Effect of pupillary dilation on intraocular lens power calculation
2016-08-08lsEgemenErkayhanUfukAdigzel
Gülsüm Egemen Erkayhan, Ufuk Adigüzel
Effect of pupillary dilation on intraocular lens power calculation
Gülsüm Egemen Erkayhan1, Ufuk Adigüzel2
1Department of Ophthalmology, Mersin Toros State Hospital, Mersin 33010, Turkey2Department of Ophthalmology, Mersin University Medical Faculty, Mersin 33330, Turkey

Received: 2015-10-19Accepted: 2016-04-21
目的:研究散瞳对人工晶状体屈光度数计算的影响。
方法:前瞻性研究。选取梅尔辛大学医学院诊断为白内障需接受白内障超声乳化联合人工晶状体植入术的患者45例52眼作为研究对象。散瞳前后,所有患者均接受术前角膜地形图测量,自动角膜曲率测量和生物特征测定。
结果:与散瞳前数值相比,散瞳后,自动角膜曲率计测得的水平曲率(Kh)明显更大,且生物超声波测定的前房深度明显更深。使用散瞳后测量计算得出的屈光度数植入人工晶状体屈光正常的几率明显较高。比较正视眼和屈光不正眼发现正视眼的前房深度明显更深。
结论:在人工晶状体屈光度计算中,角膜曲率测量与生物特征测量比公式计算更重要。如果采用接触技术进行生物超声波测定,须注意避免角膜压迫。在检测时,注意前房深度应随访,且可通过利用人工晶状体屈光度计算中的最大值将误差最小化。
引用:Erkayhan GE, Adigüzel U. 散瞳对人工晶状体屈光度数计算的影响.国际眼科杂志2016;16(8):1419-1424
Abstract
•AIM: To investigate the effect of pupillary dilation on intraocular lens power calculation.
•METHODS: This prospective study included 52 eyes of 45 patients diagnosed with cataract and indicated for phacoemulsification with intraocular lens(IOL) implantation at the Faculty of Medicine of Mersin University. For each patient, preoperative corneal topography, autokeratometric measurements and biometric measurements were performed before and after pupil dilation.
•RESULTS: Kh(horizontal) values obtained through autokeratometry and anterior chamber depth measured by biometric ultrasonography were significantly greater when pupils were dilated compared with values obtained when pupils were undilated. Implanting IOLs with power calculated using measurements taken during pupillary dilation resulted in a significantly higher rate of emmetropia. Comparison of emmetropic eyes and ametropic eyes showed significantly larger anterior chamber depth in emmetropic eyes.
•CONCLUSION: Keratometric and biometric measurements are more important in IOL power calculation than the formula used. If biometric ultrasonography is performed using contact technique, care must be taken to avoid corneal compression. Anterior chamber depth should be followed during measurement, and the margin of error can be minimized by using the highest value obtained in IOL power calculation.
KEYWORDS:•biometry; intraocular lens power calculation formulas; pupillary dilation
Citation:Erkayhan GE, Adigüzel U. Effect of pupillary dilation on intraocular lens power calculation.GuojiYankeZazhi(IntEyeSci) 2016;16(8):1419-1424
INTRODUCTION
Cataract is the progressive loss of lens transparency. Despite being treatable, cataract is the most common ocular disease and a leading cause of vision loss[1-2]. Depending on severity, cataract affects visual function and daily activities. Indications of cataract surgery are usually binocular vision impairment and low vision. Surgical procedures including intraocular lens (IOL) implantation are the only treatment for cataract[3].
Due to the important dioptric power of the crystalline lens, its removal leaves the eye extremely weak in terms of dioptric power. To regain vision it is necessary to replace the lost dioptric power. Replacement of dioptric power may be achieved with eyeglasses, contact lenses, tissue placed on the corneal surface, or IOLs. Although all of these methods result in visual recovery, they have different optic results[4]. The most common and successful method used to replace crystal lens power is to use an IOL[5]. Currently, the most popular cataract surgery method is phacoemulsification with IOL implantation[6-7]. With recent improvements in surgical technique and IOL technology, patients’ expectations have increased. In addition to the removal of their cataracts, patients expect perfect postoperative visual acuity without eyeglasses. To achieve these refractive results, the accurate calculation of IOL power preoperatively is necessary. With the increasing importance of refractive outcomes of cataract surgery, IOL power calculation has become an important focal point in recent years[8-9].
In this study, we aimed to investigate the dynamics affecting IOL power calculations and the effect of pupillary dilation on these dynamics.
SUBJECTS AND METHODS
The study was approved by the Mersin University Faculty of Medicine Ethics Committee (17 Apr. 2009, decision 2009/58) for all groups. All groups were given detailed information about their diagnosis and surgical procedures to be applied, and written informed consent was obtained.
The study group was comprised of patients who were diagnosed with cataract and underwent phacoemulsification-IOL surgery at Department of Ophthalmology, Faculty of Medicine, Mersin University between Apr. 2009 and May 2010. Fifty-two eyes of 45 patients with uncomplicated phacoemulsification-IOL procedures were included in the study. Patients with previous ocular surgeries, history of ocular trauma, corneal pathology, uveitis, glaucoma or posterior segment pathology were excluded.
