Notch3在鼻咽癌中的基础研究及临床意义
2016-08-15张军红王春华吴秀云卢新阁王莉菲杨蕾蕾王秋红
张军红 王春华 吴秀云 卢新阁 王莉菲 杨蕾蕾 王秋红
Notch3在鼻咽癌中的基础研究及临床意义
张军红王春华吴秀云卢新阁王莉菲杨蕾蕾王秋红
目的探讨Notch3是否可以作为判断鼻咽癌复发、转移和化疗预后的分子生物学指标。方法Western blot检测鼻咽癌细胞系CNE2沉默Notch3的效果,球形成实验分析沉默Notch3对癌症干细胞的影响;通过Western blot检测上皮细胞标志E-cadherin和间质细胞标志Vimentin的表达分析沉默Notch3对鼻咽癌细胞EMT的影响;检测鼻咽癌细胞上清中乳酸的浓度分析在鼻咽癌中沉默Notch3对糖酵解的影响;Western blot检测顺铂作用后Notch3的表达,判断Notch3是否可以作为鼻咽癌对化疗药物敏感的指标。结果Western blot结果显示寡核苷酸干扰序列可以明显抑制沉默鼻咽癌细胞系CNE2中Notch3的表达。通过球形成实验发现在鼻咽癌细胞系CNE2中沉默Notch3后球形结构明显增加,说明沉默Notch3后干细胞增加。通过Western blot发现鼻咽癌细胞系CNE2沉默Notch3后上皮细胞标志分子E-cadherin表达减少,间质细胞标志分子Vimentin表达升高,说明沉默促进鼻咽癌细胞EMT转化。通过检测上清液中乳酸的浓度发现鼻咽癌细胞CNE2发现沉默Notch3后乳酸浓度升高,表明糖酵解增加。Western blot发现化疗药物顺铂作用鼻咽癌细胞系CNE2 后Notch3表达减少,表明Notch3可以作为判断化疗药物有效的指标。结论在鼻咽癌细胞系CNE2中沉默Notch3后癌症干细胞增加,促进EMT转换,糖酵解增加。化疗药物顺铂作用CNE2细胞后Notch3表达减少。因此,Notch3可以作为判断鼻咽癌复发、转移和化疗预后的分子生物学指标。
鼻咽癌;Notch3;癌症干细胞;EMT;糖酵解
鼻咽癌是头颈部最常见的恶性肿瘤之一[1,2]。现今鼻咽癌治疗的主要手段是放射治疗,不过单纯的放射治疗5年生存率50%~60%,其中远处转移和局部复发是鼻咽癌治疗失败的主要原因[1,3-7]。如何降低转移和复发是当前研究的热点问题。寻找鼻咽癌转移和复发相关的特异性分子标记物对鼻咽癌治疗有重要意义。Notch信号包括Notch1~4,在癌症干细胞自我更新中发挥调控作用,但是笔者未找到相关文献表明哪个Notch蛋白在鼻咽癌癌症干细胞中调控自我更新中发挥作用。我们的目的是研究Notch3在鼻咽癌CSC、EMT、糖酵解和化疗中的作用,为临床病理学诊断,转移和预后判断提供分子学基础。
1 材料与方法
1.1细胞系人鼻咽癌细胞系CNE2由中国科学院上海细胞中心提供,生长在含10%胎牛血清、100 U/ml青霉素、100 μg/ml链霉素的高糖DMEM培养基中,在5% CO2、37℃的细胞培养箱中培养,取对数生长期的细胞开展相关的实验。
1.2干扰Notch3对鼻咽癌癌症干细胞的影响对数生长的CNE2细胞培养在60 mm培养皿中,加入50 nmol/L 的干扰序列:CAG CGT GAC CGA GAT AGG TCA及其对照:AAT TCT CCG AAC GTG TCA CGT。2 d 后取1 000细胞/孔加入96孔超低粘附培养板中。细胞培养在无血清培养基中,含有10 ng/ml EGF,10 ng/ml bFGF,1∶50 B27,10 μg/ml heparin。每个实验组含有3个样本。每3天加入100 μl培养基,6 d后对实验形成的球形结构进行计数。
1.3干扰Notch3阻止EMT转化对数生长的CNE2细胞培养在60 mm培养皿中,加入50 nmol/L的干扰序列及其对照。作用鼻咽癌细胞CNE2 2 d后,裂解蛋白,通过western blot分析干扰Notch3对E-cadherin和Vimentin表达的影响。
1.4干扰Notch3对鼻咽癌糖酵解的影响对数生长的CNE2细胞培养在60 mm培养皿中,加入50 nmol/L的干扰序列及其对照。2 d后取上清,通过乳酸检测试剂盒(eton)分析乳酸产生的数量。同时检测细胞蛋白的浓度,通过乳酸/蛋白分析干扰Notch3对糖酵解的影响。每个实验组含有3个样本。
