线型硅氧倍半聚合物的合成、形貌及稳定性
2016-07-05史亚赛周安南徐庆红
史亚赛,周安南,徐庆红
(北京化工大学 化工资源有效利用国家重点实验室, 北京 100029)
线型硅氧倍半聚合物的合成、形貌及稳定性
史亚赛,周安南,徐庆红*
(北京化工大学 化工资源有效利用国家重点实验室, 北京 100029)
摘要:利用硅氧烷试剂(10-isocyanadedecyl)triethoxysilane与含不同碳数的直链有机二胺(氨基连接于碳链的两端)反应,制备得到了系列双脲基有机硅氧烷化合物,上述化合物酸性水解得到了系列硅氧倍半聚合物. 扫描电镜照片显示,随着参与反应的直链有机二胺碳数的增加,硅氧倍半聚合物形貌由薄片状向纤维状过渡. 一个有趣的现象是,所形成的硅氧倍半聚合物形貌随着产物存放时间的延长而发生变化,并最终纤维消失. 含12碳数的二胺形成的聚合产物四个星期后纤维彻底消失而形成规整的球型. 上述结果对于研究线型硅氧倍半聚合物的合成及性质具有重要意义.
关键词:双脲基衍生物;倍半硅氧烷;形貌学;纤维
Received date: 2016-01-09.
Biography: 史亚赛(1991-),男,硕士生,研究方向为有机无机复合材料.*通讯联系人, E-mail: xuqh@mail.buct.edu.cn.
The sol-gel synthesis of bridged silsequioxanes (O1.5Si-R-SiO1.5; R=organic fragment) represents a fascinating bottom-up approach for the preparation of new hybrid materials[1-2]. Owing to the mild reaction conditions of this process, interesting properties may be tuned to these hybrids according to the incorporated organic fragment[3-5]. These materials have already been applied in many fields such as heterogeneous catalytic systems[6-8], NLO materials[9]and solid phase extraction or separation[10]. These sought-after properties are mainly due to the intrinsic properties of the organic unit.
Numerous efforts are now being made to prepare these materials with targeted morphologies in order to improve and to control their properties on different length scales. Periodic mesoporous bridged silsesquioxanes have been prepared using the surfactant mediated method but until now these are limited to small organic bridging units and are exclusively synthesized in the presence of external surfactant[11-14].
In this paper, some diureido derivatives of (10-isocyanadedecyl)triethoxysilane with different carbon linear diamines (n=2, 4, 6, 8, 10, 12) were synthesized and the morphologies of the final hydrolyzed products were studied by SEM. Results showed that these products changed from flake to fiber. Also it was found that the morphologies of these hydrolyzed products changed with the time passed. The results were supported by scanning electron microscopy (SEM),13C solid state NMR,29Si solid state NMR and IR spectrum.
1Experimental
The synthesis of the pruducts was shown in Fig.1.
P2:n=2; P4:n=4; P6:n=6; S2:n=2; S4:n=4; S6:n=6;
P8:n=8; P10:n=10; P12:n=12
S8:n=8; S10:n=10; S12:n=12
Fig.1Synthesis of the linear silsesquioxanes
1.1Synthesis of Pn (n=2,4,6,8,10,12)
The synthesis of precursors Pn (n=2,4,6,8,10,12) and the corresponding hybrid Sn(n=2,4,6,8,10,12) was accorded to the reference[15]. In a typical procedure ethylenediamine (2.0 mmol) was dissolved in CH2Cl2(30 mL) under nitrogen atmosphere. (10-isocyanadedecyl)triethoxysilane (4.0 mmol) was slowly added at room temperature and the resulted reaction mixture was stirred for 14 h. the solvent was removed and the white precipitate was washed with pentane.
P21H NMR (δ, CDCl3): 0.6 (SiCH2, 4H, t); 1.2-1.5 (CH3, 18H, t), 1.9 (CH2, 32H, m), 3.1-3.2 (NCH2, 8H, m); 3.8-3.9 (OCH2, 12H, t), 5.8 (2NH, 4H, m).13C NMR (δ, CDCl3): 10.4 (SiCH2); 18.3 (CH3); 22.8-33.7 (8CH2); 40.4 (NCH2); 40.8 (NCH); 58.3 (OCH2); 159.7 (CO). Anal. Calcd (%): C, 57.56; H, 10.47; N, 7.46. Found (%): C, 56.64; H, 10.27; N, 7.99.