Prior to cataract surgery, slit-lamp biomicroscopic examination of the anterior segment, fundus examination, autokeratometric measurements, corneal topography and biometric measurements were routinely performed. Corneal topography, autokeratometric measurements and biometric measurements were done with and without pupil dilation for each patient. Pupils were dilated using cyclopentolate drops. Patients’ preoperative corneal values obtained by Auto Kerato-Refractometer (KR8800, Topcon, Japan) and corneal topography (TMS-4, Tommy, Japan) were recorded. Corneal topography values were taken into account during measurements.
Biometric ultrasonography (EchographclassI Type B and B scan V plus BioVision, QuantelMedical, France) was conducted with a 10 mHz probe. Biometric measurements were taken by the same physician with the patient in a comfortable seated position under topical corneal anesthesia. Biometric values were calculated using the contact technique; the probe was positioned at the center of the cornea and patients used the red light in the center of the probe as a reference for fixation. Care was taken not to compress the cornea during measurement. Anterior chamber depth (ACD), crystalline lens thickness (LT), vitreous length (VL), and axial length (AL) were measured by biometric ultrasonography and recorded. Two calculations of IOL power targeting emmetropia were done, one using the SRK-T formula and an A-constant of 118.5, the other using the Binkhorst Ⅱ formula with an ACD constant of 5.26. The power of the implanted IOL was determined with the SRK-T formula.
Phacoemulsification and IOL implantation were performed by two surgeons under local or general anesthesia. During surgery, either a 6.0 mm optic diameter, A-constant 118.4 hydrophobic acrylic (Acrysof, Alcon, USA) lens or a 6.0 mm optic diameter, A-constant 118.0 aspheric hydrophilic (SoftecHD, Lenstech, USA) lens was implanted at random.
All patients were followed up at 1d, 1wk and 6wk postoperatively. At each follow-up, visual acuity evaluation using the Snellen chart, slit-lamp examination of the anterior segment and fundus examination were conducted. At the 6-week follow-up, best corrected visual acuity was measured, refractive errors were recorded and deviations from emmetropia were calculated. With the exception of patients whose calculated IOL power was the same with and without dilation, the power of the implanted IOL was randomly selected based on either dilated or undilated measurements. Accordingly, the patients were divided into three groups: Group 1: IOL power was determined with biometric measurements taken while pupil was dilated; Group 2: IOL power was determined with biometric measurements taken while pupil was not dilated; Group 3: equivalent IOL power was determined with biometric measurements taken before and during pupil dilation.
Postoperatively,spherical equivalent was calculated by adding half the cylindrical value to the spherical value. Patients with spherical equivalents between -0.50 and +0.50 were considered emmetropic.
Statistical analyses were performed using the SPSS software(USA) package. According to whether the data were parametric or nonparametric, Dependent and Independentt-test and Chi-square analyses were performed. Results are expressed as mean±SD and percentage.Pvalues less than 0.05 were accepted as significant.
RESULTS
Twenty-six (50%) of the eyes included in the study were right and 26 (50%) were left. Twenty-two (48.8%) of the patients were male and 23 (51.1%) were female. The mean age was 59.21±14y. There were 14 eyes in Group 1 (IOL power was determined using biometric measurements taken with dilated pupil), 17 eyes in Group 2 (IOL power was determined using biometric measurements taken with undilated pupil), and 21 eyes in Group 3 (calculated IOL power was the same with dilated and undilated pupils). Best corrected visual acuity was 0.15±0.17 preoperatively and 0.9±0.19 postoperatively. Postoperative refraction as mean spherical equivalent was calculated as -0.28±0.71 D (range -1.75 to +1.75) for all patients. Postoperative spherical equivalent was -0.24±0.82 D in Group 1, -0.37±0.72 D in Group 2, and -0.24±0.66 D in Group 3.
Mean preoperative values of all patients measured during pupillary dilation were: Kh(horizontal) 44.02±1.65, Kv(vertical) 44.00±1.68, and Kave(average) 43.98±1.61 (measured by autokeratometry) and Ks(steepest) 44.43±1.54,Kf(flattest) 43.28±2.09, Kave43.92±1.57 (measured by topography). Ultrasonography measurements were: ACD 3.21±0.40 mm, LT 3.90±0.46 mm, VL 16.10±1.09 mm and AL 23.21±1.03 mm.
Table 1Comparison of measurement values with and without pupil dilation

MeasuringmethodBiometricparametersDilation(+)Dilation(-)PAutokeratometryKh44.02±1.6543.85±1.690.044Kv44.00±1.6843.98±1.670.891Kave43.98±1.6143.90±1.610.081TopographyKs44.43±1.5444.30±1.620.206Kf43.28±2.0943.32±1.780.834Kave43.92±1.5743.63±2.150.150UltrasonographyACD3.21±0.403.09±0.450.002LT3.90±0.463.95±0.540.485VL16.10±1.0916.14±1.090.680AL23.21±1.0323.19±1.160.609
Kh: K horizontal; Kv: K vertical; Kave: K average; Ks: K steepest; Kf: K flattest; ACD: Anterior chamber depth; LT: Crystalline lens thickness; VL: Vitreous length; AL: Axial length; Dilation (+):Pupilla dilated; Dilation (-): Pupilla undilated.
