儿科用药发育毒性研究指标设定及中药安全性评价的特别关注点
2016-07-22孙祖越上海市计划生育科学研究所药理毒理学研究室中国生育调节药物毒理检测中心上海200032
周 莉,孙祖越(上海市计划生育科学研究所药理毒理学研究室,中国生育调节药物毒理检测中心,上海 200032)
·中药毒理学前沿——发育毒性·
儿科用药发育毒性研究指标设定及中药安全性评价的特别关注点
周 莉,孙祖越
(上海市计划生育科学研究所药理毒理学研究室,中国生育调节药物毒理检测中心,上海 200032)
周 莉,上海市计划生育科学研究所研究员,中国生育调节药物毒理检测中心(世界卫生组织人类生殖研究合作中心)副主任,复旦大学药学院硕士生导师。从事药物生殖药理毒理学、药物非临床安全性评价和前列腺疾病药理毒理学研究;作为副组长主持十二五“重大新药创制”专项等国家和部委课题研究6项;主持药理毒理学研究项目120余项。获上海市科技进步三等奖、中国实验动物学会科技进步二等奖和中国药学会科技进步三等奖各1项;授权专利8项(3项发明专利);主编出版专著《常见实验兔和大鼠畸形图谱》和《药物生殖与发育毒理学》;发表论文80余篇。现任中国毒理学会理事、中国毒理学会生殖毒理专业委员会秘书长和中国实验动物学会理事等学术职务。

摘要:目前新药开发过程中,对新生和幼龄动物非临床发育毒性研究的需求越来越迫切。本文结合作者实验室的经验,对儿科用药非临床安全性评价生长发育、摄食量等一般评价指标,以及中枢神经系统、生殖系统、行为学评估等特殊靶器官或系统评价指标的设定等进行讨论,并针对中药安评的特别关注点进行分析,以期为我国儿童用药非临床安全性研究提供支持和参考,为制定我国相关的指导原则积累素材。
关键词:儿科用药;幼龄动物;非临床安全性评价;发育毒性;中药
DOl:10.3867/j.issn.1000-3002.2016.01.004
近年来,对于儿科用药潜在毒性的关注越来越多,人们意识到儿童是一类独特的目标人群,不能根据成人用药情况来“以此类推”地判断儿童毒性。一些药物在儿童身上会呈现与成人不同的毒性表现,成熟与不成熟系统之间的差异可能导致儿科用药毒性的增加或减少。目前,临床上儿科用药缺乏来源于儿科人群安全性和有效性的信息,多半是基于成年动物或成人临床试验的安全性数据。然而,涉及到儿科用药的研发受到传统观念、伦理问题、经济和实用性等一系列的挑战,导致招募适龄儿童开展临床试验的难度增加。因此,全面而深入地开展非临床阶段儿科用药发育毒性研究就愈显重要。
儿科用药幼龄动物非临床研究中选择合适的观察指标是一个至今尚未完全解决的难题。本文结合作者实验室的实际工作经验,对儿科用药非临床安全性评价(安评)中生长发育和摄食量等一般评价指标,中枢神经、骨骼、生殖和泌尿等系统特殊靶器官和指标的设定,以及幼龄动物中药非临床安评的特别关注点进行逐一阐述。
1 一般评价指标
一般评价指标通常指的是生长发育、摄食量、血液与血清生化、大体观察和镜下观察等指标,现逐一分述如下。
1.1生长发育
幼龄动物非临床研究应测量体质量、生长速度、胫骨长度和器官质量等增长指标[1]。体质量和体质量变化是毒性的敏感指标,幼龄动物研究中体质量变化的重要原因是出生后早期增长相对快速。10周龄前的体质量每周至少称量2次,以检测其细微的变化。此外,在快速增长期增加体质量测量的频次有助于更准确地计算剂量。
除体质量外,还需要考虑幼龄动物身体成分的改变。出生后早期发育阶段全身含水量很高,至围生期随着年龄增长而降低[2]。因此,断乳前动物含水量较高而脂肪含量较少,这可能导致水溶性化合物在幼龄动物有更多的分布体积[3],而出生前即开始存储脂肪的只有豚鼠和人类。
此外,生长发育评价还应包括出生后整体生长和器官系统的功能评价(例如骨骼、肾、肺、神经、免疫、肝胆和生殖系统)。其中长骨长度(胫骨或股骨)的测量,可用于纵向评价,在人类和一些动物模型中出生后骨骼生长和发育模式是相似的。
1.2摄食量
摄食量是动物健康和潜在毒性的评价指标。断乳前,个体子代不进行摄食量评价。断乳后动物如单独饲养,则摄食量的评价比较可靠。如按每天每只动物计算,出生后28 d(postnatal day 28,PND28)开始大鼠整个生长阶段摄食量增加,但PND42时似乎处于平台期,其他实验室的结果也基本一致[4]。实际上,相对于体质量,大鼠摄食量随时间推移而逐渐减少,雄性和雌性非常相似;如按g·kg-1体质量计算,人的摄食量也在减少。设计和解释摄食量时还应考虑种属特异性,啮齿类动物的饲养模式与昼夜节律有关,大部分食物的摄入量是夜间发生,相比之下,人类、小型猪、非人灵长类动物和犬都不会受到这种方式的影响。
1.3血液与血清生化
虽然临床病理指标对于幼龄动物的研究非常有用,但会因为足够的样本量获得有限而影响技术分析的可行性,特别是啮齿类动物。对于<PND28的大鼠,整体的临床血清生化评价必须使用终点血样,<PND17的大鼠为了得到足够的样本量需要合并收集。幼龄大鼠、犬和小型猪出生后发育具有特定的模式[5-7],这3个种属胆固醇水平随年龄增长明显下降,随时间推移大鼠和犬总胆红素和尿素氮水平也明显下降。相反,犬在出生后的前20周,谷丙转氨酶增加约50%,大鼠从2~5周谷丙转氨酶增加约300%。
药物对临床生化参数的影响在观察前,需要搜集更多关于这些参数个体发生的信息。例如,发现一个药物诱导幼龄雄鼠尿液的α2-球蛋白聚集,应意识到正常情况下α2-球蛋白在青春期才在肝大量生成[8-9]。
除了评价标准的酶学参数,也可能评价与已知作用模式有关的特殊酶,如胆碱酯酶活性,从出生到PND42在大鼠脑组织中明显增加[4]。当药物靶器官是特殊的酶系统时,针对这类酶系统个体发育的过程设计和解释非临床毒性研究结果至关重要。
幼龄动物发育毒性研究中,如已知该类药物会影响造血系统或发育中的免疫系统,应考虑在特定发育阶段检测血液学指标。由于从个体动物收集足量血液存在技术挑战,缺少小动物(例如小鼠和大鼠)不同发育时间点的血液学参考值,且尚无一套完整的血液学参数用于合理解释实验结果。对于大动物(如犬、非人灵长类动物和小型猪)获得足量血液进行完整血液学检测的技术挑战不大,幼龄犬的标准血液学参数已显示从出生到青春期有一些特殊的差异[6]。
1.4大体观察和镜下观察
幼龄动物研究中,大体观察的重要性不言而喻。脏器的宏观变化与发育不良是一致的,如肾、肝、睾丸或骨骼等器官或组织具有相对的大小变化;如基于成年动物或人类的数据有已知的靶器官,则幼龄动物应检查这些器官。然而,组织病理变化与器官的成熟阶段有关(如生殖器官的睾丸),发育中的动物和成年动物之间的这些差异也是必须考虑的。新生儿肺通气量较低,肾小球和肾小管发育不全,新生儿大脑皮质和小脑表面留存有生发层,相对于胞浆骨骼肌和心肌的肌纤维较小并伴有大量核;随着发育成熟,肝髓外造血水平升高,出生后糖原消耗很快,脾和淋巴结中淋巴成分减少,骨形成和重构高度活跃,出生后甲状腺具有代谢调节作用,胶体减少和甲状腺滤泡上皮细胞的增大。
血管紧张素转换酶抑制剂的研究,可作为器官成熟受到影响的例子。如在成人观察到再生的肾小管上皮中有嗜碱小管,可认为是肾小管变性和不良影响的证据[10]。然而血管紧张素转换酶抑制剂雷米普利(ramipril)的幼龄动物研究中,发现PND17和PND28对照组和给药组动物有发育良好的嗜碱小管和片状相对不发育的嗜碱小管[5]。这项研究显示,雷米普利造成的不良影响增加了给药组动物嗜碱小管的发生率,表明动物的肾发育延缓。
2 特殊的靶器官和指标
根据药物的具体特性,以及在前期研究中注意到可能会增加特定器官或系统的毒性风险,即需要考虑对特定靶器官或系统进行观测。具体有中枢神经系统(central nervous system,CNS),生殖功能检测,性发育标志,免疫功能和泌尿、呼吸、心血管、胃肠道或骨骼系统等。
2.1中枢神经系统
对于药物非临床安全性研究来说,CNS的发育评价为通常指标。CNS是治疗儿童注意力缺陷,多动障碍和抗惊厥治疗的主要靶器官。FDA指导原则[1]关于幼龄动物研究也指出,对于发育中的神经毒性评价,应采用成熟的方法监测CNS的关键功能区域,包括评价个体发育反射、感觉运动功能、自发活动、反应性、学习和记忆功能。
