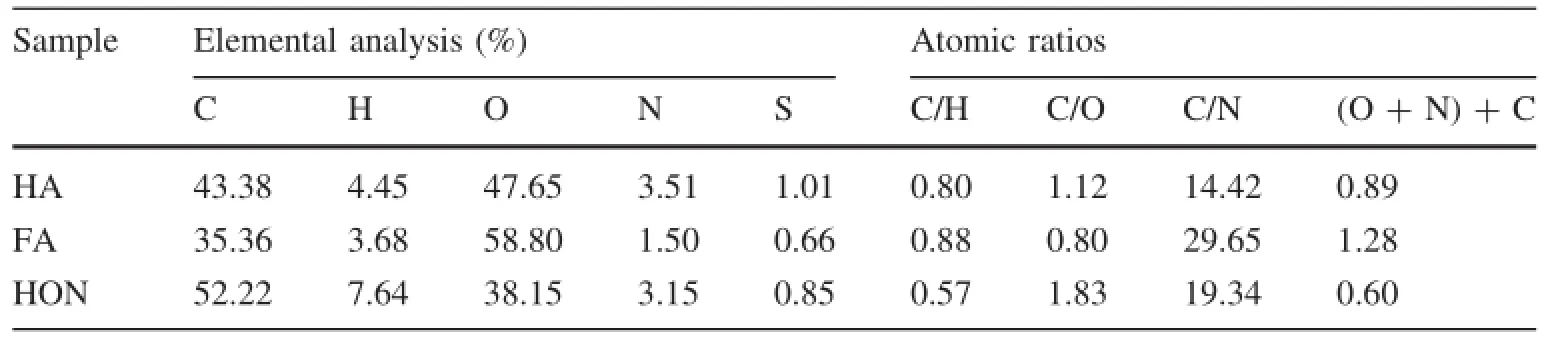
Table 1 Elemental analysis and atomic ratios of the hydrophobic fractions(HA,FA and HON)

Fig.1 Stern-Volmer plots for pyrene binding to the hydrophobic fractions(FA,HA and HON)
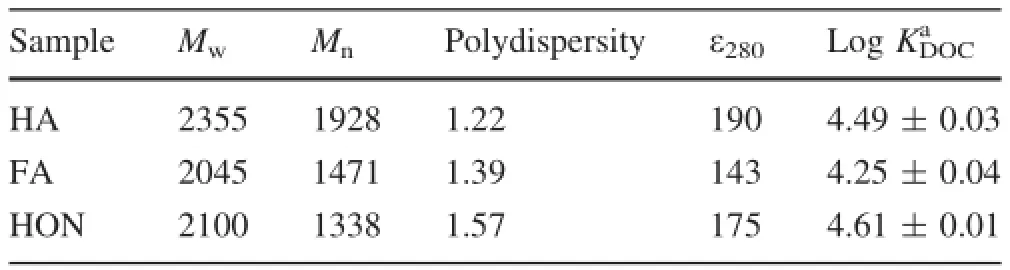
Table 2 Log KDOCvalues of pyrene with hydrophobic fractions,molecular weight and UV at 280 nm(ε280)
3.3Effect of pH on the binding characteristics
Table 3 shows the KDOCvalues of pyrene binding to HA,FA and HON at pH 4,7,and 10.The KDOCvalues of pyrene were higher at pH 4 and lower at pH 10,suggesting the strong pH-dependent sorption property.Since the DOM-fractions contain large amounts of polar and acidic function groups,any changes in pH may affect itsconformation and sorption domains,depending on its physicochemical properties and composition.
