Spectroscopic and Molecular Docking Study on Specific Binding and Inhibition of Isoniazid to Human Serum Albumin and Catalase
2016-07-12WANGYirunFANGQingHUTaoyingLIUYing
WANG Yi-run, FANG Qing, HU Tao-ying, LIU Ying,2*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China 2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
Spectroscopic and Molecular Docking Study on Specific Binding and Inhibition of Isoniazid to Human Serum Albumin and Catalase
WANG Yi-run1, FANG Qing1, HU Tao-ying1, LIU Ying1,2*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China 2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
Isonicotinic acid hydrazide (Isoniazid, INH) is one of the most commonly used first-line anti-tuberculosis drugs, which has been reported that the high concentration of INH in human body can lead to epilepsy, liver function failure, and even death. Therefore, studying the potential binding effects of INH on the structure and activity of human serum albumin (HSA) and catalase (CAT) is very essential for evaluating its toxicity and side effect. In this paper, multi-spectroscopic and molecular docking methods were used to elucidate the patterns of INH to HSA and CAT under imitated physiological conditions. The inner filter effect of all fluorescence data in the paper was eliminated to get accurate binding parameters. The Stern-Volmer quenching constants (KSV) of both HSA-INH system and CAT-INH system inversely correlated with temperatures, demonstrating that INH quench the intrinsic fluorescence of HSA and CAT via static quenching. The conformational investigation of HSA and CAT through UV-visible absorption spectroscopy, synchronous fluorescence and circular dichroism (CD) showed that INH could change the micro-environment of tryptophan residues and reduced the α-helix content of protein. These results demonstrated that the binding of INH may lead to the loosening of protein skeleton, which which may affect its physiological function. The results of molecular docking revealed that the INH was located in Sudlow’s site I of HSA. And INH bound to CAT at a cavity among the wrapping domain helical domain and β-barrel, which resulted in the inhibition of CAT activity. In addition, Levofloxacin (LVFX) is a new effective and safe second-line anti-tuberculosis drugs and can improve the curative effect on anti-TB by using with other anti-TB drugs, the result of Hill’s coefficients (nH) about synergy between INH and proved that LVFX promoted the interaction of HSA with INH. Moreover, according to the CD spectra, synergy between INH and LVFX changed the conformation of HSA and the amount of α-helix decreased about 7.9%. This work will provide important insights into the binding and toxicity mechanism of INH to HSA and CAT in vivo and is expected to be helpful in evaluating the essential information for using the INH safely.
Isoniazid; Human serum albumin; Catalase; Multi-spectroscopic techniques; Molecular modeling
Introduction
Isonicotinic acid hydrazide (Isoniazid or INH, embedded in Fig.1) is an important antibiotic, which is one of the most commonly used first-line anti-tuberculosis drugs since 1952[1]. It has been reported that high concentration of INH in human body can lead to epilepsy, liver function failure, and even death[2]. Therefore, the determination of INH in the pharmaceutical preparations is the great important part of public health programs.
Human serum albumin (HSA) is known to bind and transport many ligands. Although the interaction between INH and protein by spectroscopic methods has been studied[3], the inner filter effect was not considered, which affects the binding parameters calculated from the fluorescence data. In addition, the special binding site of INH to HSA by site marker competitive experiments and molecular docking has not been identified. These aspects hinder the comprehensive understanding of the interaction between INH and HSA. Moreover, Levofloxacin (LVFX) as a new effective and safe second-line antituberculosis drugs, can improve the curative effect on anti-TB by using with other anti-TB drugs. In this report, the effect of LVFX on the INH-HSA system was studied and Hill’s coefficients (nH) were used to analysis of synergy between INH and LVFX.
Catalase (CAT) is regard as one of the highly active enzyme of antioxidant defense systems. It is widely occurs in most aerobic organisms and animal or plant tissues, which is particularly abundant in the liver[4]. INH as the first-line drug used in antituberculosis therapy, has been known to be potentially hepatotoxic and may lead to drug-induced liver injury[2]. Therefore, study the potential binding effects of INH on the structure and activity of CAT is very essential for evaluating its toxicity and side effect. However, there is littler research data about the interaction mechanism between INH and CAT. In this study, the study on the conformation and function property of the CAT is carried out and the bovine liver catalase (BLC) was selected as the model due to 91% homological identity with human erythrocyte catalase (PDB code 1F4J)[5].
The objective of this work is to clarify the following aspects: (1) deeply understand the fluorescence quenching mechanism and conformational change of HSA and CAT induced by INH using various spectroscopic techniques after correcting the binding parameters; (2) identify the specific binding site of INH in HSA and CAT through establishment of the molecular modeling; (3) analysis of synergy between INH and LVFX by Hill’s coefficients (nH). This work will provide useful clues on realizing the transport and metabolism process of INH, clarifying the binding effects of INH on biomacromolecule from intracellular protein level (HSA) and extracellular protein level (CAT).
