一种三维四元酸锰有机框架材料的合成、结构表征及荧光性能
2016-08-16邵彩云游倩倩龙银双杨立荣
邵彩云,郭 旭,游倩倩,龙银双,高 峰,陈 一,练 晨,杨立荣*
(1.河南省多酸化学重点实验室,河南大学化学化工学院,河南 开封 475004; 2.濮阳市油田第一中学,河南 濮阳 457001)
一种三维四元酸锰有机框架材料的合成、结构表征及荧光性能
邵彩云1,郭旭1,游倩倩2,龙银双1,高峰1,陈一1,练晨1,杨立荣1*
(1.河南省多酸化学重点实验室,河南大学化学化工学院,河南 开封 475004;2.濮阳市油田第一中学,河南 濮阳 457001)
通过水热法合成了一种结构新颖的金属有机框架物{[Mn(ttac)·(bibp)·H2O]·[bibp]·H2O}n(其中H4ttac为4,5-二(3′-羧基苯基)-邻苯二甲酸, bibp为4,4′-联(咪唑基) 联二苯). 运用X射线单晶衍射、元素分析以及红外光谱对其进行了结构表征. 结构测试表明该配合物通过一维链连接成二维层状结构, 相邻的层之间存在游离的bibp分子, 并且与已配位的bibp分子之间存在π…π堆积弱作用力进而构建成三维孔状结构, 且该孔状结构中存在游离的水分子. 此外, 本文还研究了该配合物的荧光性质, 荧光实验结果显示与配体相比, 配合物的荧光发射波长发生蓝移.
金属有机框架物; 水热合成; 结构表征; 荧光性质
Biography: SHAO Caiyun (1969-), female, senior experimentalist.*Corresponding author, E-mail address: lirongyang@henu.edu.cn.
Coordination polymer, a relatively young area, has become a fast-growing and complex subject[1-2]. Synthetic chemists pay attention to this class of materials because of their interesting structures and potential applications in magnetism, optical property, molecule adsorption, drug delivery, catalysis etc[3-9]. Applications drive the development of this type of compounds and a large number of MOFs with diversity of architectures have been synthesized and characterized over the past years[10-11].Apparently, the topological structure of coordination polymers is not only influenced by coordination mode of organic linkers and coordination tendencies of metal cations, but also can be functionalized by the reaction conditions such as material ratio, pH value, temperature and reaction time, etc[12-15]. Usually, multi-dentate ligands like multicarboxylic acids are selected to fabricate extended open frameworks, because their versatile bridging fashions are benefit to construct various multidimensional frameworks. Furthermore, the flexibility of the secondary ligands plays a key role in directing the functionally-oriented complexes[16-18].Flexible multicarboxylic ligands are beneficial to the construction of frameworks with new topology, for example, 1,4-cyclohexane dicarboxylic and 1,3,5-cyclohexane tricarboxylic have been widely used for the design and synthesis of various MOFs[19-20]. Herein our research selected a flexible tetracarboxylic acid, 4,5-di(3′-carboxylphenyl)-phthalic acid (H4ttac), and 4,4′-bis(imidazolyl) biphenyl (bibp) as the auxiliary ligand to construct a 3D porous MOF. H4ttac has been proven to be an excellent candidate for building highly connected frameworks[21]. Furthermore, the introduce of the auxiliary ligand caters to the coordination needs for the metal center, so it’s an effective method for designing multiply networks[22-23]. Based on this synthetic strategy, a novel complex, namely, {[Mn(ttac)·(bibp)·H2O]·[bibp]·H2O}nwas obtained. Moreover, we investigated the FT-IR spectroscopy and luminescent property of the title complex.
1 Experimental
1.1Reagents and general techniques
All the materials and reagents were used from commercial channels without further purification. Elemental analysis (C, H and N) was measured with a Perkin-Elmer 2400-II CHNS/O analyzer. The IR spectra was collected on a Bruker VERTEX 70 IR spectrometer with KBr pellets ranging from 4 000 to 500 cm-1. Excitation and emission spectra were obtained with a HITACHI F-7000 fluorescence spectrophotometer at the ambient temperature. The structure of complex was settled by direct methods and further refined by full-matrix least-squares fitting onF2using the SHELXL-97 software, and an absorption correction was applied using the SADABS program.