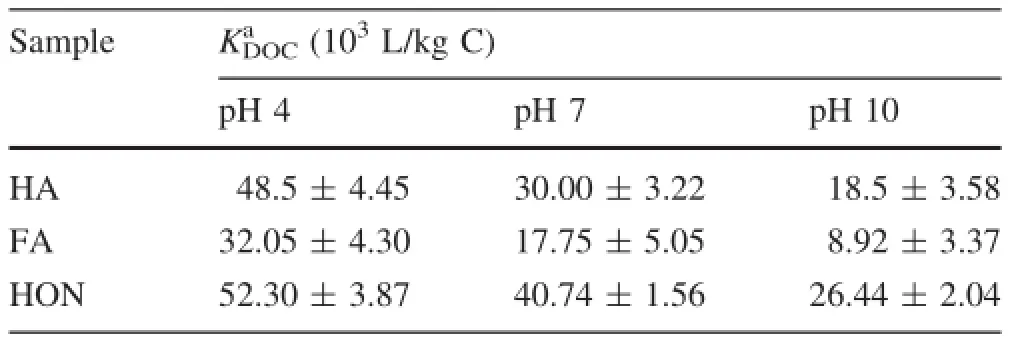
Table 3 Partitioning coefficients(KDOC)of pyrene binding to HA,FA and HON at pH 4,7 and 10
The carboxyl groups of DOM were reported to be increasingly deprotonated above pH 4,resulting in an increase of its polarity and solubility(Carter and Suffet 1982;Marschner et al.2005).Furthermore,the conformation of DOM could be affected by the change of solution pH(Jones and Tiller 1999;Ferreira et al.2002).Humic substances are in an elongated conformation in alkaline solutions due to electrostatic repulsion,but the protonation of carboxyl groups at low pHs facilitates intra-and intermolecular interactions and leads to the formation a more condensedhydrophobicstructure.Panetal.(2008)observed an aggregation of DOM molecules via atomic force microscopy and an increase in zeta potential,with decreasing pH.They suggested that,in this case,electrostatic repulsive force decrease,and the aggregation of DOM molecules formed large hydrophobic domains in which more hydrophobic compounds were adsorbed,as if individual molecules possessed lower apparent molecular sizes due to self-curling.In our previous study(Mei et al. 2009b),we studied the effects of pH on the florescence properties and molecular conformation of two humic acids by the three-dimensional excitation-emission matrix florescence(3DEEM)and steady-state florescence polarization(FP).As pH decreased,we observed a decline in the fluorescence intensity and a blue shift in the florescence maxima.In the case of the blue shift in florescence maxima,the polarity of humic substances declined and the hydrophobic domains of the DOM functional groups were exposed in the solution(Mobed et al.1996).At the same time,decreasing florescence intensities suppressed the ionization of the functional groups.The conformational change of the humic acids was corroborated by florescence polarization:the FP decreased with decreasing pH.This indicated the decreasing of the apparent molecular size,because of the self-curling of individual molecule.In addition to enhancing the hydrophobic interaction resulted from molecular aggregation,the lower pH is beneficial to polar interactions such as H-bonding and EDA interactions. Gu et al.(2007)observed the H-bonding ability of carboxyl groups of DOM declining due to deprotonation with increasing pH.Zhu et al.(2004)stated that lower pH would induce a stronger π-π EDA interaction.Furthermore,hydrogen ions could also act as the promoters for EDA interactions via aromatic moieties or heterocyclic compounds within the DOM molecules.
It is clear that a greater decrease in the KDOCvalues of pyrene binding to FA occurs with increasing pH(Table 3). With regard to the HON fraction,only a slight effect was observed with changing pH from 4 to 10,while the influence of changing pH on the pyrene sorption to HA was lower than that to FA and higher that to HON.The different extent of pH-dependent binding observed among the three DOM-fractions might be ascribed to their respective compositional and structural properties.Relative to the HON fraction,the FA fraction has a much higher polarity,as shown by the(O+N/C)ratio(Table 1),and is more enriched in polar groups.Accordingly,the distinct components of FA become tightly bound via intramolecular H-bonding at lower pHs,leading to more sensitive pH-dependent binding.While HON is less polar with a PI=0.6 and is composed of highly cross-linked,largesized hydrophobic domains,its conformation and variation of hydrophobicity would be less affected by the protonation-deprotonation transition of carboxylic groups.