1 Experimental
1.1 Materials
HSA (Sigma, Missouri, USA) and Catalase (from bovine liver, Sigma) were disolved with ultrapure water to form 1.0×10-4and 2.0×10-5mol·L-1, respectively. INH (iV Scientific, China) was dissolved with ultrapure water and diluted to 1.0×10-3mol·L-1. H2O2(Xilong Chemical Co., Ltd. China) was dissolved in ultrapure water to form a 1.0×10-2mol·L-1solution. All of the above stocking solutions were prepared with ultrapure water and kept in dark at 0~4 ℃. The 0.2 mol·L-1phosphate buffer solution (V(NaH2PO4·2H2O)/V(Na2HPO4·12H2O)=19∶81, pH 7.40) was used to control pH, 0.1 mol·L-1NaCl solution was applied to maintain the ionic strength at 0.1. All reagents were of analytical reagent grade without further purification. Millipore-Q ultrapure water was used throughout.
1.2 Methods
The methods and parameter settings on steady state fluorescence spectroscopy, UV-Vis spectroscopy, synchronous fluorescence, circular dichroism spectroscopy, competitive binding experiments and molecular docking study were according to Ref. [6].
The activity of CAT was determined by spectrophotometric method. CAT is able to decompose H2O2into O2and H2O. H2O2has a strong absorbent at 240 nm. By monitoring the absorption decreases at 240 nm, the changes in activity of CAT can be detected. The inhibition rate of enzyme activity was calculated using the following equation[7]
(1)
where ΔA1and ΔA0represent the reduction of the absorption value at 240 nm of the H2O2solution in 2 min after the addition of CAT with different concentrations of INH, respectively.
2 Results and discussion
2.1 Fluorescence quenching mechanism
Fig.1 shows the fluorescence emission spectra of HSA and CAT in the absence and presence of INH were carried out at the excitation wavelength of 280 nm at 298 K. It can be seen that HSA and CAT had a strong fluorescence emission at around 350 nm, with the addition of INH caused a gradual reduction in the fluorescence emission intensity of protein. This indicated that there were interactions between INH with HSA and CAT.
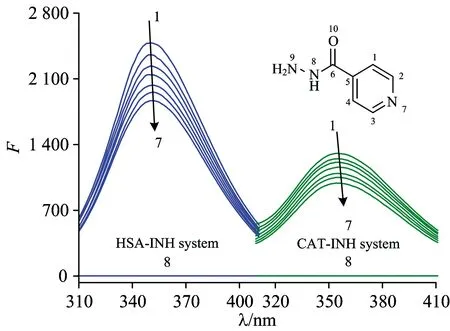
Fig.1 Fluorescence spectra of HSA and CAT by INH
cHSA=1.0×10-6mol·L-1;cCAT=2.0×10-6mol·L-1;cINH(×10-5mol·L-1) (1→7)=0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0; curve 8:cHSA=cCAT=0,cINH=1.0×10-5mol·L-1;T=298 K
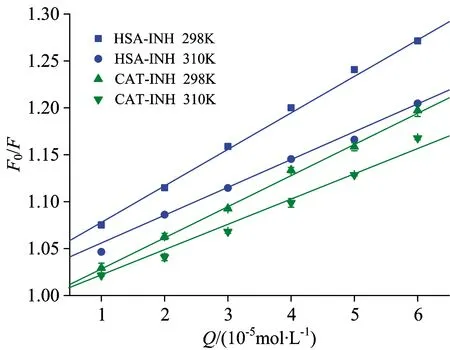
Fig.2 Stern-Volmer plots for the INH-HSA and INH-CAT system
Table 1 Stern-Volmer quenching constants for the interaction of INH with HSA and CAT at two different temperatures

SystemT/KKsv/(×103L·mol-1)Kq/(×1011L·mol-1·s-1)RHSA-INH2983103.90±0.033.03±0.013.903.030.99940.9968CAT-INH2983103.39±0.062.78±0.083.392.780.99670.9994
To elucidate the nature of fluorescence quenching, the fluorescence quenching data was analyzed by the Stern-Volmer equation and the elimination of possible inner filter effects according to Ref.[4]. and Ref.[8], respectively. The corresponding results are displayed in Table 1, the Stern-Volmer plots for the quenching of HSA and CAT by INH at 298 and 310 K are displayed in Fig.2. It can be seen that the bimolecular quenching rate constantKq(×1011L·mol-1·s-1) obtained for HSA and CAT were higher than the maximum value of diffusion controlled quenching in water (×1010L·mol-1·s-1). Moreover, the quenching constantsKsv(3.90×103L·mol-1at 298 K, 3.03×103L·mol-1at 310 K for HSA; 3.39×103L·mol-1at 298 K, 2.78×103L·mol-1at 310 K for CAT) were reversely correlated with temperatures. These results indicated that the probable quenching mechanism for HSA-INH system and CAT-INH system were static quenching.