此外,围生期发育毒性研究中,任何药物如果观察到CNS的相关变化,都应启动进一步的幼龄动物实验,还要包括CNS副作用恢复的可能性。CNS评价在标准的毒性研究设计中是最常见的附加指标,缘由既如上所述,也是监管部门的要求[11-12]。
2.1.1行为学测试指标
将行为学测试作为幼龄动物非临床研究的内容有一定困难,因为除了神经刺激外还有其他因素存在。影响因素包括给药的起始年龄、给药时期长短、选择的种属其CNS或外周神经系统是否仍在发育以及是否受试物引起成年动物某些系统的有害变化,进而导致内稳态失衡等。对某种受试物,系统的易感性有其关键窗口期,人类出生后神经系统的发育仍在继续,如儿科适应证包括一段时间的神经系统发育,监管部门可能建议进行行为学终点的评估。此外,关键系统的功能缺陷,可产生行为学上的不利影响,但要与直接的神经毒性区分。而且,不应忽视这些潜在的二次影响。行为学测试组合可灵活选择[13],如表1所列。
(1)自发活动 自发活动可通过总体活动、精细运动和步态活动进行量化,并采用运动总次数和步态活动(仅适用于啮齿类动物)表示。总体活动一般是粗略运动和精细电机运动总和(任何试验期间光束中断的情况)。步态活动仅测量大幅度运动。
PND13大鼠,通常总体活动次数相对较低,PND17达到断乳前的最大值,PND 21后活动次数减少[14]。标准的发育神经毒性研究中,大、小鼠自发活动通常是在出生后早期(PND13,PND17和PND21)进行纵向评价,进入成年期(PND60~61)再次进行评价[15]。更多的评价在围生期发育研究中进行[16],研究一般多采用纵向的方式。
(2)听觉惊愕 听觉惊愕实验中观察到的反应,应与其他行为学或生理实验指标进行比较,这对判断药物潜在的影响十分重要。一旦观察到反应的灵敏度降低与神经运动疲劳或中断相关,可能同时也会出现活动能力和握力的不良影响。实验过程中,体质量的严重下降可能干扰药物对听觉惊愕的影响,掩盖神经运动功能的细微变化。
(3)学习和记忆 学习和记忆功能包括比尔水迷宫、Morris水迷宫、主动回避和被动回避等,每个学习和记忆测试程序都有其自身的优缺点。在对整体反应评价得出结论前,分析其他行为终点与生理评价的相关性很重要。如在比尔迷宫评价中,从直道逃避的平均潜伏期延长可能与自发活动减少、听觉刺激的反应降低以及体质量下降有关。水迷宫检测,给药组与对照组比较时,首先确定给药组是否表现出游泳能力困难。当此参数有明显变化,则评价中的所有终点都可能受影响。发育迟缓会损害游泳能力。例如,宫内暴露丙硫氧嘧啶(pro-pylthiouracil)后,幼仔发育迟缓,游泳能力明显降低[17]。如断乳早期或在后续的测试中逃避时间延长,可能显示明确的运动神经障碍。
停药后或恢复期开展行为学检测,目的是评价给药后潜在的长期神经毒性结果而不是识别药物药理作用介导的影响。除了在体评价,CNS和周围神经系统病理组织学,包括形态学也需要评价[18]。大脑所有的主要区域(即嗅球、大脑皮质、海马、基底节、丘脑、下丘脑、中脑、脑干和小脑)均应检查以确保评价充分。
2.1.2身体发育指标
评价身体功能发育的常见指标是平面复正反射。虽然达到一个直立的姿态所需的时间减少与动物年龄有关,但这种反射仅出现在大鼠出生后不久。当观察到与对照组平均年龄出现统计学差异时,应考虑这些标志是与受试物相关的关键指标。
耳廓分离、出毛、门齿萌出、张耳和睁眼是广为接受的身体发育指标。耳廓分离、睁眼和牙齿萌出等断乳前的发育指标与体质量有明确的正相关,关键发育阶段仅仅收集体质量数据足以说明增长和发展的变化。这些指标可用于不同的研究设计,包括美国环境保护局(EPA)提出的多代实验研究和FDA提出的围生期发育毒性研究。对大鼠已有丰富的历史对照数据,这些指标能够也已经用于各种种属的评价,通过这些指标可洞察到发育延迟是直接给予受试物的结果。当然,这些发育指标的使用取决于暴露时的年龄和研究持续时间。

表1 个体发育反射和行为测试组合
2.2生殖功能检测
各种原因使得生殖系统评价在幼龄动物发育毒性评价研究中越来越普遍,无论生殖毒性或重复给药毒性研究中是否观察到某种“信号”,目前约有50%的幼龄大鼠研究设计中进行了生育能力评价[11]。与其他终点一样,幼龄或成年动物对药物暴露的敏感性不同导致对生殖功能的影响也不同。生殖发育和能力的评价是围生期发育毒性和生育力研究的一部分,尽管这些研究涵盖了主要的发育时期,然而在幼龄动物研究中仍存在“空白”需要填补。
生育力研究覆盖青春期到发育期,动物要待完全性成熟才能进入交配阶段,故大鼠一般在6~7周开始给药。药物的围生期发育研究是分别通过宫内暴露和乳汁暴露,评价发育中的子代生育能力,因此,评价的是药物对发育中生殖系统的影响,并不能完全覆盖从断乳到青春期。此外,乳汁中摄入的药物如未达一定水平,则通过哺乳的暴露量可能不足,且在断乳到青春期这段时间内,无法评价药物的暴露情况。因此,幼龄动物发育毒性研究要结合生育力评价研究。
啮齿类动物生殖毒性研究中上述评价是常规进行的。其评价方法有很多记载,已建立了啮齿类常用品系正常表现、时间架构等背景资料使得更易于评价,当然这并不妨碍其他合适种属的使用。然而,犬的生殖评价耗时较长,且生殖参数指标不具有良好的特点。大鼠的上述评价指标旨在筛选,而不是阐明作用机制。
后续可能需要更详细的研究,如激素水平检测(如雌二醇、孕酮、催乳激素、睾酮、促卵泡激素和促黄体激素)。这些检测的目的不是观察基因或细胞功能的细微变化,通常其细胞或分子机制尚不清楚。
普遍认为,由于啮齿动物产生大量过剩的精子,当睾丸损伤并对精子生成产生影响时,大鼠生育力是一个相对不敏感指标,但交配行为的评价是可行的(如交配前间隔时间等)。而睾丸组织病理学(包括睾丸分期)是一个敏感的指标,其他如精子数量、活动性和形态学评价(精液学)可检测出既未影响交配也未见组织病理改变的问题(如附睾精子的晚熟和精子功能的影响)。
2.3性发育标志
身体发育的最初评价是性发育和青春期的启动,包括雄性睾丸的下降、龟头包皮裂开和阴道张开。睾丸下降由雄激素和重力控制,人类在出生前,犬在5~6周,大鼠在15 d;由于主观性较高,已被主观性较少的龟头包皮裂开(包皮从龟头分离)所取代。因此,目前幼龄和成年生殖研究中两个性发育的标准指标是龟头包皮裂开和阴道张开,它们因性激素的存在而启动,故在发情期开始前检查这些指标。大鼠龟头包皮裂开发生在45 d,人类一般9个月至3岁,其中雄激素发挥着关键作用。大鼠阴道口张开发生在34~36 d,此后在首次排卵后第2天促性腺激素激增,这是青春期开始的标志。
每天检查龟头包皮裂开,直到发现包皮与阴茎分离。有人认为重复检查会使得包皮分离加快;然而为了确定不错过达标时间,许多实验室甚至在PND35~40开始检查[19]。我们实验室从PND40开始此项检查[20]。龟头包皮裂开与体质量相关,但是其他决定因素,如性激素的刺激或者化学内分泌调节物,也会干扰这一标志的正常发育。
雌性阴道张开的检测是通过检查覆盖在阴道口的被膜是否破裂。通常大鼠检查的初始年龄是PND25~30。我们实验室从PND30开始此项检查[20]。阴道张开与体质量呈正相关,生长中明显的变化可改变达标时间。除了生长的影响,雌激素和抗雌激素化合物能明显影响首次观察到阴道张开的时间。观察阴道张开时,有时会发现阴道腔偶尔出现残留细线组织。细线组织的存在并不影响动情周期或交配,它可能被观察者或在交配期去除。然而,如这一组织细线一直存在,是否应认为阴道是张开的意见似乎有不同。Gray和Ostby[21]指出,将有细线组织的动物从分析中淘汰,对2,3,7,8-四氯二苯并二噁英(TCDD)阴道张开达标的平均年龄没有影响。有些实验室认为,只要细线组织横跨阴道腔口即认为阴道张开。
2.4免疫功能检测
幼儿和成人组织(或器官)形态差异最显著的也许就是免疫系统(或器官)。新生和幼龄大鼠胸腺组织类似于年轻成年人的胸腺,新生大鼠脾和淋巴结细胞减少、缺乏滤泡和生发中心,意味着免疫反应性的过程相当于成人组织。而大鼠免疫系统约42 d才达到成人的组织形态[22]。造血方面,妊娠15 d的大鼠胎仔以肝为中心,妊娠20 d时不完全迁移至骨髓,但是新生大鼠肝仍然保存部分造血作用直至≥PND10。幼龄动物毒性研究如涉及发育免疫毒性,要考虑免疫系统具有延迟成熟的特点。