1.2Synthesis of the title complex
A mixture of manganese perchlorate, 4,5-di(3′-carboxylphenyl) phthalic acid (H4ttac) and 4,4′-bis(imidazolyl) biphenyl (bibp) in a molar ratio of 1∶1∶1 (0.075 mmol∶0.075 mmol∶0.075 mmol) was dissolved in 8 mL of distilled water. The resulted mixture was fully stirred at room temperature for 30 min, transferred into 25 mL Teflon-lined stainless steel autoclave under autogenous pressure and kept at 125 ℃ for 3 d, then cooled down to ambient temperature at a rate of 5 ℃/h. The products were harvested and dried in air, and yellowish transparent block crystals suitable for X-ray diffraction analysis were received. Anal. Calc. for C49H36MnN6O10(%): C 63.81, H 4.12, N 9.23, O 17.51; found: C 63.71, H 3.93, N 9.10, O 17.32.
2 Results and discussion
2.1Structural description of the complex
Crystallographic data and details of diffraction experiments for the complex are given in Table 1. Single-crystal X-ray structure analysis reveals that the complex crystallizes in the triclinic crystal system of theP-1 space group. The center Mn(II) is surrounded by two nitrogen atoms of bibp, one oxygen atom of water molecule and three oxygen atoms from ttac4-group, as shown in Fig.1a. The Mn-O bond lengths vary in the range of 0.212 0(2)-0.227 9(2) nm, and the Mn-N distances are between 0.222 0(3) and 0.223 6(3) nm (as listed in Table 2), which are in the normal range. Two adjacent Mn(II) cations are ligated by two oxygen atoms from two ttac4-groups forming a 1D infinite chain (Fig.1b).Meanwhile, two Mn(II) centers are linked through bibp ligands end to end generating a 1D infinite chain from another direction(Fig.1c), based on which to constitute an infinite 2D layer (Fig. 1d). Single-crystal