2.2 UV-Vis absorption spectra
The strong absorption peak au about 208 nm reflects the framework conformation of the protein and its absorbance represents the α-helical content, the weak absorption peak at about 277~280 nm appears with the polarity of the micro-environment around aromatic amino acids (Trp, Tyr and Phe)[7]. As shown in Fig.3(a), with increasing amounts of INH added to the HSA solution, the intensity of the absorption peaks of HSA at about 208 nm obviously decreased by a red shift, indicating that INH led to the loosening and unfolding of the framework conformation. In contrast, the intensity of the weak absorption peak at about 278 nm increased, which means the polarity of the microenvironment around aromatic amino acid residues changed.
At the same time, the UV-Vis absorption spectra of CAT in the presence and absence of INH was recorded [Fig.3(b)]. It can be seen that the intensity of the peak at 207 nm decreases with a slight red shift and the intensity of the peak at 277 nm also decreased. These results indicated that the interaction between INH and CAT caused the loosening and unfolding of the protein skeleton. In addition, no detectable absorption peak of the Soret band was observed at 405 nm, which belongs to π→π*transition of hematoporphyrin in CAT. Considering the deeply buried heme groups in CAT (at least 20 Å in length from the protein surface)[9], it is difficult for INH to have a direct effect on the structure of heme groups because of steric hindrance.
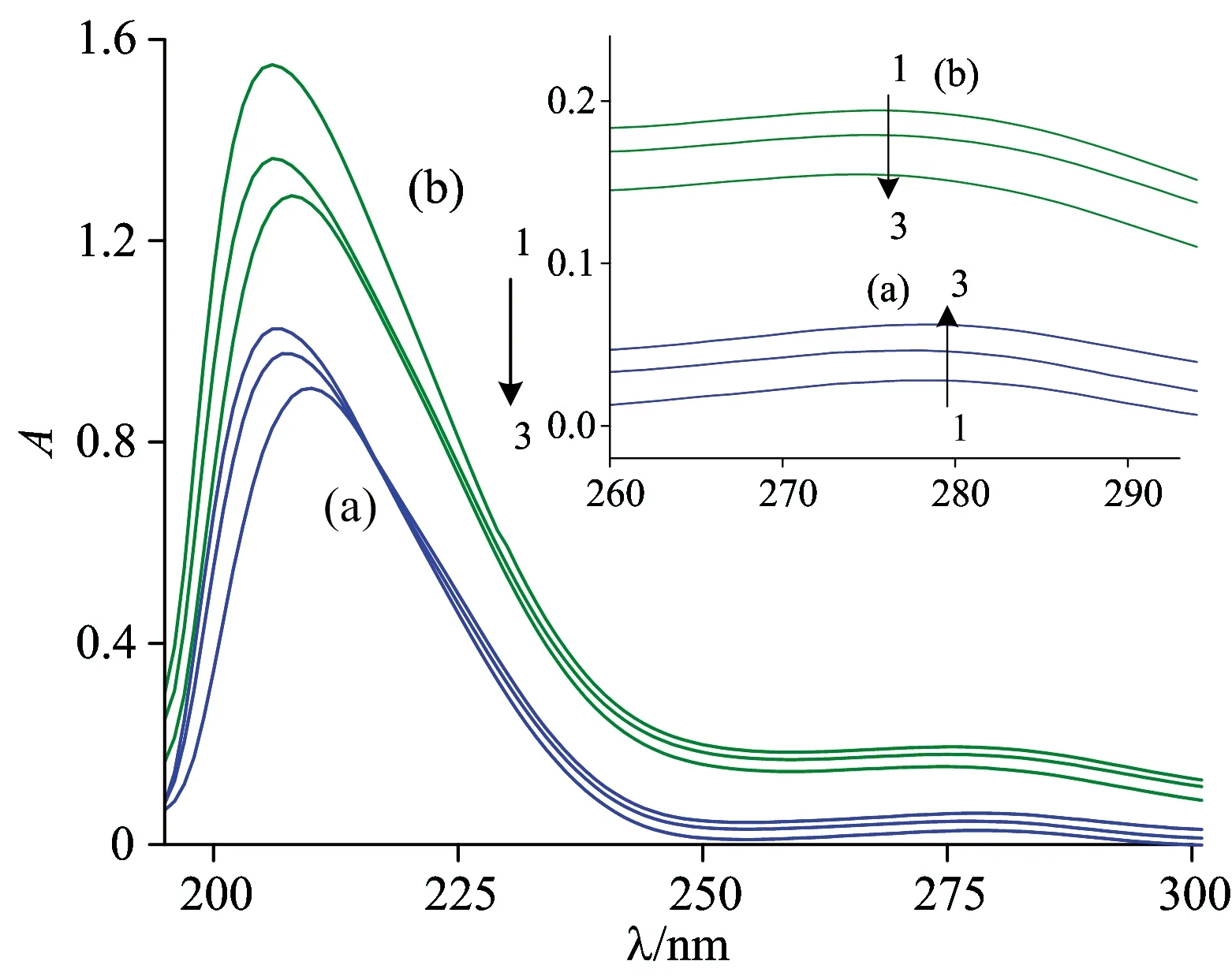
Fig.3 UV-Vis absorption spectra of HSA (a) and CAT (b) in the presence of different concentration of INH
cINH(×10-5mol·L-1) (1→3): 0.0, 1.0, 2.0;cHSA=1.0×10-6mol·L-1;cCAT=1.0×10-6mol·L-1
2.3 Synchronous fluorescence
Fig.4 illustrates the synchronous fluorescence spectra at two different wavelength intervals of HSA or CAT containing various amounts of INH. It is clearly seen that the fluorescence intensities of both Trp [Fig.4 (a)] and Tyr [Fig.4(b)] residues decreased when the concentration of INH increased gradually, and a red shift of the wavelength (λem) of the Trp residues fluorescence in HSA and CAT occurred with addition of INH, whereas the Tyr residues in HSA and CAT show a slight red shift. It is apparent that the red shift of the Trp and Tyr residues in CAT was more obvious than that of the Trp and Tyr residues in HSA. The red shift indicated that the molecular conformation of HSA or CAT was changed and Trp or Tyr residues were placed in a less hydrophobic environment by the interaction of HSA or CAT with INH.