Mean preoperative values of all patients measured when pupils were not dilated were: Kh43.85±1.69, Kv43.98±1.67, and Kave43.90±1.61 (measured by autokeratometry) and Ks43.30±1.62, Kf43.32±1.78, and Kave43.63±2.15 (measured by topography). Ultrasonography measurements were: ACD 3.09±0.45 mm, LT 3.95±0.54 mm, VL 16.14±1.09 mm and AL 23.19±1.16 mm.
When values obtained with dilated and undilated pupils were compared, values for Khand anterior chamber depth were significantly larger when pupils were dilated (P=0.044 andP=0.002, respectively) (Table 1).
Patients with mean postoperative spherical equivalent between -0.50 and +0.50 diopters were considered emmetropic. Postoperatively, 27 of the eyes were emmetropic and the other 25 eyes were determined to be ametropic. The refractive errors of 16 of the ametropic eyes were between -1.00 and +1.00 diopters and were greater than ±1.00 in 9 eyes.
In the group of eyes that were emmetropic postoperatively, mean values obtained during dilation through autokeratometry were Kh43.67±1.36, Kv43.60±1.32 and Kave43.64±1.28; mean topography values were Ks44.11±1.20, Kf43.25±1.19 and Kave43.66±1.17. Mean ACD was 3.31±0.37 mm, LT was 3.94±0.40 mm, VL was 16.17±0.83 mm, and AL was 23.43±0.89 mm. When emmetropic eyes were not dilated, mean autokeratometry values were Kh43.60±1.36, Kv43.63±1.35 and Kave43.63±1.28; mean topography values were Ks44.10±1.20, Kf43.22±1.24 and Kave43.31±2.18. Mean ACD was 3.22±0.44 mm, LT was 4.0±0.58 mm, VL was 16.27±0.90 mm, and AL was 23.49±0.86 mm (Table 2).
A comparison of values obtained from emmetropic eyes with and without pupil dilation revealed that pupillary dilation resulted in no significant differences in any of the parameters (P>0.05).
Table 2The effect of pupillary dilation on biometric parameters in emmetropic eyes

BiometricparametersDilation(+)Dilation(-)PKh43.67±1.3643.60±1.360.147Kv43.60±1.3243.63±1.350.503Kave43.64±1.2843.63±1.280.713Ks44.11±1.2044.10±1.200.920Kf43.25±1.1943.22±1.240.778Kave43.66±1.1743.31±2.180.308ACD3.31±0.373.22±0.440.107LT3.94±0.404.0±0.580.575VL16.17±0.8316.27±0.900.460AL23.43±0.8923.49±0.860.062
Kh: K horizontal; Kv: K vertical; Kave: K average; Ks: K steepest; Kf: K flattest; ACD: Anterior chamber depth; LT: Crystalline lens thickness; VL: Vitreous length; AL: Axial length; Dilation (+): Pupilla dilated; Dilation (-): Pupilla undilated.
In the group of eyes that were ametropic postoperatively, mean values obtained during dilation through autokeratometry were Kh44.41±1.88, Kv44.42±1.94 and Kave44.35±1.88; mean topography values were Ks44.78±1.81, Kf43.31±2.79 and Kave44.20±1.91. Mean ACD was 3.08±0.40 mm, LT was 3.85±0.53 mm, VL was 16.02±1.33 mm, and AL was 22.97±0.14 mm. When ametropic eyes were not dilated, mean autokeratometry values were Kh44.13±1.98, Kv44.36±1.92 and Kave44.20±1.90; mean topography values were Ks44.51±1.99, Kf43.42±2.25 and Kave43.97±2.10. Mean ACD was 2.94±0.43 mm, LT was 3.90±0.49 mm, VL was 16.00±1.27 mm, and AL was 22.85±1.36 mm (Table 3).
In ametropic eyes, pupillary dilation resulted in a statistically significant increase in anterior chamber depth value (P=0.003). Differences in the other parameters were not statistically significant (P>0.05).
When emmetropic and ametropic eyes were compared, the anterior chamber depth values from emmetropic eyes were significantly higher with and without pupillary dilation (P=0.040,P=0.031, respectively) (Table 4).
Table 3Effect of pupillary dilation on biometric parameters in ametropic eyes

BiometricparametersDilation(+)Dilation(-)PKh44.41±1.8844.13±1.980.103Kv44.42±1.9444.36±1.920.719Kave44.35±1.8844.20±1.900.087Ks44.78±1.8144.51±1.990.197Kf43.31±2.7943.42±2.250.799Kave44.20±1.9143.97±2.100.296ACD3.08±0.402.94±0.430.003LT3.85±0.533.90±0.490.676VL16.02±1.3316.00±1.270.888AL22.97±0.1422.85±1.360.274
Kh: K horizontal; Kv: K vertical; Kave: K average; Ks: K steepest; Kf: K flattest; ACD: Anterior chamber depth; LT: Crystalline lens thickness; VL: Vitreous length; AL: Axial length; Dilation (+): Pupilla dilated; Dilation (-): Pupilla undilated.