X-ray confirms that guest water and bibp molecules exist between two adjacent layers, as illustrated in Fig.1e.Owing to the π…π stacking interactions between the guest bibp molecules and the bibp ligands on the 2D layers, (with distance of 0.330 6 nm), the adjacent layers parallel to each other are further inter-connected into a stacked multilayer 3D supramolecular network[24-26].
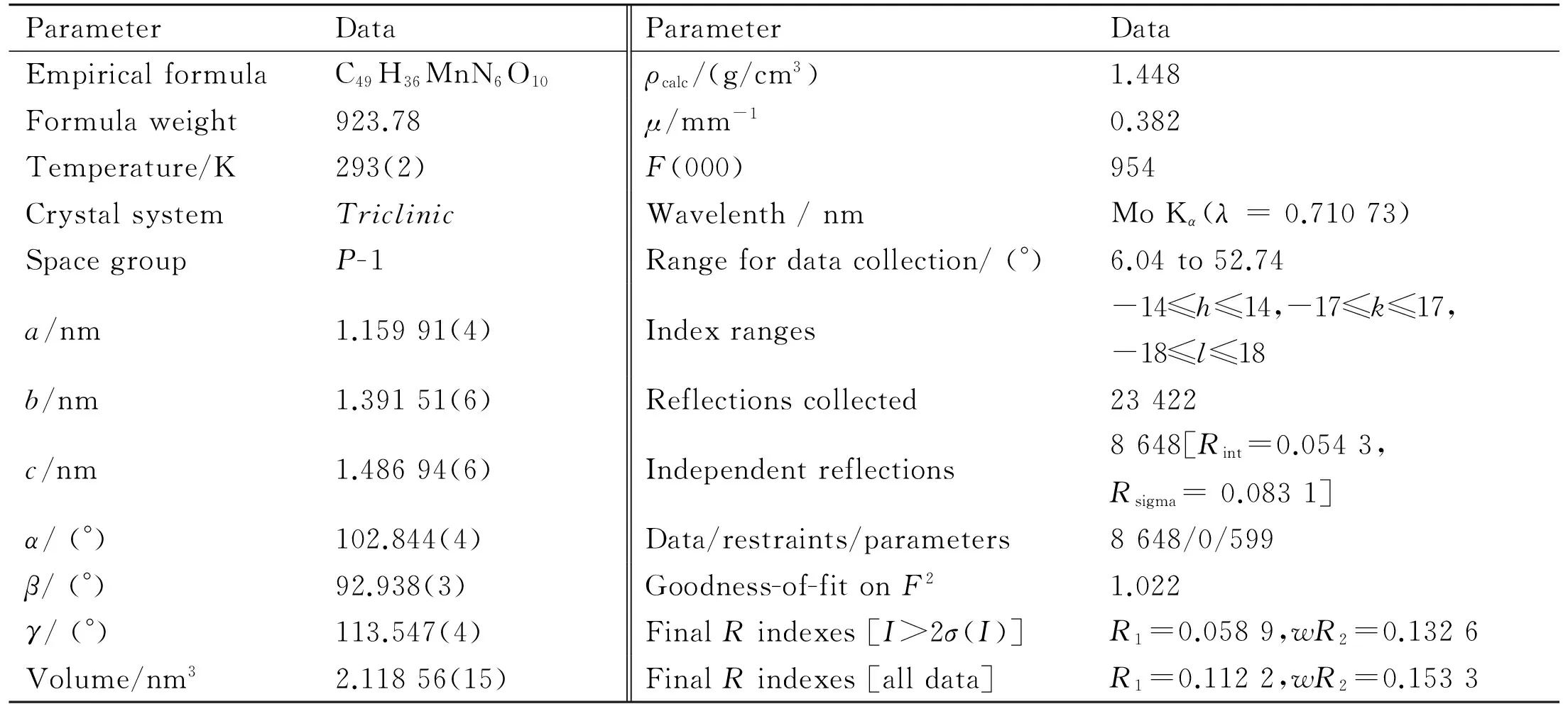
Table 1 Summary of crystallographic data for the complex
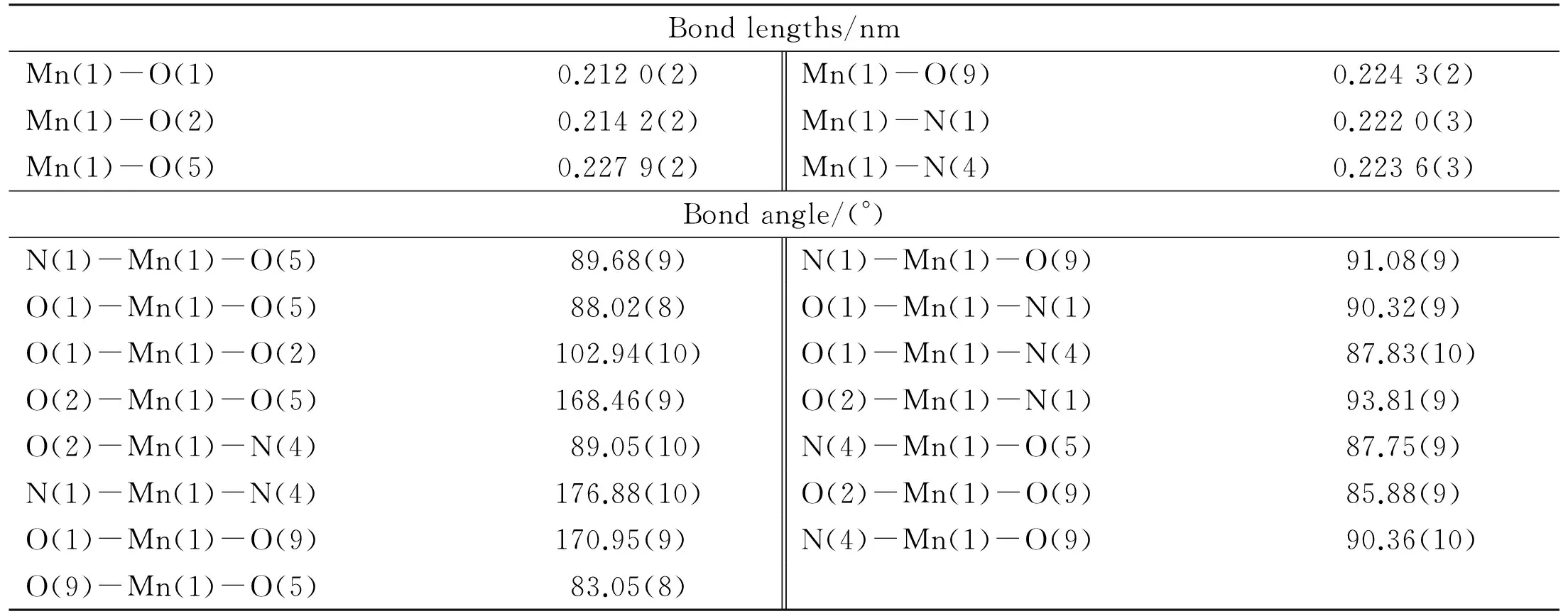
Table 2 Bond lengths and angles for complex
2.2FT-IR spectrum of the complex
The title complex is insoluble in water and most organic solvents, such as CH3CN, tetrahydrofuran (THF), CH3CH2OH, CH3OCH3and slight soluble in dimethylformamide (DMF). The structure of the complex is identified by FT-IR. The strong and board absorption of the complex at 3 421cm-1is related to the characteristic peaks of water molecules in coordination and lattice forms. The strong vibrations appear at around 1 512 cm-1and 1 350 cm-1in the complex are assigned to asymmetric (COO-) and symmetric (COO-) stretching of carboxyl groups of ttac4-ligands. The absence of characteristic bonds ranging from 1 725 to 1 825 cm-1indicates that the H2ttac ligands are completely deprotonated in the form of ttac4-an-ions upon reaction with the metal ions. The weak absorption of the characteristic bonds at 561 and 657 cm-1ascribed to the stretching of Mn-O (Fig.2)[27-28]. The results are consistent with the conclusions obtained from elemental analysis and X-ray single-crystal analysis.

Fig.1 a) Coordination environment of Mn(II) ion; b) 1D [Mn(ttac)]n chain; c) 1D [Mn(bibp)]n chain; d) view of the 2D supramolecular layer; e) 3D framework of the complex