(a): Δλ=60 nm; (b): Δλ=15 nm;cHSA=1.0×10-6mol·L-1;cCAT=2.0×10-6mol·L-1; (cINH×10-5mol·L-1) (1→7)=0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0;T=298 K
2.4 Circular dichroism spectra
To ascertain the possible influence of INH binding on the secondary structure of proteins, CD measurements were performed in the presence of different INH concentrations. As can be seen from Fig.5, the CD spectra of HSA and CAT exhibited two negative bands at 209 and 222 nm as the characteristic of an α-helix structure of proteins[7]. The secondary structure of HSA and CAT was calculated and the outcomes were shown in Fig.6.
cINH(×10-5mol·L-1) (1→5): 0.0, 5.0, 10.0, 5.0, 10.0;cHSA=cCAT=2.0×10-6mol·L-1;cLVFX=6.0×10-6mol·L-1

Fig.6 The secondary structure contents of HSA and CAT in the absence and presence of INH
It was noted that the free HSA had 43.7% α-helix, 9.2% β-sheet, 13.6% β-turn, and 33.5% random coil. When the molecular ratio of HSA to INH was 1∶25 and 1∶50, the α-helix decreased by 1.6% and 3.5%. These results demonstrated that the binding of INH may lead to the loosening of protein skeleton, which increased the hydrophobicity around the Trp residue of HSA. As shown in Fig.6, CAT has the secondary structures of 14.7% α-helix, 33.7% β-sheet, 13.4% β-turn, and 38.2% random coil. With the addition of INH to CAT, the α-helix decreased by 2.3% and 3.5%, the random coil increased by 2.3% and 2.4%, respectively. Because the function is related to the structure of the enzyme, the INH bound to CAT and led to the secondary structure changes, which may be responsible for the activity decrease of CAT.
2.5 Effect of INH on CAT activity
It can be seen from the above data that the effect of INH on CAT conformation is obvious. The structure of an enzyme is closely related to its functions so that a structural variation may affect its normal physiological function.
The impacts of different concentrations of INH on CAT activity are presented in Fig.7. As the molar ratio of INH to CAT increased to 25∶1, the activity of CAT decreased to 84.3%±1.3% of the initial level. At a higher molar ration 125∶1, the activity of CAT was only 61%±0.8% of the initial level [Fig.7(a)]. Effect of interaction time of INH with CAT on the activity of CAT was also investigated. As shown in Fig.7(b), the relative CAT activities were reduced to 84.8%±1.1% and 74%±1.2% after treatment with the 50 and 100 μmol·L-1INH for 20 min, respectively. As the exposure time increased to 80 min, the CAT activity decreased to 81.7%±1.2% and 68.6±1.4% of the initial level, respectively. The results indicated that the incubation time had little effect on the activity of CAT, while the INH concentration had significant effect on CAT activities.