1.5化疗药物降低Notch3的表达对数生长的CNE2细胞培养在60 mm培养皿中,加入5 μmol/L的顺铂及其对照。作用鼻咽癌细胞CNE2 2 d后,通过western blot分析顺铂对Notch3表达的影响。
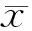
2 结果
2.1干扰Notch3促进鼻咽癌癌症干细胞的生成越来越多的实验证明癌症干细胞是癌症发生和转移的基础[22]。我们在鼻咽癌细胞系中干扰Notch3观察对鼻咽癌癌症干细胞的影响。在鼻咽癌细胞中干扰Notch3 2 d后,各取对照组和干扰组细胞1 000个/孔培养在96孔超低粘附培养板中,6 d后发现对照组形成球形结构12个,而干扰组形成球形结构25个。结果说明干扰Notch3后鼻咽癌细胞形成球形结构增加,可以促进癌症干细胞的生成。见图1~3,表1。

图1Western blot显示在鼻咽癌细胞CNE2中干扰Notch3能明显抑制其表达
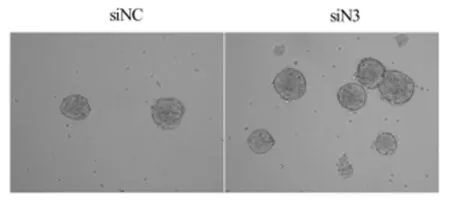
图2球形成实验显示鼻咽癌细胞CNE2干扰Notch3后球形结构形成增加(×100)
图3 鼻咽癌细胞系CNE2干扰Notch3后球形结构增加标状图
/200细胞,
注:与CNE2-siNc比较,*P<0.05
2.2干扰Notch3阻止鼻咽癌EMT转化我们在鼻咽癌细胞CNE2干扰Notch3后通过检测上皮细胞标志分子E-cadherin和间充质细胞标志分子Vimentin分析沉默Notch3对鼻咽癌EMT的影响。我们发现,鼻咽癌细胞CNE2沉默Notch3后上皮细胞标志分子E-cadherin表达减少,间质细胞标志分子Vimentin表达升高。上述结果说明在鼻咽癌细胞中沉默Notch3促进上皮间充质转化,能够促进鼻咽癌的转移。见图4。

图4Western blot显示鼻咽癌细胞CNE2干扰Notch3后上皮细胞标志分子E-cadherin表达减少,间质细胞标志分子Vimentin表达升高
2.3干扰Notch3促进鼻咽癌细胞CNE2糖酵解的增加在多种癌症中发现肿瘤的恶性程度和糖酵解的增加呈正相关。我们检测鼻咽癌细胞CNE2沉默Notch3后对糖酵解程度的影响,分析Notch3的表达和鼻咽癌细胞恶性程度的关系。鼻咽癌细胞CNE2沉默Notch3 2 d后,取上清检测乳酸分泌量的变化,发现鼻咽癌细胞沉默Notch3后乳酸分泌增加。上述结果说明鼻咽癌细胞沉默Notch3后糖酵解程度增加,恶性程度增加。见图5,表2。

图5 鼻咽癌细胞CNE2沉默Notch3后对糖酵解的影响

类别乳酸分泌量蛋白比例CNE2-siNc8620±104CNE2-siN315641±474*
注:与CNE2-siNc比较,*P<0.05
2.4化疗药物顺铂(cisplatin)降低鼻咽癌细胞系CNE2细胞Notch3的表达我们前期试验显示沉默Notch3后CSC增多,因此高表达Notch3可能作为鼻咽癌化疗有效的指标。我们使用5 μmol/L顺铂作用鼻咽癌细胞系CNE2 2 d后,通过western blot检测Notch3的表达。结果发现顺铂可以引起鼻咽癌细胞CNE2中Notch3表达减少。这些结果说明化疗药物cisplatin可以降低鼻咽癌Notch3表达增加,高表达的Notch3可以作为监测化疗药物有效治疗鼻咽癌的分子生物学指标。见表6。

图6western blot显示5 μmol/L顺铂作用鼻咽癌细胞系CNE2后Notch3表达降低
3 讨论
越来越多的文献支持癌症干细胞(cancer stem cell)是癌症发生和转移的原因[8-10]。在乳腺癌、胰腺癌、结肠癌和黑色素瘤中发现可以引起癌症发生和转移的癌症干细胞[11-14]。在鼻咽癌中也发现存在癌症干细胞[15-17]。最近文献表明可以通过体外实验sphere-forming assay验证鼻咽癌癌症干细胞的存在[18]。最近研究显示癌症干细胞比非癌症干细胞具有抗化疗的作用,是化疗后癌细胞复发和转移的原因之一。不同的癌症干细胞对放疗的敏感性也不同[19]。多个实验室研究返现鼻咽癌干细胞具有抗化疗的作用[19]。上皮间充质转化(epithelial-mesenchymal transition,EMT)是癌症细胞转移的关键步骤,可以诱导癌症干细胞的生成[20]。肿瘤组织获取能量的主要方式是糖酵解,且随着恶性程度增加糖酵解的程度也增加。癌症干细胞,抗化疗作用,EMT和糖酵解反映了癌症的发生,转移和恶性程度的指标。