Table 4Parameters affecting emmetropia
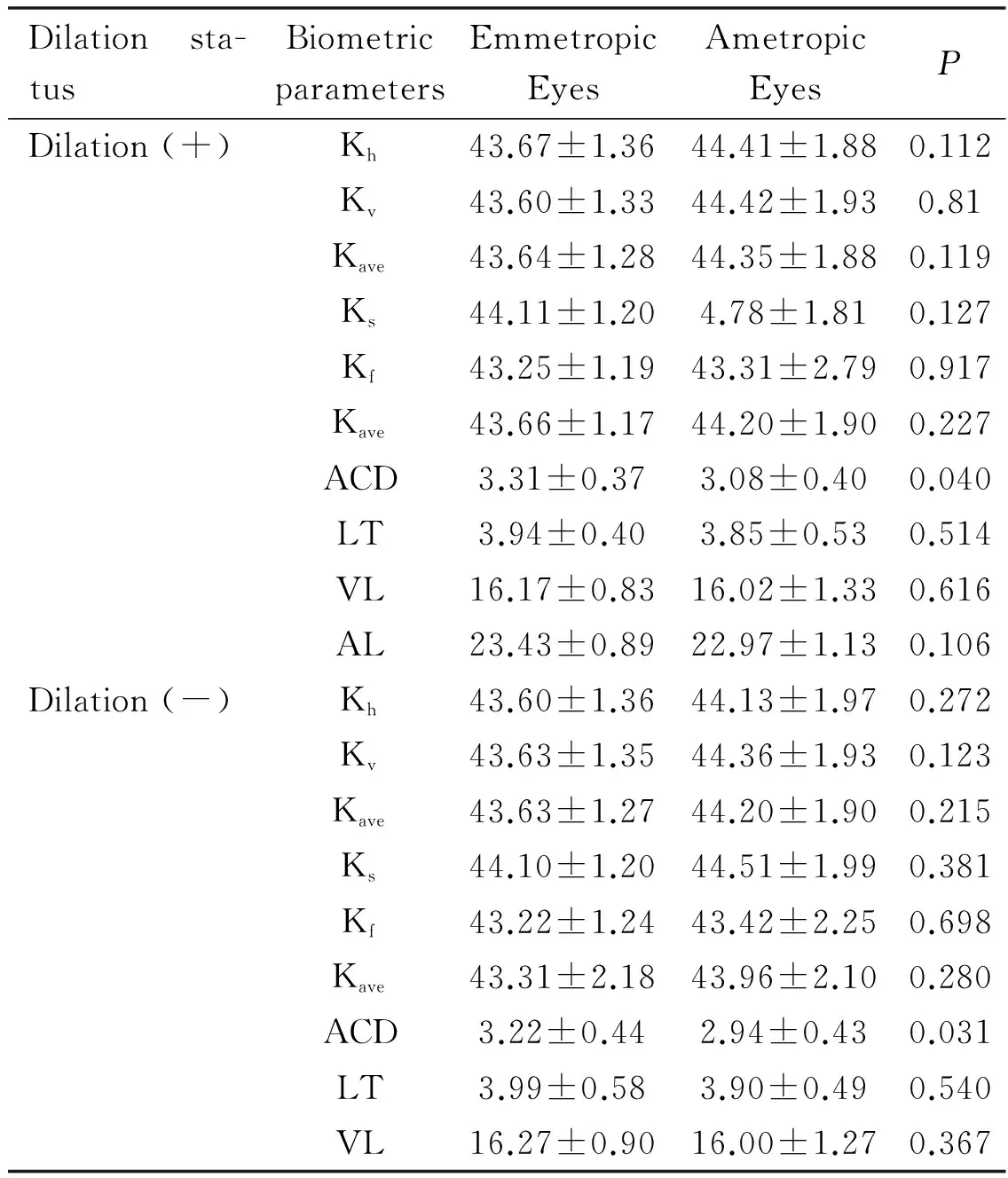
Dilationsta-tusBiometricparametersEmmetropicEyesAmetropicEyesPDilation(+)Kh43.67±1.3644.41±1.880.112Kv43.60±1.3344.42±1.930.81Kave43.64±1.2844.35±1.880.119Ks44.11±1.204.78±1.810.127Kf43.25±1.1943.31±2.790.917Kave43.66±1.1744.20±1.900.227ACD3.31±0.373.08±0.400.040LT3.94±0.403.85±0.530.514VL16.17±0.8316.02±1.330.616AL23.43±0.8922.97±1.130.106Dilation(-)Kh43.60±1.3644.13±1.970.272Kv43.63±1.3544.36±1.930.123Kave43.63±1.2744.20±1.900.215Ks44.10±1.2044.51±1.990.381Kf43.22±1.2443.42±2.250.698Kave43.31±2.1843.96±2.100.280ACD3.22±0.442.94±0.430.031LT3.99±0.583.90±0.490.540VL16.27±0.9016.00±1.270.367
Kh: K horizontal; Kv: K vertical; Kave: K average; Ks: K steepest; Kf: K flattest; ACD: Anterior chamber depth; LT: Crystalline lens thickness; VL: Vitreous length; AL: Axial length; Dilation (+): Pupilla dilated; Dilation (-): Pupilla undilated.