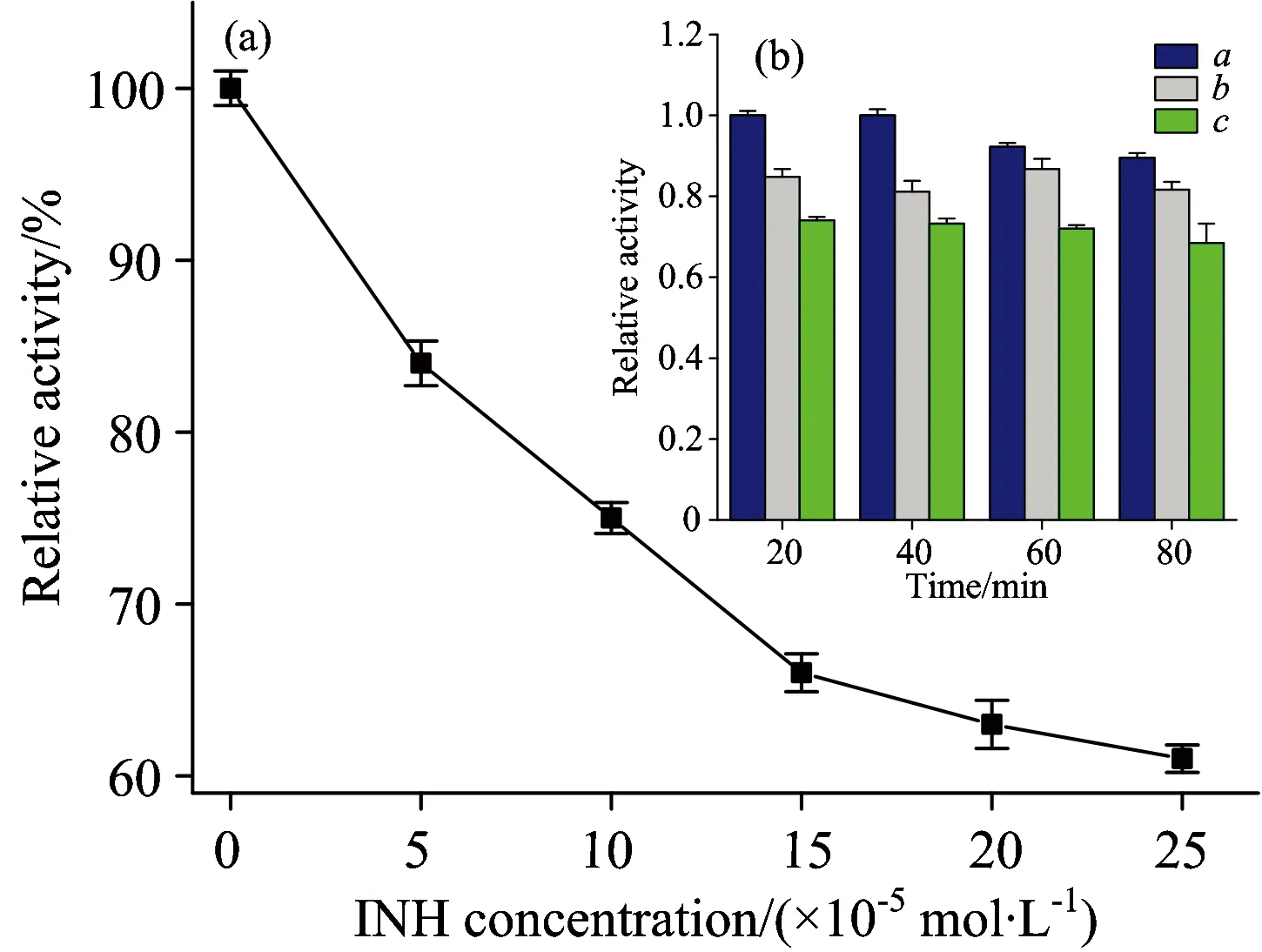
Fig.7 Effect of INH on CAT activity (a) Effect of INH concentration on the activity of CAT (b) Effect of interaction time of INH with CAT on the activity of CAT
cINH(×10-5mol·L-1): A, 0; B, 5; C, 10;cCAT=2×10-6mol·L-1
2.6 Effect of LVFX on the HSA-INH system
In this paper, the effect of INH and HSA with or without LVFX was studied by fluorescence spectra and CD spectra. Fig.8(a) shows the fluorescence spectra in the presence of the LVFX, which could decrease the fluorescence intensity of the HSA-INH system. To make the trends more clear, the normalized fluorescenceF/F0of the spectra is plotted in Fig.8(b), whereF0andFare the fluorescence intensities of HSA before and after the addition of INH, respectively. The general trends of the two curves were compared in Fig.8, the capability of INH quenching the fluorescent group of HSA was enhanced in the presence of LVFX.