Fig.2 IR spectrum of the complex
2.3Luminescent property
MOFs possessing excellent luminescence properties present potential applications as luminescent sensing materials. The luminescent spectra of the free H4ttac and the title complex in solid state, immersed in water (10-4mol/L) were studied at room temperature (shown in Fig.3a and Fig.3b). The H4ttac displays emission bands with maxima at 399 nm upon excitation at 335 nm. The emission bands of the free ligand may be ascribed to the π*→ n or π*→ π transitions. When the title compound was excited at 300 nm, the emission maximum at 340 nm was observed. The maximum luminescent emission and excitation of the complex shows a blue shift compared with the free H4ttac, which can be assigned to the mixture efforts of ligand-to-ligand charge transitions (LLCT) and ligand-to-metal charge transfer (LMCT)[29-32].
3 Conclusions
In conclusion, we have successfully synthesized a novel 3D MOF assembled from Mn(II), H4ttac, and bibp under hydrothermal reactions. From a structural point of view, the neighboring 2D layers composed of 1D channels are further assembled into a 3D supramolecular architectureviaπ…π interactions. Furthermore, the complex shows complex fluorescence properties, which indicates that the complex may be a potential fluorescent material. The hydrothermal synthesis is an effective method for the design and synthesis of interesting MOFs with attractive structures and properties.

Fig.3 a) Emission and excitation spectra for the free H4ttac at room temperature; b) Emission and excitation spectra for the complex at room temperature
References:
[1] KITAGAWA S. Metal-organic frameworks (MOFs) [J]. Chem Soc Rev, 2014, 43(16): 5415-5418.