癌症干细胞指具有干细胞性质的癌细胞,也就是具有自我更新和多细胞分化能力的细胞[21,22]。少量数量的癌症干细胞能够形成癌症,是癌症形成、复发和转移的基础,具有抗放化疗,糖酵解程度比较高的特点[23]。经过二十多年的研究,在乳腺癌、胶质瘤、黑色素瘤、前列腺癌、胰腺癌、鼻咽癌等癌症中均发现癌症干细胞。球形成实验室体外检测癌症干细胞存在的,可以用来检测神经干细胞、乳腺干细胞、乳腺癌症干细胞的存在[24]。在多个实验中也用来检验鼻咽癌癌症干细胞的存在[18,25,26]。我们的实验结果显示,在鼻咽癌细胞系CNE2中沉默Notch3后球形结构增加,表明低表达的Notch3和鼻咽癌癌症干细胞的数量呈正相关。这些结果说明鼻咽癌中高表达的Notch3可以作为低复发和低转移的鼻咽癌分子指标。
EMT是指上皮细胞转化为间质表型细胞的过程,主要特征是细胞粘附分子如E-cadherin表达减少和骨架蛋白如Vimentin表达增加。通过EMT过程,上皮细胞失去了极性,获得了更高的迁徙和侵袭,抗凋亡和降解细胞外基质的能力,在癌症转移中发挥了重要作用[27]。研究发现在乳腺癌、结肠癌、肺癌、前列腺癌、肝癌、胰腺癌均存在EMT发生,且在这些癌症的原发性浸润和继发性转移中起着举足轻重的作用[28]。在鼻咽癌中也发现存在EMT存在[29]。我们研究发现在鼻咽癌细胞沉默Notch3后上皮细胞标志E-cadherin表达降低,间质细胞标志Vimentin表达升高,说明沉默Notch3后鼻咽癌发生EMT转化,促进鼻咽癌的转移。因此,高表达的Notch3预示着鼻咽癌的转移概率低。
肿瘤细胞的糖酵解程度和恶性程度呈正相关。肿瘤细胞即使在供养充足的条件下,也仍然以糖酵解作为主要的能量供应方式,摄取葡萄糖生成乳酸,成为Warburg效应[30]。这是肿瘤细胞的基本特点,也是目前临床用来诊断肿瘤的依据。临床上采用的18氟标记脱氧葡萄糖(18F-FDG)PET显像技术正是利用Warburg效应开展恶性肿瘤的诊断,基于良、恶性病变之间的葡萄糖代谢水平差异,F-FDG可被用于区别良、恶性病变。PET显像技术应用在肿瘤分期、分型、复发、转移的诊断,肿瘤生物学特征的预测以及对治疗反应的监控等方面有重要意义[31]。因此,我们可以通过肿瘤细胞产生乳酸的程度判断癌症的恶性程度。我们的研究结果发现,鼻咽癌细胞DNE2沉默Notch3后乳酸分泌增加。总上所述,鼻咽癌细胞沉默Notch3后糖酵解程度增加,恶性程度增加。
最近研究认为肿瘤细胞化疗耐受和肿瘤干细胞有关。经过化疗的肿瘤残存组织或者体外培养的肿瘤细胞系癌症干细胞增加[32]。多个实验室也发现,不同来源的肿瘤干细胞对目前临床治疗的化疗药物耐药,导致临床治疗失败和癌症复发[33]。因此,我们可以通过分析化疗药物对肿瘤细胞的耐受分析癌症干细胞。我们研究发现鼻咽癌细胞系CNE2细胞在5 μmol/L顺铂作用2 d后Notch3表达下降,说明高表达Notch3的鼻咽癌细胞对化疗药物敏感。结合前述实验,我们认为高表达Notch3是反映鼻咽癌细胞化疗药物敏感的靶标。
沉默Notch3导致鼻咽癌癌症干细胞数量增加,发生EMT转化和糖酵解的程度增加。化疗药物cisplatin作用鼻咽癌细胞后Notch3表达降低。这些结果说明高表达的Notch3表示鼻咽癌复发和转移的概率低,恶性程度低,预示很好的抗化疗作用,因此可以作为一个鼻咽癌诊断和治疗的良性指标。
尽管我们实验结果显示Notch3干扰后干细胞增加,促进EMT转换,促进糖酵解和化疗药物作用鼻咽癌细胞后Notch3后,说明Notch3是反映鼻咽癌的一个良性的指标。不过,沉默Notch3后影响鼻咽癌恶性转化的机制未明,是我们下一步研究的目标。
1Bensouda Y.Treatment for metastatic nasopharyngeal carcinoma.Eur Ann Otorhinolaryngol Head Neck Dis,2011,128:79-85.