Hill’s coefficients (nH) were used to analysis of synergy between INH and LVFX, which were calculated graphically on the basis of the following equation (2) and (3)[10-11]
(2)
whereYis the fractional binding saturation; fraction of sites occupied with the ligand; 1-Yis the number of free sites;nHis the Hill’s coefficient;Kais the binding constant.
For fluorescence measurement
(3)
whereL=(F0-F)F0; 1/Lm=intercept of OX axis on the plot 1/Lvs. 1/[Q]. WhennH=1.0, there is no cooperativity;nH>1.0, there is a positive cooperativity;nH<1.0, there is a negative cooperativity.
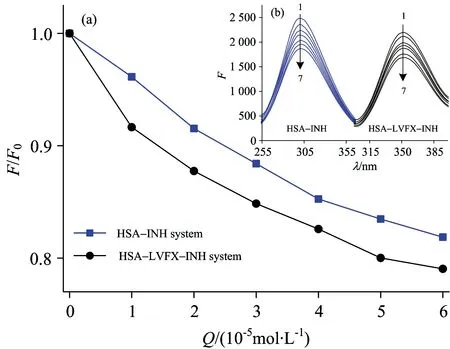
Fig.8 (a) Fluorescence spectra of HSA by INH in the presence of LVFX (b) Normalized fluorescence intensity of HSA with INH in the presence of LVFX or not
cHSA=1.0×10-6mol·L-1;cLVTX=6.0×10-6mol·L-1;cINH(×10-5mol·L-1) (1~7)=0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0;T=298 K
Hill’s coefficients of the HSA-INH systems (nH(HSA-INH)=0.98) less than 1 meant that a negative cooperativity was found when INH bound to HSA. However, Hill’s coefficients of HSA-INH system increased (nH(HSA-INH-LVFX)=1.09) with the addition of LVFZ,nH>1 indicated that there existed a certain positive cooperativity effect when INH and LVFX simultaneously bound to HSA. The results further proved that LVFX promoted the interaction of HSA with INH to result in the longer storage time of INH in blood plasma.
According to the CD spectra Fig.5 and Fig.6, in the presence of LVFX, the intensity of the negative peak increased and the amount of α-helix (%) decreased from 42.1% (HSA∶INH=1∶25) to 34.2% (HSA∶LVFX∶INH=1∶3∶25), which demonstrated that synergy between INH and LVFX changed the conformation of HSA.
2.7 Identification of the specific binding site
The molecular docking approach was adopted to further determine the specific binding sites of INH to HSA and CAT, respectively.
The exceptional capability of HSA to bind various substances is predominantly dependent on the existence of two primary binding regions, namely subdomain ⅡA (Sudlow’s site Ⅰ) and subdomain ⅢA (Sudlow’s site Ⅱ). The crystal structure of HSA was taken from the Protein Data Bank (PDB code 1h9z). The binding behavior between the INH and HSA was shown in Fig.9. It was noted that the INH was located in the binding pocket of site Ⅰ. And there were five hydrogen bonds between INH and Gln29, Pro147, Phe149, Cys245, and Cys253 of HSA with the distance in the range of 2.69~3.35 Å. And the hydrophobic force (C5 of INH with Cys253) and other forces (N2 and N3 of INH with Ala 151 and Tyr150) with a distance in the range of 3.40~3.85 Å were also presented.