通常幼龄动物毒性研究不包含免疫毒性实验,尽管FDA指导原则[1]对免疫系统和器官发育提供了一些信息以及发育时期对照表,但并未对儿科用药的安评提出具体的实验建议。欧洲药品管理局(EMA)(人用药品委员会,CHMP)指导原则[23]指出,如某类药物或此前进行的研究中显示应关注发育中的免疫系统,则要进行免疫毒性实验;实验设计应基于验证分析,但正如所有幼龄动物的研究一样,要保持灵活性。除了功能验证,如T细胞依赖抗体反应、细胞介导免疫测定和组织病理等,应符合人用药品注册技术要求国际协调会议(ICH)免疫毒性指导原则[24]中描述的方法。
筛查包括常规指标如血液学(白细胞分类计数)、脏器质量和组织病理〔如胸腺、脾、淋巴结、Peyer板(肠道集合淋巴结)和骨髓涂片等〕,还应包括胸腺发育(如B细胞和T细胞)以及通过上述功能的检测以评价体液免疫和细胞免疫的完整性[25]。
迟发型超敏反应被认为是最敏感的实验,刚断乳大鼠可以进行评价[26]。其他不经常使用的测试方法包括流式细胞仪或ELISA技术检测细胞因子和免疫球蛋白水平[25]。
2.5泌尿系统
人类怀孕34周时肾发生已经完成,但出生后第1年内肾单位的成熟和肾小管的延长仍在继续。小鼠和豚鼠类似,出生时肾发生已完成,因此采用这些种属进行出生后肾发育毒性的评价是不合适的。大鼠从出生到第8天肾发生开始,4~6周完成肾发生[27]。犬2周时完成肾发生。
肾小球滤过率是测量肾功能的合适指标。人类肾小球滤过功能从出生前的胎儿期开始启动,持续增加,1~2岁时达成人水平。大鼠肾小球滤过率在出生6周内快速增加,犬出生后1~6周也会增加[27]。
针对这些差异,幼龄动物实验中关于肾发育研究的设计,需要考虑肾成熟时间的变化及种属间解剖和功能方面的不同,这对于种属选择(大鼠或犬)以及给药开始的年龄可能产生很大影响。
2.6呼吸系统
不同种属呼吸系统的解剖结构和功能成熟时间不同。大鼠和人类的肺脏发育阶段很相似,故认为幼龄大鼠是可接受的模型,如表2所示。很少用犬进行此类研究,由于出生时的生物差异性,犬出生后肺发育一直表现相互矛盾的结果。但对于<2岁的儿童吸入制剂的安全评价,仍然认为是合适的种属。兔和非人灵长类动物出生时肺发育已达到比较高级的阶段,因此不适合作为幼龄动物肺发育的评价模型。
对于吸入制剂,大鼠最早可在出生后第1天通过全身暴露开始给药,最大暴露时间约6 h。由于母鼠可能也不得不置于吸入装置内,而随后开始的梳理活动以及通过母乳,药物可能通过皮肤和口服混合暴露,故这是一个相当不精确的暴露途径。犬在大约2周时,可进行吸入暴露研究;而此时幼鼠可离开母鼠长达4 h,大鼠一般在断乳后4周时进行。
2.7心血管系统
在生化和电生理学方面,人类、犬和大鼠有很多细节的不同[28]。大鼠功能性神经分布发生在出生后,伴随副交感神经系统首先成熟。出生2~3周发生阳离子对交感神经的反应。犬出生时心的神经分布在结构上和功能上还不成熟,交感和副交感神经系统大约7周时功能完善。标准的重复给药毒性实验是检测血压、心率、心电图(PQ、QT和QRS间期)这些指标。如果有特殊考虑以心脏作为靶器官,选择种属之前建议进行详细文献检索。
2.8胃肠道系统
通常认为实验动物和人类胃肠道系统的生长和发育模式相似,虽然没有一个实验动物种属是人类出生后胃肠道发育的完美模型,但通过适当的动物实验设计,可评估出生后胃肠道的发育情况。人类新生儿的胃液pH值高,随着时间的推移显著降低,2岁时达到成人水平。大鼠6周时达到成年的胃液分泌水平,而犬是在13周。人类胰腺功能约在2岁成熟,实验动物则在断乳时成熟。
2.9骨骼系统
实验动物出生后骨骼生长和发育模式与人类相似,通过简单的顶臀长或长骨长度的活体测量,如股骨和胫骨进行骨骼发育的初筛,表3是我们实验室开展的药物安评中部分骨骼检测数据,大剂量受试物可影响幼龄动物的骨骼发育。如果特殊关注骨骼系统,血液和尿液的骨转换生物标志物(如骨钙素、胶原Ⅰ型氨基端前肽键、羧基端肽和氨基端肽等)、解剖后检测的如骨矿物质含量和骨矿物质密度(骨密度)、骨组织形态学和骨强度的评价(生物力学)等可作为深层次的评价指标。

表3 AAA小儿颗粒浸膏对幼龄大鼠骨密度的影响
骨组织形态测量学是对骨骼组织的定量评价,它通过观察未脱钙骨骼架构部分提供结构、静态、动态和微结构参数。生物力学测试包括测量长骨的抗弯和抗扭强度,以及椎体和股骨颈的抗压强度,强度是几何形状、大小、皮质厚度、密度、结构和组成成分的函数。何时评价这些参数取决于受试药物的药理活性以及前期的成年动物重复给药毒性研究或幼龄动物的剂量探索性研究资料。建议使用逐级方法,先期数据可用来启动进一步的观察[29]。
上述分析清楚地发现,同种动物不同系统或器官,其发育成熟的终结点不全相等,如神经系统发育成熟的时间点较早,而生殖系统发育成熟的时间点较晚。因此,幼龄动物发育毒性研究中指标的观测点可以先后不一,具体要根据目标系统或器官发育成熟时间段的长短而设定。这将决定了同一个剂量组幼龄动物可能要再分列出好几个不同的观测指标亚组。这样的“卫星组”设定方式是区别于一般毒理学研究中剂量组设定方式的主要方面。
3 中药非临床安全性评价的特别关注点
鉴于中药儿科用药非临床安评的特殊性,我们要更加关注其与化学药的不同之处,特别在观察指标的选择上,要针对目标适应证和中药处方组成及特性进行设计,主要考虑如下。
3.1全面设定指标
许多中药既无成年动物非临床安全性毒性数据,又无规范的临床人群毒性研究的数据,此时开展幼龄动物中药安评时,就要设立较为全面的指标,以提高毒性阳性结果的检出率。
3.2加强激素水平的检测
许多中药往往具有激素样活性成分,临床服用时,可能导致成人的生殖与发育的异常。对这类中药的研究,可重点检测幼龄动物体内激素水平。
3.3重点采用临床提示的相关指标
众多中药有一定的临床用药经验,并积累了一定的毒性数据。即便是成人的临床数据,对开展幼龄动物发育毒性研究也极具参考价值,要重点采用已知的临床提示指标。
3.4及时清除给药动物的中药异味
中药时常带有强烈的药物异味,在开展离乳前动物给药时,为了避免母鼠因异味而拒绝哺乳的可能,实验人员在给药时应采取措施,尽量清除幼龄动物身上中药的残留气味。
3.5鼓励开展伴随毒代动力学研究
中药较多的是复方用药,成分复杂,物质基础及作用机制不甚明确,有效成分或毒性成分不清,开展伴随毒代动力学研究难度较大。但是,为了更好地开展安全性评价,应选用有毒成分或含量较高的指示成分进行检测,开展伴随毒代动力学研究可更好地解释安评中出现的毒性反应。
4 结语
当决定开展幼龄动物研究时,最重要的考虑是药物的药理作用、预期的毒性靶器官或系统及对成年动物前期的毒性研究和成人研究的临床经验。由于幼儿生长发育迅速,许多细胞、组织和器官系统的结构和功能特点与成人有很大差异,不同的生理和代谢因素、药代动力学和行为模式可能使儿童的反应比成人更加敏感或迟钝。因此,当关注到药物有可能影响生长发育,特别是如果有可能增加器官系统毒性的风险因素,基于人群预期的临床发展,在指标设定时考虑和分析应更周到。
参考文献:
[1]FDA.Guidance for Industry:Nonclinical Safety EvaluationofPediatricDrugProducts[Z/OL].Silver Spring,MD:US Department of Health and Human Services,Center for Drug Evaluation and Research.(2006-02)[2015-12-18]http://www. fda.gov/downloads/drugs/guidancecompliancereg-ulatoryinformation/guidances/ucm079247.pdf
[2]Adolph EF,Heggeness FW.Age changesin body water and fat in fetal and infant mammals [J].Growth,1971,35(1):55-63.