Of the eyes that received an IOL with power determined using SRK-T with measurements made during pupillary dilation, postoperative mean spherical equivalent was -0.50 to +0.50 D (emmetropic) in 30 (57.6%) eyes, and greater than ±0.50 D (ametropic) in 22 (42.27%) eyes. Of the eyes that received an IOL with power determined using SRK-T with measurements made while pupils were not dilated, postoperative mean spherical equivalent was -0.50 to +0.50 D (emmetropic) in 24 (46.15%) eyes, and greater than ±0.50 D (ametropic) in 28 eyes (53.8%) (Table 5).
Table 5Emmetropia status in all eyes with and without pupillary dilation
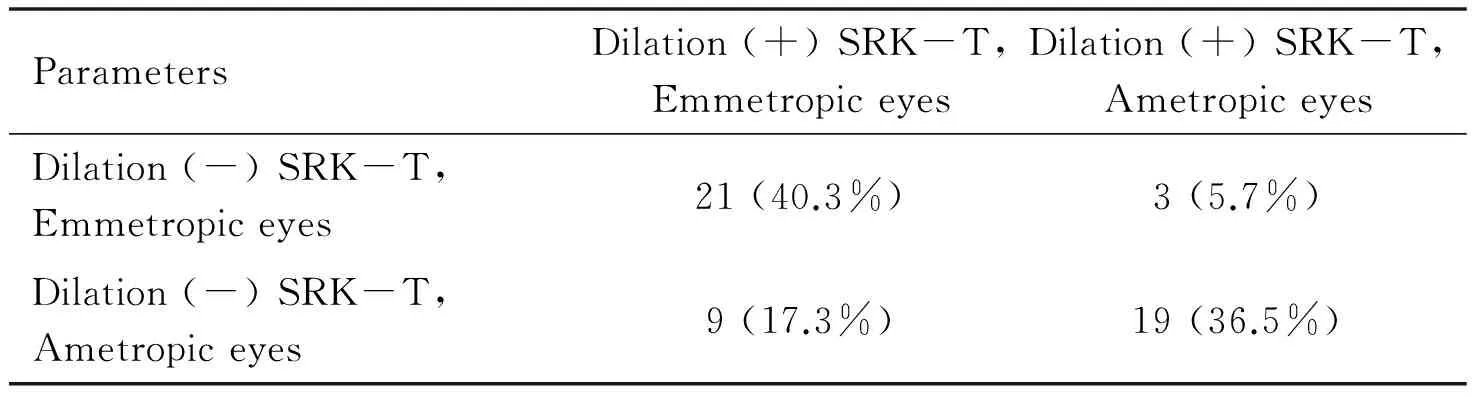
ParametersDilation(+)SRK-T,EmmetropiceyesDilation(+)SRK-T,AmetropiceyesDilation(-)SRK-T,Emmetropiceyes21(40.3%)3(5.7%)Dilation(-)SRK-T,Ametropiceyes9(17.3%)19(36.5%)
Dilation (+): Pupilla dilated; Dilation (-): Pupilla undilated.
Table 6Emmetropia and anterior chamber depth according to dilation status

ParametersEmmetropiceyesAmetropiceyesPDilation(+)ACD(mm)3.31±0.373.07±0.410.037Dilation(-)ACD(mm)3.17±0.463.02±0.460.246
ACD: Anterior chamber depth; Dilation (+): Pupilla dilated; Dilation (-): Pupilla undilated.
Table 7Emmetropization based on ACD values less than or greater than 3mm
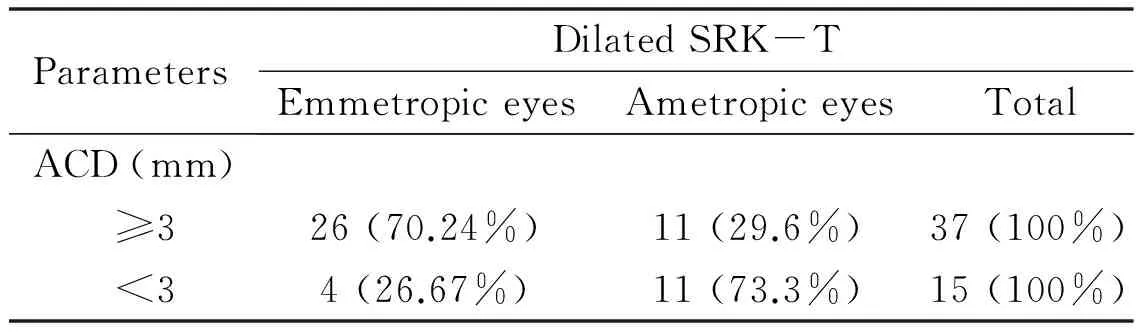
ParametersDilatedSRK-TEmmetropiceyesAmetropiceyesTotalACD(mm) ≥326(70.24%)11(29.6%)37(100%) <34(26.67%)11(73.3%)15(100%)
ACD: Anterior chamber depth.