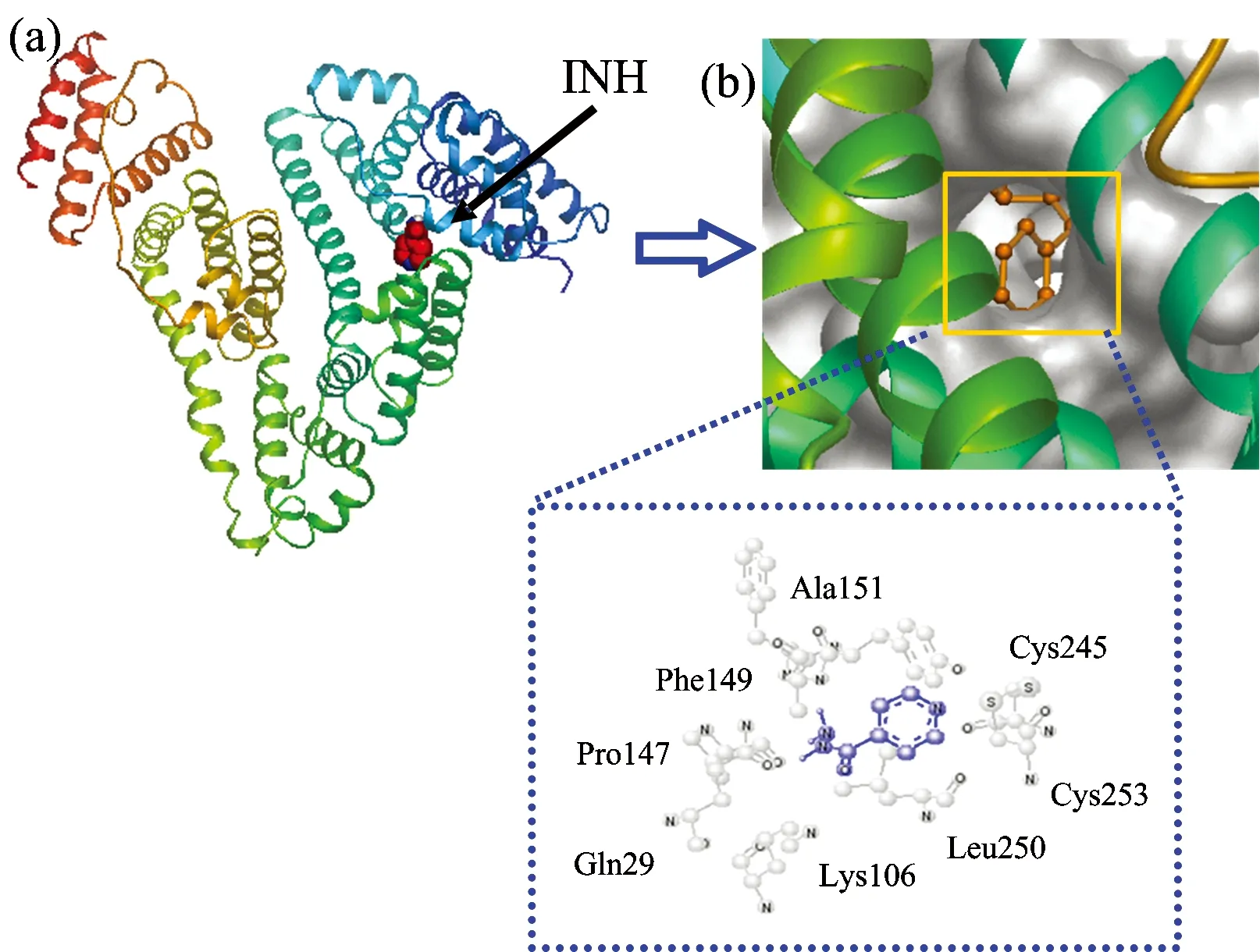
Fig.9 (a) The binding site of INH in HSA;(b) Detailed illustration of the binding between HSA and INH in hydrophobic surface model;(c) Molecular modeling of the interaction between INH and HSA. The atoms of INH are marked with blue and the atoms of amino acid residues of HSA are labeled with gray
The crystal structure of bovine liver catalase has revealed that it is a tetramer with four identical subunits and each subunit contains a heme group in the active site[12]. Therefore, we choose to focus our molecular docking study on one monomer of CAT. The results were shown in Fig.10. As shown in Fig. 10, INH bound to CAT at a cavity among the wrapping domain helical domain and β-barrel. The hydrogen bonds existed between the N8 of INH and the oxygen atom OE1 on Glu287, the N7 of INH and the O atom on Pro308, and N9 of INH and Val282. In addition, the hydrophobic force (C2 of INH with Val282, C3 of INH with Pro308, C5 of INH with Ile310) and other forces (N7 of INH with Val282, C6 of INH with Glu287) with a distance in the range of 3.75~3.79 Å were also presented. From the CAT activity measurement, even when the concentration of INH reached 1.0×10-4mol·L-1, the activity of CAT remains about 75% of the initial level. Based on the above results, INH did not directly bind into the CAT activity sites, but the binding of INH influenced the microenvironment of the CAT activity sites, which caused the inhibition of CAT activity.

Fig.10 (a) The binding site of INH in a CAT monomer;(b) Molecular modeling of the interaction between INH and CAT. The atoms of INH are marked with blue and the atoms of amino acid residues of CAT are labeled with gray
3 Conclusions
After elimination of the inner filter effects, the fluorescence of HSA and CAT were quenched by INH through static quenching mechanism, respectively. As demonstrated by the synchronous fluorescence spectroscopy, UV-Vis absorpion and CD spectra, the molecular conformation of HSA and CAT were changed and Trp or Tyr residues were placed in less hydrophobic environment in the presence of INH. In addition, the binding site results for INH with HSA and CAT were successfully generated by molecular docking. The results showed that the INH was located in Sudlow’s site I of HSA and bound to CAT at a cavity among the wrapping domain helical domain and β-barrel, which influenced the micro-environment of the CAT activity sites to cause the inhibition of CAT activity. The fluorescence spectra and CD spectra of INH and HSA in the presence of LVFX indicated that the capability of INH quench HSA was enhanced and synergy between INH and LVFX changed the conformation of HSA. Hill’s coefficients (nH>1) of HSA-INH system with the addition of LVFZ showed that there existed a certain positive cooperativity effect when INH and LVFX simultaneously bound to HSA. This study raises critical concerns regarding the transport, distribution and toxicity effects of INH in the human body.