2Chan AT.Nasopharyngeal carcinoma.Ann Oncol,2010,21:308-312.
3Liu T.Radiation therapy for nasopharyngeal carcinoma using simultaneously integrated boost (SIB) protocol: a comparison planning study between intensity modulated arc radiotherapy vs. intensity modulated radiotherapy.Technol Cancer Res Treat,2012,11:415-420.
4Tan BC,Khor TH,Chia KB.Radiotherapy in treatment of nasopharyngeal carcinoma.Ann Acad Med Singapore,1980,9:347-349.
5Tian Y,Guo Z,Zhu M.Radiation encephalopathy in nasopharyngeal carcinoma patients in mainland China:a systematic evaluation.Zhonghua Zhong Liu Za Zhi,2002,24:471-473.
6Setton J.Definitive treatment of metastatic nasopharyngeal carcinoma:Report of 5 cases with review of literature.Head Neck,2012,34:753-757.
7Venkatramani R,Mascarenhas L.Successful treatment of recurrent metastatic nasopharyngeal carcinoma with oxaliplatin and doxorubicin.J Pediatr Hematol Oncol,2014,36:e307-309.
8Ailles LE,Weissman IL.Cancer stem cells in solid tumors.Curr Opin Biotechnol,2007,18:460-466.
9Vermeulen L.Cancer stem cells-old concepts,new insights.Cell Death Differ,2008,15:947-58.
10McCarthy N.Cancer stem cells: Underground movement.Nature Reviews Cancer,2007,7:812-813.
11Schillace RV,Skinner AM,Pommier RF,et al.Estrogen receptor,progesterone receptor, interleukin-6 and interleukin-8 are variable in breast cancer and benign stem/progenitor cell populations.BMC Cancer,2014,14:733.
12Zhong Y.ALDH1 is a better clinical indicator for relapse of invasive ductal breast cancer than the CD44+/CD24- phenotype.Med Oncol,2014,31:864.
13Lu Y.MiR-200a inhibits epithelial-mesenchymal transition of pancreatic cancer stem cell.BMC Cancer,2014,14:85.
14Vincent Z.CD133-positive cancer stem cells from Colo205 human colon adenocarcinoma cell line show resistance to chemotherapy and display a specific metabolomic profile.Genes Cancer,2014,5:250-260.
15Yang CH,Wang HL,Lin YS,et al.Identification of CD24 as a cancer stem cell marker in human nasopharyngeal carcinoma.PLoS One,2014,9:e109495.
16Yang CH.Identification of CD24 as a cancer stem cell marker in human nasopharyngeal carcinoma.PLoS One,2014,9:e99412.
17Wei P.Cancer stem-like cell:a novel target for nasopharyngeal carcinoma therapy.Stem Cell Res Ther,2014,5:44.
18Lun SW.CD44+ cancer stem-like cells in EBV-associated nasopharyngeal carcinoma.PLoS One,2012,7:e52426.
19Zhao Y.Stem cell-like side populations in esophageal cancer: a source of chemotherapy resistance and metastases.Stem Cells Dev,2014,23:180-192.
20Lamouille S,Xu J,Derynck R.Molecular mechanisms of epithelial-mesenchymal transition.Nat Rev Mol Cell Biol,2014,15:178-196.
21Espinoza I.Notch signaling: targeting cancer stem cells and epithelial-to-mesenchymal transition.Onco Targets Ther,2013,6:1249-1259.
22Liao WT.Metastatic cancer stem cells: from the concept to therapeutics.Am J Stem Cells,2014,3:46-62.