P41H NMR (δ, CDCl3): 0.6 (SiCH2, 4H, t); 1.2-1.5 (CH3, 18H, t), 1.8 (CH2, 36H, m), 3.1 (NCH2, 8H, m); 3.8-3.9 (OCH2, 12H, t), 5.4 (2NH, 4H, m).13C NMR (δ, CDCl3): 10.4 (SiCH2); 18.3 (CH3); 22.8-33.2 (10CH2); 40.0 (NCH2); 40.4 (NCH); 58.3 (OCH2); 159.2 (CO). Anal. Calcd (%): C, 58.57; H, 10.61; N, 7.19. Found (%): C, 58.6; H, 10.56; N, 7.26.
P61H NMR (δ, CDCl3): 0.6 (SiCH2, 4H, t); 1.2-1.3 (CH3, 18H, t), 1.5 (CH2, 40H, m), 3.1-3.2 (NCH2, 8H, m); 3.8-3.9 (OCH2, 12H, t), 5.1 (2NH, 4H, m).13C NMR (δ, CDCl3): 10.4 (SiCH2); 18.3 (CH3); 22.7-33.2 (12CH2); 39.6 (NCH2); 40.4 (NCH); 58.3 (OCH2); 159.2 (CO). Anal. Calcd (%): C, 59.59; H, 10.74; N, 6.94. Found (%): C, 59.01; H, 10.51; N, 7.37.
P81H NMR (δ, CDCl3): 0.6 (SiCH2, 4H, t); 1.2-1.3 (CH3, 18H, t), 1.4-1.7 (CH2, 44H, m), 3.1-3.2 (NCH2, 8H, m); 3.8-3.9 (OCH2, 12H, t), 4.9 (2NH, 4H, m).13C NMR (δ, CDCl3): 10.4 (SiCH2); 18.3 (CH3); 22.7-33.2 (12CH2); 39.1 (NCH2); 40.2 (NCH); 58.3 (OCH2); 159.0 (CO). Anal. Calcd (%): C, 60.39; H, 10.86; N, 6.71. Found (%): C, 60.13; H, 10.72; N, 6.98.
P101H NMR (δ, CDCl3): 0.6 (SiCH2, 4H, t); 1.2-1.3 (CH3, 18H, t), 1.4-1.7 (CH2, 46H, m), 3.1-3.2 (NCH2, 8H, m); 3.8-3.9 (OCH2, 12H, t), 4.5 (2NH, 4H, m).13C NMR (δ, CDCl3): 10.4 (SiCH2); 18.3 (CH3); 22.7-30.3 (14CH2); 39.3 (NCH2); 40.4 (NCH); 58.3 (OCH2); 158.8 (CO). Anal. Calcd (%): C, 61.21; H, 10.97; N, 6.49. Found (%): C, 57.30; H, 10.08; N, 6.59.
P121H NMR (δ, CDCl3): 0.6 (SiCH2, 4H, t); 1.2-1.3 (CH3, 18H, t), 1.4-1.7 (CH2, 48H, m), 3.1-3.2 (NCH2, 8H, m); 3.8-3.9 (OCH2, 12H, t), 4.6 (2NH, 4H, m).13C NMR (δ, CDCl3): 10.4 (SiCH2); 18.3 (CH3); 22.7-30.3 (16CH2); 39.0 (NCH2); 40.6 (NCH); 58.3 (OCH2); 158.5 (CO). Anal. Calcd (%): C, 61.97; H, 11.09; N, 6.29. Found (%): C, 60.00; H, 10.67; N, 6.55.
1.2Synthesis of Sn (n=2,4,6,8,10,12)
In a typical procedure, a suspension of Pn(n=2,4,6,8,10,12) in DMSO were refluxed until all of solid was dissolved and formed colloid at room temperature, and some of HCl solution was added to the colloid (molar ratio of Pn∶H2O∶HCl is 1∶600∶0.2). The mixture was stirred for 1.5 h and heated statically for 4 d at 80 ℃, it was then filtered, washed with water and dried at room temperature.