[3]Ginsberg G,Hattis D,Sonawane B,Russ A,Banati P,Kozlak M,et al.Evaluation of child/ adult pharmacokinetic differences from a data-base derived from the therapeutic drug literature [J].Toxicol Sci,2002,66(2):185-200.
[4]Hood RD.DevelopmentalandReproductive Toxicology:APractical Approach[M].3rd ed. London:CRC Press,2012:302-339.
[5]Beck MJ,Ching SV,Leung E,Moorman AR,Lai AA,Radovsky A,et al.An acute toxicity and pharmacokineticstudyoftheaceinhibitor,ramipril(R),in juvenile rats[J].Birth Defects Res A,2004,70:294-323.
[6]Robinson K,Smith SY,Viau A.Dog juvenile toxicity [M]//Hoberman AM,Lewis EM.Pediatric Nonclin-ical Drug Testing:Principles,Requirements,and Practices.Hoboken,New Jersey:John Wiley& Sons,2012:183-212.
[7]Svendsen O.The minipig in toxicology[J].Exp Toxicol Pathol,2006,57(5):335-339.
[8]Roy AK,Schiop MJ,Dowbenko DJ.Regulation of the hepatic synthesis of alpha2u globulin and its corresponding messenger RNA in maturing male rats[J].FEBS Lett,1976,70(1):137-140.
[9]Neuhaus O W,Flory W.Age-dependent changes in the excretion of urinary proteins by the rat[J]. Nephron,1978,22(4-6):570-576.
[10]Gopinath C,Prentice DE,Lewis DJ.The Urinary System[M]//Gopinath C,Prentice DE,Lewis DJ. Atlas of Experimental Toxicological Pathology. Boston:Kluwer Academic Publishers,1987:78.
[11]Bailey GP,Mariën D.The value of juvenile animal studies″What have we learned from preclinical juvenile toxicity studies?Ⅱ″[J].Birth Defects Res B,2011,92(4):273-291.
[12]Cappon GD,Bailey GP,Buschmann J,Feuston MH,Fisher JE,Hew KW,et al.Juvenile animal toxicity study designs to support pediatric drug development [J].Birth Defects Res B,2009,86(6):463-469.
[13]Moser VC.Applicationsofa neurobehavioral screening battery[J].Int J Toxicol,1991,10(6):661-669.
[14]Campbell BA,Lytle LD,Fibiger HC.Ontogeny of adrenergicarousalandcholinergicinhibitory mechanisms in the rat[J].Science,1969,166 (395):635-637.
[15]Stump DG,Beck M J,Radovsky A,Garman RH,Freshwater LL,Sheets LP,et al.Developmental neurotoxicitystudyofdietarybisphenolAin Sprague-Dawley rats[J].Toxicological sciences,2010:kfq025.
[16]FDA.ICH Harmonized Tripartite Guideline.Detection of Toxicity to Reproduction for Medicinal Products& Toxicity to Male Fertility S5(R2).Fed Regist[EB/OL]. (1994-10)[2015-12-18]http://www.ich.org/prod-ucts/guidelines/safety/article/safety-guidelines.html
[17]Sun ZY,Zhou L.Drug Reproductive and Develop-mental Toxicology(药物生殖与发育毒理学)[M]. Shanghai:Shanghai Science and Technology Press,2015:368-388.
[18]De Groot DM,Bos-Kuijpers MH,Kaufmann WS,Lammers JH,O′callaghan JP,Pakkenberg B,et al. Regulatory developmental neurotoxicity testing:a model study focusing on conventional neuropa-thology endpoints and other perspectives[J]. Environ Toxicol Pharmacol,2005,19(3):745-755.
[19]Clark RL.Endpointsofreproductivesystem development[M]//Daston G and Kimmel C.An Evaluation and InterpretationofReproductive Endpointsfor Human Health Risk Assessment. Washington,DC:ILSI Press,1998.
[20]Juan J,Li Z,Sun ZY.The exploration of stan-dardized experimental method in drug prenatal toxicity test[J].Chin J New Drugs,2015,24 (14):1631-1635.
[21]Gray LE,Ostby JS.In utero 2,3,7,8-tetrachlorod-ibenzo-p-dioxin(TCDD)alters reproductive mor-phology and function in female rat offspring[J]. Toxicol Appl Pharmacol,1995,133(2):285-294.
[22]Burns-Naas LA,Hastings KL,Ladics GS.What′s so special about the developing immune system?[J].Int J Toxicol,2008,27:223.