[1] Zargar B, Hatamie A. Spectrochim Acta A,2013, 106: 185.
[2] Swamy N, Basavaiah K, Vinay K B. J. Anal. Chem.,2015, 70: 696.
[3] Markarian S A, Aznauryan M G. Mol. Biol. Rep.,2012, 39: 7559.
[4] Wang R Q, Zhang L, Wang R, et al. Spectrochim. Acta A,2013, 102: 88.
[5] Qin P F, Liu R T. J. Hazard. Mater.,2013, 252: 321.
[6] Hu T Y, Liu Y. J. Pharm. Biomed. Anal.,2015, 107: 325.
[7] Otto D M, Moon T W. Arch. Environ. Contam. Toxicol.,1996, 31: 141.
[8] Hao F, Jing M Y, Liu R T. J. Photochem. Photobiol. B,2015, 143: 100.
[9] Zhou X M, Lu W J, Su L, et al. J. Agri. Food. Chem.,2012, 60: 1135.
[11] Hill A V. J. Physiol.,1910, 40: iv.
[12] Yang B J, Hao F, Liu R T, et al. J. Photoch. Photobio. B,2013, 128: 35.
*通讯联系人
R113
A
光谱法和分子对接模拟技术研究异烟肼对人血清白蛋白和过氧化氢酶的特异性结合及抑制作用
王艺润1,方 庆1,胡涛英1,刘 颖1, 2*
1. 中央民族大学生命与环境科学学院,北京 100081 2. 中央民族大学北京市食品环境与健康工程技术研究中心,北京 100081
异烟肼(Isoniazid, INH)是最常用的一线抗结核药物之一。据报道,人体内高浓度的异烟肼可导致癫痫, 肝功能衰竭, 甚至死亡。因此,研究异烟肼对人血清白蛋白(HSA)和过氧化氢酶(CAT)的结构和活性的潜在结合影响有利于评估其毒性和副作用。在模拟生理条件下,利用多种荧光光谱和分子对接技术研究INH与HSA和CAT之间的相互作用。所有荧光数据均进行了内滤光校正以获得更准确的结合参数。结果表明,INH-HSA和INH-CAT体系的猝灭常数(Ksv)随着温度的升高而降低,表明INH对HSA及CAT的荧光猝灭机理为静态猝灭。利用紫外-可见吸收光谱、同步荧光光谱、和圆二色(CD)光谱法研究了INH对HSA和CAT构象的影响。结果发现,INH可改变色氨酸残基的微环境并降低HSA和CAT中α-螺旋结构,导致蛋白质结构发生伸展,进而可能影响其生理功能。分子对接结果表明,INH与HSA的结合位点位于HSA的site Ⅰ,ⅡA子域。INH可以进入CAT中β-折叠的桶状空腔,从而抑制CAT的活性。Hill系数结果表明,INH与左氧氟沙星(LVFX,一种安全有效的二线抗结核药物,与其他抗结核药物联合使用可以提高抗结核疗效)之间存在药物协同性,促进INH与HSA的相互作用。另外,CD光谱测定表明INH与LVFX的协同作用改变HSA的二级结构,使α-螺旋结构降低约7.9%。该研究探讨了INH与HSA和CAT之间的结合作用和毒性机制,为INH的安全使用提供重要依据。
异烟肼;人血清白蛋白;过氧化氢酶;多光谱法;分子对接
2016-03-30,
2016-07-28)
Foundation item: The National Natural Science Foundation of China (21177163), 111 Project B08044, First-class University First Class Academic Program of Minzu University of China (YLDX01013), Special Guidance Fund of Building World First-class Universities (Disciplines) and Characteristic Development of Minzu University of China (2016), Coordinate Development of First-Class and First-Class University Discipline Construction Funds(10301-0150200604), The Academic Team Construction Project of Minzu University of China (2015MDTD25C&13C), First-class Universities and First-class Discipline Construction Transitional Funds under Special Funding(2016, Ph.D), 2015MDTD08C
10.3964/j.issn.1000-0593(2016)11-3789-07
Received: 2016-03-30; accepted: 2016-07-28
Biography: WANG Yi-run, (1989—), Doctor of College of Life and Environmental Sciences, Minzu University of China e-mail: yrwang8@126.com *Corresponding author e-mail: liuying4300@163.com