4 Conclusions
Our data reveals that the sorption of pyrene to lake-derived hydrophobic fractions is strongly correlated to the aromatic domains in DOM macromolecules.Relative to the more polar acidic DOM-fractions,the hydrophobic neutral fraction(HON)exhibits a more efficient sorption ability to pyrene.Generally,the sorption of pyrene to the three hydrophobic fractions is strongly pH-dependent.The partitioning coefficients(KDOC)of pyrene generally increase with decreasing pH,which is especially obvious with the sorption of pyrene to the FA fraction,suggesting the binding is controlled by hydrophobic interactions.Among the three hydrophobic fractions,HON exhibited the highest sorption ability to pyrene,which may result from its lower polarity and stronger EDA ability.The effect of pH on the sorption of pyrene to HON is relatively limited due to its less polar groups;furthermore,HON possesses larger-sized hydrophobic domains whose conformation and hydrophobicity would not be much affected by the protonation-deprotonation transition of the carboxyl groups.As the carboxyl groups of DOM are protonated with decreasing pH(i.e.,increasing H+),the electrostatic repulsion between the DOM molecules decrease and the molecular aggregation happen;furthermore,the aggregation results in the formation of larger hydrophobic domains in which moreHOC are adsorbed,especially those with large hydrophobicity.Our data are beneficial to further understanding the binding of HOCs to DOM and how it has been affected,which may lead to a more accurate prediction of KDOC.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China(No.41173128).
References
Akkanen J,Kukkonen JVK(2001)Effects of water hardness and dissolved organic material on bioavailability of selected organic chemicals.Environ Toxicol Chem 20:2303-2308
Borisover M,Laor Y,Bukhanovsky N,Saadi I(2006)Fluorescence based evidence for adsorptive binding of pyrene to effluent dissolved organic matter.Chemosphere 65:1925-1934
Carter CW,Suffet IH(1982)Binding of DDT to dissolved humic materials.Environ Sci Technol 16:735-740
Chefetz B,Chen Y,Hadar Y(1998)Purification and characterization of laccase from chaetomium thermophilim and its role in humification.Appl Environ Microbiol 64:3175-3179
Chen S,Inskeep WP,Williams SA,Callis P(1994)Fluorescence lifetime measurements of fluoranthene,1-naphthol and napropamide in the presence of dissolved humic acids.Environ Sci Technol 28:1582-1588
Chin YP,Aiken GR,O`Loughlin E (1994)Molecular weight,Polydispersity and spectroscopic properties of aquatic humic substances.Environ Sci Technol 28:1853-1858
Cox L,Velarde P,Cabrera A,Hermosin MC,Cornejo J(2007)Dissolved organic carbon interaction with sorption and leaching of diuron in organic-amended soils.Eur J Soil Sci 58(3):714-721
Ferreira JA,Martin-Neto L,Vaz CMP,Regitano JB(2002)Sorption interactions between imazaquin and a humic acid extracted from a typical Brazilian Oxisol.J Environ Qual 31(5):1665-1670
Gauthier TD,Seitz WR,Grant CL(1997)Effects of structural and compositional variations of dissolved humic materials on pyrene Kocvalues.Environ Sci Technol 21:243-248
Gu C,Karthikeyan KG,Sibley SD,Pederson JA(2007)Complexation of the antibiotic tetracycline with humic acid.Chemosphere 66(8):1494-1501
Gunasekara A,Simpson MJ,Xing B(2003)Identification and characterization of sorption domains in soil organic matter using structurally modified humic acids.Environ Sci Technol 37:852-858
Ilani T,Schulz E,Chefetz B(2005)Interactions of organic compounds with wastewater dissolved organic matter:role of hydrophobic fractions.J Environ Qual 34(2):552-562
JonesKD,TillerCL(1999)Effectofsolutionchemistryontheextentof binding of phenanthrene by a soil humic acid:a comparison of dissolvedandclayboundhumic.EnvironSciTechnol33:580-587
Laor Y,Rebhun M(2002)Evidence for nonlinear binding of PAHs to dissolved humic acids.Environ Sci Technol 36:955-961
Leeheer JA(1981)Comprehensive approach to preparative isolation and fractionation of dissolved organic carbon from natural waters and wastewaters.Environ Sci Technol 15:578-587