S213C MAS NMR (δ): 15.6; 32.6; 40.7; 159.9.29Si MAS NMR (δ): -65.9, -56.8, -48.2, -41.7, -39.7. Anal. Calcd (%): C, 54.51; H, 9.15; N, 10.60; Si, 10.59. Found (%): C, 53.77; H, 9.31; N, 9.67; Si, 9.95.
S413C MAS NMR (δ): 13.6; 18.3; 31.9; 38.0; 42.3; 159.6.29Si MAS NMR (δ): -66.4; -57.8; -48.4; Anal. Calcd (%): C, 56.12; H, 9.35; N, 10.07; Si, 10.07. Found (%): C, 56.35; H, 9.28; N, 9.99; Si, 9.95.
S613C MAS NMR (δ): 13.8; 31.3; 41.8; 160.0.29Si MAS NMR (δ): -67.5; -57.3; -48.1; -40.0. Anal. Calcd (%): C, 57.49; H, 9.65; N, 9.58; Si, 9.60. Found (%): C, 53.76; H, 9.51; N, 9.17; Si, 9.02.
S813C MAS NMR (δ): 14.1; 31.3; 43.0; 159.8.29Si MAS NMR (δ): -66.6; -57.6; -48.4; -40.0. Anal. Calcd (%): C, 58.78; H, 9.87; N, 9.14; Si, 9.16. Found (%): C, 56.15; H, 9.84; N, 8.56; Si, 8.94.
S1013C MAS NMR (δ): 14.7; 24.7; 32.9; 42.6; 159.9.29Si MAS NMR (δ): -68.7; -57.5; -48.3; -40.0. Anal. Calcd (%): C, 59.96; H, 10.06; N, 8.74; Si, 8.76. Found (%): C, 57.13; H, 10.01; N, 7.99; Si, 8.18.
S1213C MAS NMR (δ): 14.1; 31.4; 41.4; 159.7.29Si MAS NMR (δ): -67.1; -57.6; -49.4; -40.0. Anal. Calcd (%): C, 61.03; H, 10.25; N, 8.38; Si, 8.37. Found (%): C, 54.47; H, 9.68; N, 7.72; Si, 9.90.
1.3Characterizations
Solid-state13C MAS NMR and29Si NMR spectra of the samples were obtained with a Bruker AV600 (made in Germany), and the chemical shifts recorded on the d-scale were referenced through external tetrakistrimethylsilane (TTMS). The elemental contents were analyzed on a Vario-EL elemental analyzer. Scanning electronic microscopy (SEM) images of the product were observed on a Shimadzu SS-550 microscope at 15 keV.
2Results and discussion
We first synthesized the pure diureido precursors Pn by the reaction of (10-isocyanadedecyl)triethoxysilane with corresponding linear diamines (n= 2, 4, 6, 8, 10, 12). Next, the diureido precursors were hydrolyzed undergo acidic hydrolysis in water at 80 ℃ (Fig.1). In these cases, the reactions occurred in a heterogeneous solution since these precursors were not dissolved in water. A mixture of DMSO and water was used to promote the hydrolysis-condensation reaction in the case.
The29Si and13C solid-state NMR spectra of all the solid materials confirmed the presence of a covalently bonded organosilicate network. In all cases, the29Si spectra exhibited two signals at around -57 and -67 assigned to SiC(OH)(OSi)2(T2) and SiC(OSi)3(T3). The13C spectra showed a peak at about 160 (C=O) and several sp3carbon atoms characteristic of the organic fragments.
SEM images for all the fresh hydrolyzed pro-ducts were studied (shown in Fig.2). For S2 obtained from P2, it has flake shape morphology, and the flakes overlaped each other. Diameter of one flake is about 2.5 um. But for the products from S4 to S12, the morphologies of the products change into fibers. For S4, diameter of the fiber is about 1.0 um. Compared to S4, much thinner fibers are found in S6 (diameter of the fiber is about 500 nm), and the fibers composite a meshwork structures. However the meshwork structure has a trend to form amorphous state that can be found in S8 and S10. In S8, the fiber and meshwork changed illegibly, and many wide fibers are found in S10 (diameter of the fiber is about 1.5 um). That is to say, the morphologies of the hydrolyzed products change from flake to fiber and from fiber to amorphous states withCnincrease. The above pheno-menon is very important to study the synthesis of linear silsesquioxanes.