[23]European Medicines Agency(EMA),Committee for Human Medicinal Products(CHMP).Guideline on the Need for Non-clinical Testing in Juvenile Animals on Human Pharmaceuticals for Pediatric Indications[EB/OL].(2008-01)[2015-12-18]http://www.ema.europa.eu/docs/en_GB/document_li-brary/Scientific_guideline/2009/09/WC500003305.pdf
[24]ICH S8:Immunotoxicity Studies for Human Phar-maceuticals,ICH[EB/OL].(2005-09)[2015-12-18]http://www.ich.org/products/guidelines/safety/article/ safety-guidelines.html
[25]Barrow PC,Ravel G.Immune assessments in developmental and juvenile toxicology:practical considerations for the regulatory safety testing of pharmaceuticals[J].Regul Toxicol Pharmacol,2005,43(1):35-44.
[26]Bunn TL,Dietert RR,Ladics GS,Holsapple MP. Developmental immunotoxicology assessment in the rat:age,gender,and strain comparisons after exposure to lead[J].Toxicol Method,2001,11:1-18.
[27]Zoetis T,Hurtt ME.Species comparison of ana-tomical and functional renal development[J].Birth Defects Res B,2003,68(2):111-120.
[28]Hew KW,Keller KA.Postnatal anatomicaland functional development of the heart:a species comparison[J].Birth Defects Res B,2003,68 (4):309-320.
[29]De Schaepdrijver LM,Bailey GP, Coogan TP,Ingram-Ross JL.Juvenile animal toxicity assess-ments:decision strategies and study design[J]. Pediatr Drug Development:Concept Appl,2013:201-221.
(本文编辑:齐春会)
Abstract:OBJECTlVE To study the therapeutic effect and the underlying mechanism of asiatico-side on bleomycin-induced rat interstitial pulmonary fibrosis(IPF).METHODS Male Sprague-Dawley (SD)rats were divided into normal control group,bleomycin 5 mg·kg-1model group and asiaticoside 50 mg·kg-1group.The model and asiaticoside group were administrated with bleomycin 5 mg·kg-1to induce IPF,while the asiaticoside group was administrated with asiaticoside 50 mg·kg-1by gastric perfusion. Hematein eosin(HE)and Masson staining were carried out to analyze the histopathological changes in the lung.Lung homogenates were used to examine hydroxyproline(HYP)content,and serum samples were used to measure the concentration of interferon-γ(IFN-γ),interleukin-4(IL-4)and tumor necrosis factor-α(TNF-α).In addition,immunohistochemical methods were used to locate lung transforming growth factor-β1(TGF-β1)and adenosine 2A receptor(A2AR)expression,and Western blotting was used to examine the expression levels of TGF-β1and A2AR.RESULTS On the 7th,14th and 28th days,the scores of pulmonary inflammation were higher in model group than in control group (P<0.01),and the asiaticoside group showed mitigated alveolitis(P<0.01,P<0.05)compared with model group.Compared with control group,the scores of pulmonary fibrosis in model group were elevated(P<0.01),and the asiaticoside group showed reduced pulmonary fibrosis(P<0.05).On the 14th and 28th days,HYP content in the model group〔1.85±0.10,(2.48±0.18)mg·g-1〕was higher than in the control group〔0.79±0.07,(0.84±0.08)mg·g-1〕(P<0.01),but HYP content in the asiaticoside group〔1.32±0.131,(1.71±0.13)mg·g-1〕was lower than in the model group(P<0.05).IL-4 and TNF-α in the asiaticoside group were lower than in model group(P<0.05),but were higher in the model group than in the control group(P<0.01,P<0.05).The expression level of TGF-β1protein in the asiaticoside group was lower than in the model group(P<0.05),but was higher in the model group than in the control group(P<0.05).The expression level of A2AR protein in the asiaticoside group was higher than in the model group(P<0.05),but was lower in the model group than in the control group(P<0.05). CONCLUSlON Asiaticoside can mitigate bleomycin-induced IPF by inhibiting the expression of IL-4,TNF-α and TGF-β1,and raising the level of A2AR.
Key words:asiaticoside;pulmonary fibrosis;interferon-gamma;interleukin-4;tumor necrosis factor-alpha;adenosine A2 receptor
CLC number:R285.5Document code:AArticle lD:1000-3002(2016)01-0029-09
DOl:10.3867/j.issn.1000-3002.2016.01.005
Biographies:YE Wen-jing,female,postgradute,main research field is interstitial pulmonary fibrosis and pulmonary hypertension;XIE Xu-ying,female,pharmacist,main research field is the pharmacology mechanism of traditional Chinese medicine monomer.
Interstitial pulmonary fibrosis(IPF)is a type of chronic inflammatory disease characterized by alveolitis,interstitial pneumonia,alveolar epithelial damage,and abnormal accumulation of collagen. However,the etiology of IPF remains unclear[1-2]. Fibrosis induced by inflammatory cascade is importanttothepathogenesisofpulmonary fibrosis.IPF is a progressive disease whose primary clinical symptoms include progressive dyspnea,wheezing,shortness of breath,coughing and difficulty breathing.Conventional glucocorti-coids and cytotoxic drugs show poor effects. Lack of a specific treatment leaves most patients to die of respiratory failure.It is of great importance to find treatments and drugs that are efficacious and have few side effects for treatment of IPF.
Centella asiatica(also known as Luo-de-da,Beng-da-wan,and Ban-bian-qian in China)belongs to the subfamily Mackinlayoideae of the family Apiaceae,and it is a traditional Chinese medicine (TCM).It is of a cold nature and tastes bitter and acrid and has the effect of clearing heat,removing dampness,reducing swelling,and detoxification.Shen[3]considered that interstitial pneumonia belongs to the wind-warm-dampness diseases in TCM and found that C.asiatica can alleviate the symptoms,promote lung lesions absorption,and improve lung function.Asiatico-side is the main active ingredient of Centella asiatica.It is a triterpenoid saponin.Used mainly in the treatment of wound healing,keloids,and scleroderma, asiaticosideshowsespecially good clinical efficacy in the treatment of sclero-derma skin fibrosis,but the exact mechanism is not clear.Studies have shown that asiaticoside can inhibit fibroblast proliferation and suppress protein and mRNA levels of typeⅠandⅢcolla-gens[4-5].Another study showed that asiaticoside can significantly improvelunglesions,inhibit mitogen-activated protein kinase activity,sup-press the expression of tumor necrosis factor (TNF)-α and interleukin(IL)-6,and increase the expression of peroxisome proliferatoractivated receptor(PPAR)-γtoantagonizenuclear factor(NF)-κB activity in the lungs of mice with sepsis[6].It is here speculated that this herb may be able to prevent the progress of IPF by pre-venting lung inflammation and fibrosis.In this study,asiaticoside was found to ameliorate bleo-mycin-induced IPF in rats and a possible mecha-nism was explored.
1 MATERlALS AND METHODS
1.1Drug,reagents and equipments
Bleomycin was purchased from Hebei Phar-maceutical Factory,Tianjin(Tianjin,China). Rat IL-4,IFN-γ and TNF-α ELISA kits were purchased from Invitrogen(California,USA). The hydroxyproline assay kit was purchased from Nanjing Jiancheng(Nanjing,China).The immunohistochemical analysis kit was purchased fromBoshideBiologicalProductsCompany (Wuhan,China).Asiaticoside was purchased from Sigma(St.Louis,MO,USA).Transforming growth factor-β1(TGF-β1)andadenosine 2A receptor(A2AR)antibodies(ab92486,ab3461)were purchased from Abcam(Cambridge,UK). Mini-PROTAN Vertical Electrophoresis Cell and xMark Microplate Absorbance Spectrophotometer were purchased from Bio-Rad(California,USA). Electric-heated Thermostatic Water Bath was purchased from HEDE Labovatory Equipment Co. Ltd(Shanghai,China).