23Prud'homme GJ.Cancer stem cells and novel targets for antitumor strategies.Curr Pharm Des,2012,18:2838-2849.
24Rota LM.Determining mammosphere-forming potential:application of the limiting dilution analysis.J Mammary Gland Biol Neoplasia,2012,17:119-123.
25Cheng Y.Physiological beta-catenin signaling controls self-renewal networks and generation of stem-like cells from nasopharyngeal carcinoma.BMC Cell Biol,2013,14:44.
26Kondo S.Epstein-Barr virus latent membrane protein 1 induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell lines.J Virol,2011,85:11255-11264.
27Kothari AN.Novel clinical therapeutics targeting the epithelial to mesenchymal transition.Clin Transl Med,2014,3:35.
28Steinestel K.Clinical significance of epithelial-mesenchymal transition.Clin Transl Med,2014,3:17.
29Shen Y.Resveratrol Impedes the Stemness,Epithelial-Mesenchymal Transition, and Metabolic Reprogramming of Cancer Stem Cells in Nasopharyngeal Carcinoma through p53 Activation. Evid Based Complement Alternat Med,2013,2013: 590393.
30Elf SE,Chen J.Targeting glucose metabolism in patients with cancer.Cancer,2014,120:774-180.
31Bensinger SJ,Christofk HR.New aspects of the Warburg effect in cancer cell biology.Semin Cell Dev Biol,2012,23:352-161.
32Easwaran H,Tsai HC,Baylin SB.Cancer epigenetics: tumor heterogeneity,plasticity of stem-like states,and drug resistance.Mol Cell,2014,54:716-127.
33Wu Q.Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches.Cancer Lett,2014,347:159-166.
The significance of Notch3 in nasopharyngeal carcinoma
ZHANGJunhong*,WANGChunhua,WUXiuyun,etal.
*Desect1mentofPathology,HebeiProvincialOphthalmologyHospital,Hebei,Xingtai054001,China
ObjectiveTo explore the possibility of Notch3 as a molecular biological index to evaluate the recurrence,metastasis and prognosis of nasopharyngeal cancer. MethodsWestern Blot was used to detect the effects of silencing Notch3 in nasopharyngeal carcinoma cell lines-CNE2 by small interfering RNA (siRNA). The sphere forming assay was performed to analyze the influence of silencing Notch3 in CNE2 on the number of cancer stem cells. The expression levels of E-cadherin and VImentin were detected by Western Blot to observe the influence of silencing Notch3 in CNE2 on EMT. The effects of silencing Notch3 in CNE2 on glycolysis were observed by detecting the concentration of lactic acid. The expression levels of Notch3 were determine after CNE2 cells were treated by cisplatin in order to evaluate whether Noth3 could be regarded as an index of chemotherapy sensitivity.ResultsThe results by Western Blot showed that the siRNA could effectively silence the expressions of Notch3 in CNE2 cells. The results by sphere forming assay showed that the sphericity structure was obviously increased after silencing Notch3 in CNE2 cells,which suggested that silencing Notch3 increased the number of cancer stem cells. After silencing Notch3 in CNE2 cells the expression levels of epithelial marker-E-cadherin were decreased, however, the expression levels of mesenchymal cell marker molecule-Vimentin were increased, which indicated that silencing Notch3 promoted EMT transformation of CNE2 cells. Moreover lactate concentration was increased in CNE2 cells after silencing Notch3,which suggested that silencing Notch3 in CNE2 cells could increase glycolysis. Western Blot results showed that the expression levels of Notch3 were decreased after CNE2 cells were interfered by cisplatin,which indicated that Notch3 could be regarded as an index to evaluate the efficiency of chemotherapeutics.ConclusionIn nasopharyngeal carcinoma cell lines-CNE2 cells, silencing Notch3 can increase the number of cancer stem cell, promote EMT transition and enhance glycolysis. The expression levels of Notch3 are decreased after CNE2 cells are interfered by cisplatin.Thus Notch3 can be regarded as an index to evaluate the recurrence, metastasis and prognosis of nasopharyngeal cancer.
nasopharyngeal cancer; Notch3; cancer stem cell; EMT;glycolysis
2015-11-08)
10.3969/j.issn.1002-7386.2016.15.006
054001河北省邢台市,河北省眼科医院病理科(张军红、杨蕾蕾),耳鼻喉科(王春华、吴秀云、卢新阁),眼科学重点实验室(王莉菲),检验科(王秋红)
R 739.6
A
1002-7386(2016)15-2262-05
项目来源:邢台市科学技术研究与发展支撑计划项目(编号:2013ZC062 )