When postoperative emmetropia status was compared in eyes with IOL power determined from dilated and undilated measurements, it was found that using measurements taken during pupillary dilation resulted in a significantly higher number of emmetropic eyes postoperatively (P=0.000).
In the eyes for which IOL power was calculated by SRK-T using measurements taken while dilated and emmetropization was achieved (postoperative spherical equivalent within ±0.50 D), ACD was 3.31±0.37 mm; in ametropic eyes (over ±0.50 D), ACD was 3.07±0.41 mm. ACD values were significantly larger in emmetropic eyes compared with ametropic eyes (P=0.037).
In cases where IOL power was calculated using measurements taken while pupils were not dilated, mean ACD values in the eyes with and without postoperative emmetropia were 3.17±0.46 mm and 3.02±0.46 mm, respectively. However, the difference was not statistically significant (P=0.246) (Table 6).
Of the 52 eyes measured during pupillary dilation, ACD was 3 mm or over in 37 eyes, and emmetropization was achieved in 26 (70.24%) of those eyes. ACD was under 3 mm in 15 eyes, and emmetropization was achieved in 4 (26.67%) of those eyes. Emmetropization occurred at a significantly higher rate in the group of eyes with ACD of 3 mm or over (P=0.005) (Table 7).
Using measurements taken during pupillary dilation yielded a mean IOL power of 21.24±2.81 D with the Binkhorst Ⅱ formula and 21.01±2.50 D with the SRK-T formula. When comparing the Binkhorst Ⅱ and SRK-T formulas independent of refractive error, the Binkhorst Ⅱ formula resulted in significantly higher IOL power (P=0.005). Using measurements taken without pupillary dilation in the Binkhorst Ⅱ and SRK-T formulas, mean IOL power was 21.01±2.50 D and 21.01±2.54 D, respectively. The same comparison between formulas revealed a statistically significant difference (P=0.005) (Table 8).
DISCUSSION
Today, the aim of cataract surgery is not just the removal of an opacifying crystalline lens, but also, parallel to the developments in surgical techniques and IOL technology, to achieve the best uncorrected visual acuity possible postoperatively[10]. In recent years, there has been increased demand from patients for cataract treatment to reduce the need for eyeglasses in the postoperative period. Cataract surgery has begun to be considered in a sense as refractive surgery. Patients’ vision quality as well as life quality have gained prominence[11]. Before the invention of IOLs, the eyeglasses and contact lenses used for postoperative refractive correction had many disadvantages. One positive aspect of refractive corrections using eyeglasses and contact lenses is that the examinations are performed after surgery and can be repeated if necessary for modification of the lenses[12]. In contrast, calculation of IOL power is done before cataract surgery. A mistake in this calculation necessitates a second surgical procedure. Therefore, the correct calculation of IOL power is extremely important[13-14].
Despite advances in surgical technique and technology, we may still face unexpected refractive outcomes after cataract surgery. The causes of these refractive deviations are errors in biometry, surgical complications, changes in lens localization, reverse placement of the lens, incorrect lens and manufacturer error[15-16]. In their study of the effect of pupillary dilation on IOL power calculation, Bansaletal[17]were unable to demonstrate an effect of pupillary dilation on axial length and postoperative refraction. Heatleyetal[18]used IOL Master and the SRK-T formula in their study and only found significant differences in Kvand average K values between measurements with and without dilation; no effect of pupillary dilation on axial length was detected. Similar to the results of Heatleyetal[18]we also found no statistically significant differences in the axial length, lens thickness, K values measured by topography and vitreous length when pupils were dilated and undilated. However, Khvalues measured by autokeratometry and anterior chamber thickness values measured during dilation were significantly larger. As there is no question of corneal contact before keratometric and topographic measurements, we tried to eliminate external factors that could affect corneal refractivity. However, the effect being in only one keratometric axis and the lack of effect in the K values obtained via topography suggested an error in keratometry measurement. Our results indicate that corneal refractivity measurements performed with topography are more reliable.
Table 8Comparison of BinkhorstⅡ and SRK-T formulas in terms of IOL power
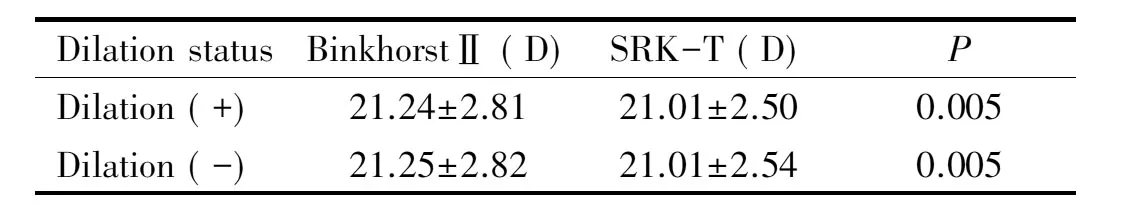
Dilationsta-tusBinkhorstⅡ(D)SRK-T(D)PDilation(+)21.24±2.8121.01±2.500.005Dilation(-)21.25±2.8221.01±2.540.005
Dilation (+): Pupilla dilated; Dilation (-): Pupilla undilated.