[2] STOCK N, BISWAS S. Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites [J]. Chem Rev, 2011, 112(2): 933-969.
[3] HABIB H A, SANCHIZ J, JANIAK C. [Cu2(μ5-btb)(μ-OH)(μ-H2O)]: a two-dimensional coordination polymer built from ferromagnetically coupled Cu2units (btb = benzene-1,2,3-tricarboxylate) [J]. Dalton Trans, 2008(36): 4877-4884.
[4] MUELLER U, SCHUBERT M, TEICH F, et al. Metal-organic frameworks prospective industrial applications [J]. J Mater Chem, 2006, 16(7): 626-636.
[5] LI R J, LI M, ZHOU X P, et al. A highly stable MOF with a rod SBU and a tetracarboxylate linker: unusual topology and CO2adsorption behaviour under ambient conditions [J]. Chem Commun, 2014, 50(31): 4047-4049.
[6] ZHANG W H, DONG Z, WANG Y Y, et al. Synthesis, structural diversity and fluorescent characterisation of a series of d10metal-organic frameworks (MOFs): reaction conditions, secondary ligand and metal effects [J]. Dalton Trans, 2011, 40(11): 2509-2521.
[7] KRENO L E, LEONG K, FARHA O K, et al. Metal-organic framework materials as chemical sensors [J]. Chem Rev, 2011, 112(2): 1105-1125.
[8] YAMADA T, OTSUBO K, MAKIURA R, et al. Designer coordination polymers: dimensional crossover architectures and proton conduction [J]. Chem Soc Rev, 2013, 42(16): 6655-6669.
[9] HARBUZARU B V, CORMA A, REY F, et al. Metal-organic nanoporous structures with anisotropic photoluminescence and magnetic properties and their use as sensors [J]. Angew Chem Int Ed, 2008, 47(6): 1080-1083.
[10] LONG J R, YAGHI O M. The pervasive chemistry of metal-organic frameworks [J]. Chem Soc Rev, 2009, 38(5): 1213-1214.
[11] JANIAK C, VIETH J K. MOFs, MILs and more: concepts, properties and applications for porous coordination networks (PCNs) [J]. New J Chem, 2010, 34(11): 2366-2388.
[12] QIN L, ZHENG M X, GUO Z J, et al. One non-interpenetrated chiral porous multifunctional metal-organic framework and its applications for sensing small solvent molecules and adsorption [J]. Chem Commun, 2015, 51(12): 2447-2449.
[13] SUENAGA Y, YAN S G, WU L P, et al. Self-assembly of copper(I) and silver(I) complexes with square-grid and channel structures [J]. J Chem Soc Dalton Trans, 1998(7): 1121-1126.
[14] XU J, SUN X, FAN Y, et al. Synthesis, crystal structures and luminescent properties of pH-dependent Zn(II) coordination polymers [J]. J Inorg Organomet Polym, 2012, 22(4): 910-915.
[15] HIRSCH K A, WILSON S R, MOORE J S. Coordination networks of 3,3′-dicyanodiphenylacetylene and silver (I) salts: structural diversity through changes in ligand conformation and counterion [J]. Inorg Chem, 1997, 36(14): 2960-2968.
[16] LIU Y Y, MA J F, YANG J, et al. Syntheses and characterization of six coordination polymers of zinc(II) and cobalt(II) with 1,3,5-benzenetricarboxylate anion and bis(imidazole) ligands [J]. Inorg Chem, 2007, 46(8): 3027-3037.
[17] HU T L, ZOU R Q, LI J R, et al. d10Metal complexes assembled from isomeric benzenedicarboxylates and 3-(2-pyridyl)pyrazole showing 1D chain structures: syntheses, structures and luminescent properties [J]. Dalton Trans, 2008 (10): 1302-1311.
[18] DU M, JIANG X J, ZHAO X J. Molecular tectonics of mixed-ligand metal-organic frameworks: positional isomeric effect, metal-directed assembly, and structural diversification [J]. Inorg Chem, 2007, 46(10): 3984-3995.