1.2Animals,group and treatment
Male Sprague-Dawley(SD)rats,aged 2 to 3 months,180-250 g,were purchased from ShanghaiExperimentalAnimalCenterand housed there and fed standard food.Animal experiments were approved by the Institutional Animal Ethics Committee for Experimentation on Animals of Wenzhou Medical University and complied with the animal management rule of theex-Ministry of Health,China and the US National Institutes of Health Guide.The rats were randomly divided into three groups:normal control group,model group,and asiaticoside group.Each group had 15 rats.The rats were anesthetized by chloral hydrate.The model group and the asiaticoside group were given bleomycin5 mg·kg-1and normal control group was given 1 mg·kg-1saline by intratracheal perfusion.From the 1st day of the experiment,the control group and model group were given saline 10 mg·kg-1per day and the asiaticoside group was given asi-aticoside 50 mg·kg-1per day by gastric perfusion. After bleomycin administration,5 rats were anes-thetized by chloral hydrate per 7 d,blood samples were taken from abdominal aorta,and trachea and lungs were isolated.Lungs were rinsed with saline.The left lung was stored at-80℃to prevent RNase contamination;the right lower lung was fixed in 4%paraformaldehyde,embedded with paraffin,sectioned for HE,Masson staining and immunohistochemical analysis.
1.3Alveolitis and fibrosis grade for semiquantitative pathological analysis
Sections of the right upper lobe of the lung were checked by Hematein eosin(HE)and Mas-son staining.The extent of alveolitis and fibrosis was determined using methods described by Szapiel et al[7].It was dividedinto four grades for semi-quantitative pathological analysis.
1.3.1Alveolitis grade criteria
None(0),no alveolitis;mild(1+),thickening of the alveolar septum by a mononuclear cell infiltrate,with involvement limited to focal,pleuralbased lesions occupying less than 20%of the lungs and with good preservation of the alveolar architecture;moderate(2+),a more wide-spread alveolitis involving 20%to 50%of the lungs,although still predominantly pleural based;severe(3+),a diffuse alveolitis involving more than 50%of the lungs,with occasional consolidation of air spaces by the intra-alveolar mononuclear cells and some hemorrhagic areas within the interstitium and/or alveolus.
1.3.2Fibrosis grade criteria
None(0),no evidence of fibrosis;mild (1+),focal regions of fibrosis involving less than 20%of the lungs.Fibrosis involved the pleura and the interstitium of the subpleural parenchyma with some distortion of alveolar architecture;moderate(2+),more extensive fibrosis involving 20%to 50%of the lung and fibrotic regions mostly extending inward from the pleura and still focal;severe(3+),widespreadfibrosis, involving more than 50%of the lungs.Confluent lesions withextensivederangementofparenchymal architecture,including cystic air spaces lined by cuboidal epithelium.
1.4Hydroxyproline content in lung tissue by biochemical method
HYP content of lung tissue was measured bycolorimetryusingbiochemicalmethods according to kit instructions.Results are presented as HYP(mg)per gram of lung tissue.
1.5Concentration of lFN-γ ,lL-4 and TNF-α in serum by ELlSA
The levels of IFN-γ,IL-4 and TNF-α in serum were measured by ELISA according to the manu-facturer′s instructions(eBioscience).Data were quantified using a standard curve.
1.6Location of TGF-β1and A2AR expression in lungs by immunohistochemistry
Paraffin-embeddedsectionsof the right lower lobe of the lung were used for immunohisto-chemical analysis using the streptavidin-biotinperoxidase(SABC)method according to the man-ufacturer′s instructions.The first antibodies were anti-TGF-β1and A2AR,both of which were poly-clonal antibodies,and the secondary antibody was goat anti-rabbit.
1.7Expression level of TGF-β1and A2AR by Western blotting
Frozen tissue samples were homogenized in lysis buffer followed by three pulses of sonication and a centrifugation at 1509×g for 20 min.Total protein concentration was determined by Bradford assay.Proteins were separated by SDS-PAGE andtransferredtoapolyvinylidenefluoride membrane.The membrane was blocked in skim milk and incubated overnight with primary anti-body at 4℃.The next day,the membrane was incubatedwithsecondaryantibodyatroom temperature for 1 h and then with a chemilumi-nescent substrate.The membrane was exposed to an X-ray film,which was developed in a dark-room.Relative TGF-β1and A2AR protein levels were calculated according to the intensity ofWestern blotting bands using GAPDH as the internal control.Two replicates were performed for each protein.Data were represented by the histogram.
1.8Statistical analysis
All statistical data were presented as±s. Between-group means comparisons were performed using one-way analysis of variance(ANOVA)followed by a Student-Newman-Keuls test.P-values less than 0.05 were considered statisti-cally significant.
2 RESULTS
2.1Histopathological analysis of interstitial pulmonary fibrosis
2.1.1HE staining
As shown in Fig.1A, compared with the normal control group,the model group showed varying degrees of alveolitis at different time points:on the 7th day after bleomycin perfu-sion,there was a large number of inflammatory cells infiltrating pulmonary alveolar and intersti-tial lung,apparent inflammation,but no alveoli and severe epithelial loss in the lungs;on the 14th day,alveolitis was alleviated,fibroblasts began to increase,and alveolar septa were widening;on the 28th day,alveolitis was signifi-cantlyreduced,somealveolicollapsedand were replaced by collagen fibers and fibroblasts,alveolar septa widened,diffuse inflammation of lung tissue was significantly reduced,and lung tissue structures were severely damaged.Over the three days,the scores of pulmonary inflam-mation were more higher in model group than in control group(Fig.1B)(P<0.01).The asiaticoside groupshowedsignificantlyreducedalveolitis compared with model group,and semi-quantita-tive scoring results found that the difference was significant(P<0.01,P<0.05).
2.1.2Masson staining
As shown in Fig.2A,the model group showed more collagen fibers position than the normal control group.On the 28th day,pulmo-nary fibrosis was more obvious.The scores of pulmonary fibrosis in the model group were higher than in normal control group(Fig.1B)(P<0.01). Theasiaticosidegroupsignificantlyreduced pulmonary fibrosis compared with the model group (P<0.05).
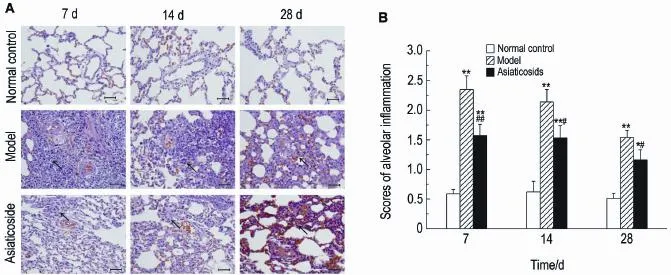
Fig.1 Effect of asiaticoside on interstitial pulmonary alveolitis of rats by HE staining(×400).
2.2Hydroxyproline content in lung tissue
As shown in Fig.3,on the 7th day after bleo-mycin administration,HYP content of lung tissue of the model group showed no significant differenceswith normal control group.On the 14th and 28th days,HYP content was higher in model groups 〔1.85±0.10,(2.48±0.18)mg·g-1〕than that in control group〔0.79±0.07,(0.84±0.08)mg·g-1〕(P<0.01).On the 7th day,HYP content in asiaticosidegroupshowednosignificant differenceswithmodelgroup.Therewas significantly lower HYP content in the asiaticoside group〔1.32±0.131,(1.71±0.13)mg·g-1〕than in model group on the 14th and 28th days (P<0.05).

Fig.2 Effect of asiaticoside on interstitial pulmonary fibrosis of rats by Masson staining(×400).A:See Fig.1 for the rat treatment.Arrows showed different levels of collagen fibers in different groups at different time.B:scores of lung fibrosis.±s,n=15,**P<0.01,compared with the normal control group;#P<0.05,compared with the model group.