In this study we investigated the effect of pupillary dilation in emmetropic and ametropic eyes, and we found significantly larger anterior chamber depth values in emmetropized eyes with and without dilation. In ametropic eyes, smaller anterior chamber depth was found with undilated pupils. Considering these results, it appears that low anterior chamber depth measurements prevent emmetropization. Low ACD measurement suggests that pressure was applied to the cornea. Corneal pressure during biometric measurements using contact technique causes lower axial length measurement, resulting in an incorrect calculation of IOL power. IOL power was calculated with the SRK-T formula using both measurements taken while pupils were dilated and undilated. When emmetropization was evaluated postoperatively, IOL implants with power calculated using dilated pupil measurements corresponded to a significantly higher ratio of postoperative emmetropia. This indicates that calculating IOL power based on dilated eyes increases the likelihood of emmetropization, which may be attributed to larger anterior chamber depth measurements. In that case, the increase in anterior chamber depth values emerges as an advantage in the calculation of IOL power. In cases with IOL power calculated using measurements taken during pupillary dilation in the SRK-T formula, the eyes in which postoperative emmetropization was achieved had larger ACD values than the eyes which were not emmetropic postoperatively. There was a statistically significant increase in the rate of emmetropia among eyes with ACD values of 3 mm or greater as measured during pupillary dilation. This suggests that ACD value has an important role in the calculation of IOL power. Using the highest ACD value over 3 mm in biometric calculations increases the reliability of IOL power calculations. The accurate prediction of IOL power is closely related to both accurate biometric measurements as well as choosing an appropriate formula. Appropriate choice of formula reduces postoperative refractive errors and therefore the number of unsatisfied patients[19]. There are many formulas for calculating IOL power in order to minimize postoperative refractive errors. The superiority of theoretical versus empirical formulas in IOL power determination is a subject of debate. For eyes with average axial length (22-24 mm), no significant differences between second generation theoretical and empirical formulas in terms of postoperative refractive success are evident[20-21].
Donosoetal[22]compared the SRK Ⅱ, Binkhorst Ⅱ, Hoffer Q, SRK-T and Holladay formulas according to axial length and reported no significant differences between the formulas in patients with axial length between 22 and 28 mm. They emphasized that the Binkhorst Ⅱ and Hoffer Q formulas yielded the best results in eyes with axial length less than 22 mm, while the SRK-T formula was best for eyes with axial length over 28 mm. Retzlaffetal[23]compared the SRK-T, Holladay, SRK Ⅱ, Hoffer and Binkhorst Ⅱ formulas in a study of 1677 patients. They found that errors smaller than 0.5 D occurred at rates of 50%, 50%, 48%, 42% and 47%, respectively, and concluded that the Hoffer formula was significantly less accurate, while the other formulas were statistically equivalent. They determined that errors smaller than 1.00 D occurred at rates of 80%, 80%, 77%, 78% and 78%; SRK Ⅱ was significantly less accurate than the SRK-T and Holladay formulas, but differences between the other formulas were insignificant. The differences between formulas in terms of IOL power estimation and postoperative refractive outcomes are not large. However, unexpected postoperative refractive results do occur, and it has been shown that these are caused more by biometric measurement errors than the formulas themselves. Holladayetal[24]reported that differences of 1.00 D or greater between IOL power calculations from theoretical and empirical formulas were due to preoperative measurement errors in 92% of cases. With proper preoperative biometric measurement, unexpected postoperative high ametropia can be avoided. Using the SRK-T formula we achieved postoperative refractive errors within ±0.5 D in 51.9%, within ±1.00 D in 82.6%, and over ±1.00 D in 17.3% of the eyes in this study. Our results are similar to those of Retzlaffetal[23]When the Binkhorst Ⅱ and SRK-T formulas were compared independent of refractive errors, Binkhorst Ⅱ yielded significantly higher IOL power. The possibility of postoperative myopia should be considered when using the Binkhorst Ⅱ formula.
In conclusion, the results of our study indicate that the most important step in accurate IOL power calculation is keratometric and biometric measurement, rather than formula selection. Topography was shown to be a more reliable method of keratometric measurement. Taking biometric measurements after pupillary dilation increases the rate of emmetropia; furthermore, when conducting measurements, special attention should be given to anterior chamber depth, and in cases with an ACD less than 3 mm, repeating the biometric measurements is recommended. In cases with ACD values over 3 mm, using the largest value while calculating IOL power will minimize the margin of error. Using the SRK-T formula instead of the Binkhorst Ⅱ formula in the calculation of IOL power was shown to increase the rate of postoperative emmetropia.