Leenheer JA,Croue´JP(2003)Characterizing aquatic dissolved organic matter.Environ Sci Technol 37(1):19A-26A
Maoz A,Chefetz B(2010)Sorption of the pharmaceuticals carbamazepine and naproxen to dissolved organic matter:role of structural fractions.Water Res 44:981-989
Marschner B,Winkler R,Jodemann D(2005)Factors controlling the partitioning of pyrene to dissolved organic matter extracted from different soils.Eur J Soil Sci 56:299-306
Mei Y,Wu FC,Wang LY,Bai YC,Li W,Liao HQ(2009a)Binding characteristics of perylene,phenanthrene and anthracene to different DOM fractions from lake water.J Environ Sci 21:414-423
Mei Y,Wang LY,Wu FC(2009b)Effects of water chemistry and concentration of dissolved organic matter on its florescence characteristics and molecular conformation.Chin J Geochem 28:413-420
Mobed JJ,Hemmingsen SL,Autry JH(1996)Florescence characterization of IHSS humic substances:total luminescence spectra with absorbance correction.Environ Sci Technol 30:3061-3065
Pan B,Ghosh S,Xing BS(2008)Dissolved organic matter conformation and its interaction with pyrene as affected by water chemistry and concentration.Environ Sci Technol 42:1594-1599
Perre CD,Me´nach KL,Ibalot F,Parlanti E,Budzinski H(2014)Development of soild-phase microextraction to study dissolved organic matter-polycyclic aromatic hydrocarbon interactions in aquatic environment.Anal Chem Acta 807:51-60
Peuravuori J(2001)Binding of pyrene on lake aquatic humic matter:the role of structural descriptors.Anal Chim Acta 429:75-89
Polubesova T,Sherman-Nakache M,Chefetz B(2007)Binding of pyrene to hydrophobic fractions of dissolved organic matter:effects of polyvalent metal complexation.Environ Sci Technol 41(15):5389-5394
Salloum MJ,Chefetz B,Hatcher PG(2002)Phenanthrene sorption by aliphatic-rich natural organic matter.Environ Sci Technol 36:1953-1958
Schlautman MA,Morgan JJ(1993)Effects of aquatic chemistry on the binding of polycylic aromatic-hydrocarbons by dissolved humic materials.Environ Sci Technol 27:961-969
Simpson MJ,Chefetz B,Hatcher PG(2003)Phenanthrene sorption to structurally modified humic acids.J Environ Qual 32:1750-1758 Tanaka S,Oba K,Fukushima M,Nakayasu K,Hasebe K(1997)Water solubility enhancement of pyrene in the presence of humic substances.Anal Chim Acta 337:351-357
Tranina SJ,Novak J,Smeck NE(1990)An ultraviolet absorbance method of estimating the percent aromatic carbon content of humic acids.J Environ Qual 19:151-153
Wijnja H,Pignatello JJ,Malekani K (2004)Formation of π-π complexes between phenanthrene and model π-π acceptor humic subunits.J Environ Qual 33:265-275
Xi BD,Geng CM,Yue Z,Wei ZM,He XS(2012)Interaction of phenanthrene with dissolved organic matter and its fractions from leachate of different landfill ages.Environ Earth Sci 67:1861-1867
Xing B,McGill WB,Dudas MJ(1994)Cross-correlation of polarity curves to predict partition coefficients of nonionic organic contaminants.Environ Sci Technol 28:1929-1933
Zhu QD,Hyun S,Pignatello JJ(2004)Evidence of π-π electron donor-acceptor interactions between π-donor aromatic compounds and π-acceptor sites in soil organic matter through pH effects on sorption.Environ Sci Technol 38:4361-4368
2 December 2015/Revised:31 December 2015/Accepted:25 January 2016/Published online:10 February 2016
✉ Yi Mei meiyi@mail.gyig.ac.cn
1State Key Laboratory of Environmental Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences,Guiyang 550081,China
2State Environmental Protection Key Laboratory for Lake Pollution Control,Research Center of Lake Environment,Chinese Research Academy of Environmental Sciences,Beijing 100012,China
杂志排行
Acta Geochimica的其它文章
- Initiation and evolution of the South China Sea:an overview
- Nuclear field shift effects on stable isotope fractionation:a review
- Characteristics and distributions of atmospheric mercury emitted from anthropogenic sources in Guiyang,southwestern China
- The effects of soil sand contents on characteristics of humic acids along soil profiles
- Solubilization of potassium containing minerals by high temperature resistant Streptomyces sp.isolated
- A hydrochemical study of the Hammam Righa geothermal waters in north-central Algeria