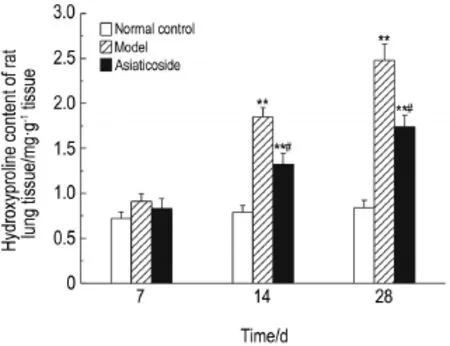
Fig.3 Effect of asiaticoside on the rat hydroxyproline content in lung tissue of rats.See Fig.1 for the rat treatment.±s,**P<0.01,compared with the normal control group;#P<0.05,compared with the model group.
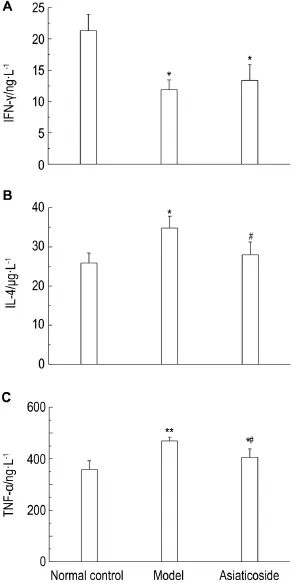
Fig.4 Effect of asiaticoside on concentration of inter-feron-γ (lFN-γ )(A),interleukin-4(lL-4)(B)and tumor necrosis factor-α (TNF-α )(C) in serum of rats by ELlSA.See Fig.1 for the rat treatment.±s,n=15.*P<0.05,**P<0.01,compared with the normal control group;#P<0.05,compared with the model group.
2.3Concentration of lL-4 and TNF-α in serum
Compared with normal control group,con-centration of IFN-γ was decreased in model group (Fig.4A)(P<0.05),but there was no significant dif-ference between model group and asiaticoside group.Compared with normal control group,concentration of IL-4 and TNF-α was higher than that in model group(P<0.01 or P<0.05),but was significantly lower in the asiaticoside group than thatinthemodelgroup(Fig.4Aand4C)(P<0.05).
2.4Expression and location of TGF-β1and A2AR in rat lung
As shown in Fig.5,TGF-β1expression was mainly detected in pulmonary artery smooth muscle cells and more pronounced in the model group than in normal control group.A2AR was mianly detected around pulmonary artery and decreased in the model group,but increased in the asiatico-side group.

Fig.5:Effect of asiaticoside on location of transforming growth factor-β1(TGF-β1)and adenosine 2A receptor (A2AR)detected by immunohistochemistry(×400).See Fig.1 for the rat treatment.Arrows for the expression and location of A2AR and TGF-β1.
2.5Expression level of TGF-β1and A2AR in rat lung
As shown in Fig.6,TGF-β1expression in lung tissue was significantly higher in model group than that in normal control groups(P<0.05)and lower than that in the asiaticoside group(Fig.6A).A2AR levels were lower in mod-el group than in normal control group(P<0.05) and asiaticoside group(P<0.05)(Fig.6B).

Fig.6 Effect of asiaticoside on the expression level of TGF-β1and A2AR by Western blotting.A1 and B1:West-ern blotting.A2 and B2:semi-quantitative of A1 and B1.±s,n=15.*P<0.05,compare with the normal control group;#P<0.05,compared with the model group.
3 DlSCUSSlON
A variety of factors including pathogenic microorganisms,dust,and certain drugs can induce pulmonary fibrosis.Bleomycin is an anti-cancer peptide drug,and can induce a dosedependent pulmonary fibrosis in both humans and animals.Rat models of pulmonary fibrosis induced by intratracheal injection of bleomycin share similarities with the human disease[7].In the present study,bleomycin was used to generate rat models.HE and Masson staining of lung tissue revealed that 7 d after bleomycin adminis-tration,there was significant inflammatory cell infiltration in the alveoli and the interstitial lungs of model group rats and that 28 d after bleomycinadministration,alveolar interstitial fibroblasts and collagen were increased significantly,and alveo-lar septa widened,which is consistent with the pathologicalprocessofIPF,indicatingthat animal models of pulmonary fibrosis had been successfully generated.The asiaticoside group showedsignificantlybetterinflammationand fibrosis than model group,suggesting that asiati-cosidecaneffectivelyamelioratepulmonary fibrosis.HYP content is an indicator of collagen metabolism and the extent of tissue fibrosis. HYP content was consistent with the pathological results of the present study.
Recent studies have shown that Th1/Th2 imbalance may play an important role in the development of pulmonary fibrosis[8].Th2-type cytokines can cause or aggravate pulmonary fibrosis.It is believed that in the process of lung damage repair and the accompanying inflamma-tory reactions,type 1 cytokines,such as IFN-γ,can inhibit lung fibroblast proliferation and extracellular matrix(ECM)formation,thus promoting the repair of normal tissue structure.Type 2 cyto-kines,such as IL-4,can promote activation and proliferation of fibroblasts and causing excessive damage repair response,resulting in deposition of ECM proteins and fibrosis.Many studies have shown the presence of Th2 advantage during pulmonary fibrosis[9-10].Reversing this advantage can reduce pulmonary fibrosis[11].The current study showed that pulmonary fibrosis induced by bleomycincancauseTh1/Th2imbalancein rats,as indicated by IFN-γ down-regulation and IL-4 up-regulation.Asiaticoside had an inhibitory effect on IL-4 expression but no significant effect on IFN-γ.
TGF-β is a pleiotropic factor secreted by various cells,and studies have indicated that the expresssion of TGF-β1mRNA is significantly elevated in pulmonary fibrosis of rats[12-13].TGF-β induces fibroblasts to secrete ECM molecules such as collagen and cadherins.As a member of TGF-β family,TGF-β1plays an important role in the PIF process[14].Studies have shown that the TGF-β soluble receptor has high affinity for TGF-β and can decrease proline levels and proline hydroxylase activity,but significantly reduce the extent of bleomycin-induced pulmonary fibrosis[15-16]. Our previous study showed that asiaticoside can prevent the thickening of the pulmonary arteries and inhibit fibrosis of the media wall in hypoxiainduced pulmonary hypertension rats[17].In this study,immunohistochemistry and Western blot results revealed that TGF-β1expression levels of lung tissue in the asiaticoside group were signifi-cantly lower than in the model group,suggesting that the antifibrotic effect of asiaticoside may have been achieved at least in part through inhibition of TGF-β1expression.
TNF-α is asecretedpolypeptidemainly producedbythepulmonaryalveolarmacro-phages(PAM),and studies have found that infliximab,a TNF-α inhibitor,can attenuate bleo-mycin-induced pulmonary fibrosis,which means TNF-α upregulationisrelatedtopulmonary fibrosis[18].Intravenous injection of TNF-α can cause diffuse alveolar damage,which was mainly manifested by alveolar epithelia and endothelial cell necrosis[19].Prior studies have shown that asiati-coside can improve sepsis-induced lung injury by inhibiting lung tissue and serum inflammatory factors like TNF-α and IL-6[6].This is consis-tent with the results of this study.A2AR,an ade-nosine receptor,is widely distributed in the lung. It has been reported that adenosine binding to A2AR can lead to inhibition of inflammatory cyto-kines such as TNF-α,regulate endothelial cell function,and slow down the inflammatory pro-cesses,which causes pulmonary fibrosis[20]. A2AR-/-mice were found to be more sensitive than A2AR+/+mice to bleomycin-induced lung injury[21].The study showed there to be less A2AR expression in bleomycin-inducedpulmo-nary fibrosis in rats,but asiaticoside was found to mitigate this decrease.
In summary,asiaticoside is found to inhibit the expression of IL-4,TNF-α,and TGF-β1,raise the level of A2AR,and inhibit pulmonary inflammation and fibrosis,thus ameliorating bleo-mycin-induced interstitial pulmonary fibrosis.Theunderlying mechanism is also briefly explored,thus paving a way for the revelation of more therapeutic indications of asiaticoside and providing patients with pulmonary fibrosis new treatment options.