REFERENCES
1 Zhao W, Zhao W, Zhao J, Wang D, Li J. Screening of potential target genes for cataract by analyzing mRNA expression profile of mouse Hsf4-null lens.BMCOphthalmol2015;15:76
2 Petrash JM. Aging and age-related diseases of the ocular lens and vitreous body.InvestOphthalmolVisSci2013;54(14):ORSF54-9
3 Woodfield AS, Gower EW,Cassard SD, Ramanthan S. Intraoperative phacoemulsification complication rates of second- and third-year ophthalmology residents a 5-year comparison.Ophthalmology2011;118(5):954-958
4 Sahin A, Hamrah P. Clinically relevant biometry.CurrOpinOphthalmol2012;23(1):47-53
5 Eleftheriadis H.IOLMaster biometry: refractive results of 100 consecutive cases.BrJOphthalmol2003;87(8):960-963
6 Haigis W. Challenges and approaches in modern biometry and IOL calculation.SaudiJOphthalmol2012;26(1):7-12
7 Lawuyi LE, Gurbaxani A. The clinical utility of new combination phenylephrine/ketorolac injection in cataract surgery.ClinOphthalmol2015;9:1249-1254
8 Alió JL, Abdou AA, Puente AA, Zato MA, Nagy Z. Femtosecond laser cataract surgery: updates on technologies and outcomes.JRefractSurg2014;30(6):420-427
9 Moon SW, Lim SH, Lee HY. Accuracy of biometry for intraocular lens implantation using the new partial coherence interferometer, AL-scan.KoreanJOphthalmol2014;28(6):444-450
10 Preussner PR, Olsen T, Hoffmann P, Findl O.Intraocular lens calculation accuracy limits in normal eyes.JCataractRefractSurg2008;34(5):802-808
11 Behndig A, Montan P, Lundström M, Zetterström C, Kugelberg M. Gender differences in biometry prediction error and intra-ocular lens power calculation formula.ActaOphthalmol2014;92(8):759-763
12 Lee AC, Qazi MA, Pepose JS. Biometry and intraocular lens power calculation.CurrOpinOphthalmol2008;19(1):13-17
13 Ianchulev T, Hoffer KJ, Yoo SH, Chang DF, Breen M, Padrick T, Tran DB. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery.Ophthalmology2014;121(1):56-60
14 Olsen T. Calculation of intraocular lens power: a review.ActaOphthalmolScand2007;85(5):472-485
15 Kirwan C, Nolan JM, Stack J, Moore TC, Beatty S. Determinants of patient satisfaction and function related to vision following cataract surgery in eyes with no visually consequential ocular co-morbidity.GraefesArchClinExpOphthalmol2015;253(10):1735-1744
16 Turhan SA, Toker E. Predictive Accuracy of Intraocular Lens Power Calculation: Comparison of Optical Low-Coherence Reflectometry and Immersion Ultrasound Biometry.EyeContactLens2015;41(4):245-251
17 Bansal S, Quah SA, Turpin T, Batterbury M. Biometric calculation of intraocular lens power for cataract surgery following pupil dilatation.ClinExperimentOphthalmol2008;36(2):156-158
18 Heatley CJ, Whitefield LA, Hugkulstone CE. Effect of pupil dilation on the accuracy of the IOLMaster.JCataractRefractSurg2002;28(11):1993-1996
19 Hoffmann PC, HÜTz WW, Eckhardt HB, Heuring AH.Intraocular lens calculation and ultrasound biometry: immersion and contact procedures.KlinMonblAugenheilkd1998;213(3):161-165
20 Carifi G, Aiello F, Zygoura V, Kopsachilis N, Maurino V. Accuracy of the refractive prediction determined by multiple currently available intraocular lens power calculation formulas in small eyes.AmJOphthalmol2015;159(3):577-583
21 Miraftab M, Hashemi H, Fotouhi A, Khabazkhoob M, Rezvan F, Asgari S. Effect of anterior chamber depth on the choice of intraocular lens calculation formula in patients with normal axial length.MiddleEastAfrJOphthalmol2014;21(4):307-311
22 Donoso R, Mura JJ, López M, Papic A. Emmetropization at cataract surgery. Looking for the best IOL power calculation formula according to the eye length.ArchSocEspOftalmol2003;78(9):477-480
23 Sanders DR, Retzlaff JA, Kraff MC, Gimbel HV, Raanan MG. Comparison of the SRK/T formula and other theoretical and regression formulas.JCataractRefractSurg1990;16(3):341-346
24 Holladay JT, Prager TC,Chandler TY, Musgrove KH, Lewis JW, Ruiz RS. A three-partsystem for refining intraocular lens power calculations.JCataractRefractSurg1988;14(1):17-24
(作者单位:133010土耳其梅尔辛梅尔辛Toros州医院眼科;233330土耳其梅尔辛梅尔辛大学医学院眼科)
通讯作者:Gülsüm Egemen Erkayhan. drerkayhan@yahoo.com.tr
DOI:10.3980/j.issn.1672-5123.2016.8.05
关键词:生物统计学;人工晶状体屈光度计算公式;散瞳
散瞳对人工晶状体屈光度数计算的影响
Gülsüm Egemen Erkayhan1, Ufuk Adigüzel2
摘要