[19] ZHANG M L, WANG J J, CHEN X L. Synthesis, structure, and luminescent properties of two 3D pillared-layer networks with instopology [J]. J Inorg Organomet Polym, 2014, 24(5): 879-883.
[20] WANG J, ZHANG Y H, TONG M L. Two new 3D metal-organic frameworks of nanoscale cages constructed by Cd(II) and conformationally flexible cyclohexanehexacarboxylate [J]. Chem Commun, 2006(30): 3166-3168.
[21] YANG L, LIU L, LIAN C, et al. Zero-, one-, two-and three-dimensional coordination polymers based on tetracarboxylic acid: Syntheses, structures, magnetic and luminescent properties [J]. Dyes Pigm, 2015, 122: 246-256.
[22] LIU J Q, WANG Y Y, ZHANG Y N, et al. Topological diversification in metal-organic frameworks: secondary ligand and metal effects [J]. Eur J Inorg Chem, 2009, 2009(1): 147-154.
[23] MA L F, WANG L Y, HUO X K, et al. Chain, pillar, layer, and different pores: an-[(3-carboxyphenyl)-sulfonyl] glycine ligand as a versatile building block for the construction of coordination polymers [J]. Cryst Growth Des, 2008, 8(2): 620-628.
[24] ZHOU X, LIU P, HUANG W H, et al. Solvents influence on sizes of channels in three fry topological Mn(II)-MOFs based on metal-carboxylate chains: syntheses, structures and magnetic properties [J]. Cryst Eng Comm, 2013, 15(40): 8125-8132.
[25] KAR P, IDA Y, ISHIDA T, et al. Formation of two drastically different MOFs based on Mn(II)-benzoate and pyrazine with a change in seasonal temperature: structural analysis and magnetic study [J]. Cryst Eng Comm, 2013, 15(2): 400-410.
[26] LIU Y, LI H, HAN Y, et al. Template-assisted synthesis of Co, Mn-MOFs with magnetic properties based on pyridinedicarboxylic acid [J]. Cryst Growth Des, 2012, 12(7): 3505-3513.
[27] LUO R, XU H, GU H X, et al. Four MOFs with 2,2′-dimethoxy-4,4′-biphenyldicarboxylic acid: syntheses, structures, topologies and properties[J]. Cryst Eng Comm, 2014, 16(5): 784-796.
[28] LIU M S, YU Q Y, CAI Y P, et al. One-, two-, and three-dimensional lanthanide complexes constructed from pyridine-2,6-dicarboxylic acid and oxalic acid ligands [J]. Cryst Growth Des, 2008, 8(11): 4083-4091.
[29] ZHONG D C, DENG J H, LUO X Z, et al. Two cadmium-cluster-based metal-organic frameworks with mixed ligands of 1,2,3-benzenetriazole (HBTA) and 1,4-benzenedicarboxylic acid (H2BDC) [J]. Cryst Growth Des, 2012, 12(4): 1992-1998.
[30] ZOU J P, PENG Q, WEN Z, et al. Two novel metal-organic frameworks (MOFs) with (3,6)-connected net topologies: syntheses, crystal structures, third-order nonlinear optical and luminescent properties [J]. Cryst Growth Des, 2010, 10(6): 2613-2619.
[31] GONG Y N, XIE Y R, ZHONG D C, et al. A Two-fold interpenetrating porous metal-organic framework with a large solvent-accessible volume: gas sorption and luminescent properties [J]. Cryst Growth Des, 2015, 15(7): 3119-3122.
[32] ERER H, YESILEL O Z, ARICI M. A Series of zinc(II) 3D → 3D interpenetrated coordination polymers based on thiophene-2,5-dicarboxylate and bis(imida-zole) derivative linkers [J]. Cryst Growth Des, 2015, 15(7): 3201-3211.
[责任编辑:刘红玲]
date: 2016-01-03.
The Development of Science and Technology Projects (162300410010), Natural Science Foundation of Henan Province, P.R. China (13A15006).