REFERENCES:
[1]Olson AL,Swigris JJ.Idiopathic pulmonary fibrosis:diagnosis and epidemiology[J].Clin Chest Med,2012,33(1):41-50.
[2]Rafii R,Juarez MM,Albertson TE,Chan AL.A review of current and novel therapies for idiopathic pulmonary fibrosis[J].J Thorac Dis,2013,5(1):48-73.
[3]Shen QL.A report of 35 cases of interstitial pneu-monia treated by Qing-Run-Hua-Jie decoction[J]. J Emerg Tradit Chin Med(中国中医急症),2006,15(2):199-200.
[4]Wan J,Gong X,Jiang R,Zhang Z,Zhang L.Anti-pyretic and anti-inflammatory effects of asiaticoside in lipopolysaccharide-treated rat through up-regulation of heme oxygenase-1[J].Phytother Res,2013,27(8):1136-1142.
[5]Guo JS,Cheng CL,Koo MW.Inhibitory effects of Centella asiatica water extract and asiaticoside on inducible nitric oxide synthase during gastric ulcer healing in rats[J].Planta Med,2004,70(12):1150-1154.
[6]Zhang LN,Zheng JJ,Zhang L,Gong X,Huang H,Wang CD,et al.Protective effects of asiaticoside on septic lung injury in mice[J].Exp Toxicol Pathol,2011,63(6):519-525.
[7]Szapiel SV,Elson NA,Fulmer JD,Hunninghake GW,Crystal RG.Bleomycin-induced interstitial pulmonary disease in the nude,athymic mouse[J].Am Rev Respir Dis,1979,120(4):893-899.
[8]Lukacs NW,Hogaboam C,Chensue SW,Blease K,Kunkel SL.Type 1/type 2 cytokine paradigm and the progression of pulmonary fibrosis[J].Chest,2001,120(1 Suppl):5S-8S.
[9]Pignatti P,Brunetti G,Moretto D,Yacoub MR,Fiori M,Balbi B,et al.Role of the chemokine receptors CXCR3 and CCR4 in human pulmonary fibrosis[J].Am J Respir Crit Care Med,2006,173 (3):310-317.
[10]Yoshinouchi T,Naniwa T,Shimizu S,Ohtsuki Y,Fujita J,Sato S,et al.Expression of chemokine receptors CXCR3 and CCR4 in lymphocytes of idiopathic nonspecific interstitial pneumonia[J]. Respir Med,2007,101(6):1258-1264.
[11]Kikuchi N, Ishii Y,Morishima Y,Yageta Y,Haraguchi N,Itoh K,et al.Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance[J].Respir Res,2010,11(1):31.
[12]Hamid T,Guo SZ,Kingery JR,Xiang X,Dawn B,Prabhu SD.Cardiomyocyte NF-κB p65 promotes adverse remodelling,apoptosis,and endoplasmic reticulum stress in heart failure[J].Cardiovasc Res,2011,89(1):129-138.
[13]Jiang HY,Wek RC.Phosphorylation of the alphasubunit of the eukaryotic initiation factor-2 alpha (eIF2alpha)reduces protein synthesis and enhances apoptosis in response to proteasome inhibition [J].J Biol Chem,2005,280(14):14189-14202.
[14]Coker RK,Laurent GJ,Shahzeidi S,Lympany PA,Du Bois RM,Jeffery PK,et al.Transforming growth factors-beta 1,-beta 2,and-beta 3 stimu-late fibroblast procollagen production in vitro but aredifferentiallyexpressedduringbleomycininduced lung fibrosis[J].Am J Pathol,1997,150 (3):981-991.
[15]Wang Q, Wang Y, Hyde DM,Gotwals PJ,Koteliansky VE,Ryan ST,et al.Reduction of bleo-mycin induced lung fibrosis by transforming growth factorbetasolublereceptorinhamsters[J]. Thorax,1999,54(9):805-812.
[16]Bonniaud P,Margetts PJ,Kolb M,Schroeder JA,Kapoun AM,Damm D,et al.Progressive trans-forming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor [J].Am J Respir Crit Care Med,2005,171(8):889-898.
[17]Wang XB,Wang W,Zhu XC,Ye WJ,Cai H,Wu PL,et al.The potential of asiaticoside for TGF-β1/ Smadsignalinginhibitioninpreventionand progression of hypoxia-induced pulmonary hyper-tension[J].Life Sci,2015,137:56-64.
[18]Altintas N,Erboga M,Aktas C,Bilir B,Aydin M,Sengul A,et al.Protective effect of infliximab,a tumor necrosis factor-alfa inhibitor,on bleomycininduced lung fibrosis in rats[J].Inflammation,2015,1-14.
[19]Jirik FR, Podor TJ, Hirano T, Kishimoto T,Loskutoff DJ,Carson DA,et al.Bacterial lipopoly saccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells[J].J Immunol,1989,142(1):144-147.
[20]Scheibner KA,Boodoo S,Collins S,Black KE,Chan-Li Y,Zarek P,et al.The adenosine a2a receptor inhibits matrix-induced inflammation in a novel fashion[J].Am J Respir Cell Mol Biol,2009,40(3):251-259.
[21]Xu MH,Gong YS,Su MS,Dai ZY,Dai SS,Bao SZ,et al.Absence of the adenosine A2A receptor confers pulmonary arterial hypertension and increased pulmonary vascular remodeling in mice[J].J Vasc Res,2011,48(2):171-183.
Determination of parameters of developmental toxicity study on pediatric drugs and focus of attention for safety evaluation of traditional Chinese medicine
ZHOU Li,SUN Zu-yue
(Shanghai Institute of Planned Parenthood Research,National Evaluation Center for Toxicology of Fertility Regulating Drugs,Shanghai 200032,China)
Abstract:There is an increasing demand for neonatal and juvenile animal toxicity studies during the research and development of new drugs.In this paper,we discussed general evaluation parameters of pediatric non-clinical safety with pediatric drugs,such as growth and development and food intake,and paramenters of other organs and systems,such as the central nervous system,reproductive system,behavior evaluation in combination with our own experience.In addition,the characteristics of non-clin-ical safety evaluation of new traditional Chinese medicine materia medica used for juvenile animals were analyzed.This paper is intended reference for non-clinical safety evaluation of pediatric drugs and to gain some experience related to formulation of new guidelines.
Key words:pediatric drugs;juvenile animal;non-clinical safety evaluation;developmental toxicity;traditional Chinese materia medica
Asiaticoside attenuates bleomycin-induced interstitial pulmonary fibrosis
YE Wen-jing1,ZHU Xiao-chun1,WANG Xiao-bing1,WANG Liang-xing2,XIE Xu-ying3
(1.Department of Rheumology,2.Department of Respiratory Medicina,3.Pharmaceutical Department,the First Affiliated Hospital of Wenzhou Medical University,Wenzhou 325000,China)
中图分类号:R99
文献标志码:A
文章编号:1000-3002-(2016)01-0021-08
Foundation item:The project supported by Shanghai Technical Services Platform for Non-clinical Evaluation of Drug Against Male Reproductive and Urinary Diseases(15DZ2290400);and Shanghai Experimental Animal Scientific and Technological Innovative Action Plan(14140901302) SUN Zu-yue,E-mail:sunzy64@163.com The project supported by National Natural Science Foundation of China(81470250);National Natural Science Foundation of China(81270110);National Natural Science Foundation of China(81473406);Zhejiang Natural Science Foundation(Q16H010010);and Science and Technology Project of Wenzhou City(Y20140048) XIE Xu-ying,E-mail:386178454@qq.com,Tel:13967743354,Fax:(0577)88805703
收稿日期:(2015-12-13接受日期:2015-12-17)
基金项目:上海市男性生殖与泌尿疾病药物非临床评价专业技术服务平台(15DZ2290400);上海市实验动物创新行动计划项目(14140901302)
通讯作者:孙祖越,E-mail:sunzy64@163.com