The reason of the above changeable rule on morphologies of the hydrolyzed products can be explained. WhenCnis small, polymerization is not dominant, H-bonds between the molecules make the short precursor molecules form plate in many directions. But whenCnis increased, formation of H-bonds between the original molecules becomes difficulty, polymerization becomes a dominating factor, and fibers are formed easily. In such case, whenCnis not so large, some H-bonds can be still formed between the polymer molecular chains, wild fibers are formed. But whenCnis larger, formation of H-bond between the polymeric chemical chains becomes more difficulty, and thin fibers are formed. Course of these kinds of hydrolyzed products is illustrated in Fig.3. WithCnincreasing, much longer fibers of hydrolyzed products will be bended, and these bended fibers will be adsorbed with each other by H-bonds between the polymerizations, it induces that the fiber becomes wider than that ofCnis small. It’s the possible reason that the fibers become wider whenCnis larger enough.

Fig.2 SEM images for the fresh products: a, S2; b, S4; c, S6; d, S8; e, S10; f, S12

Fig.3 Formation courses of hydrolyzed products to small (a) and big (b) carbon number in precursors
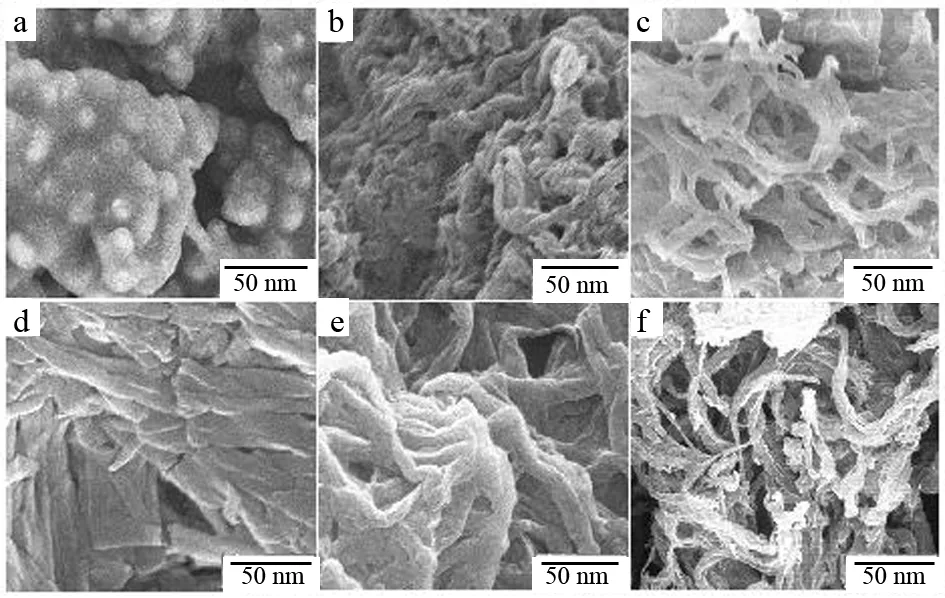
Fig.4 SEM images for the products remained for two weeks: a, S2; b, S4; c, S6; d, S8; e, S10; f, S12
Stability of Sn(n= 2, 4, 6, 8, 10, 12) was studied. The results indicate that the stabilities of these products are different. The morphologies of the hydrolyzed products after two weeks and four weeks are shown in Fig.4 and Fig.5, respectively. To S2, the flakes changed into balls and these balls gathered with each other after two weeks. Four weeks later, the product changed into amorphous. Similar phenomenon happened to S4, S6, S8 and S10, but the changes of the hydrolyzed products from fibers to amorphous are slower than S2. However for S12, different phenomenon appeared, the fibers gathered together to form column after two weeks, and the column changed into ball four weeks later. Considering much longer fibers in S12, H-bonds made the fibers gathered breadthwise to form column. With the time increased, the column shrank to form ball, a stable state, under the force of H-bonds.
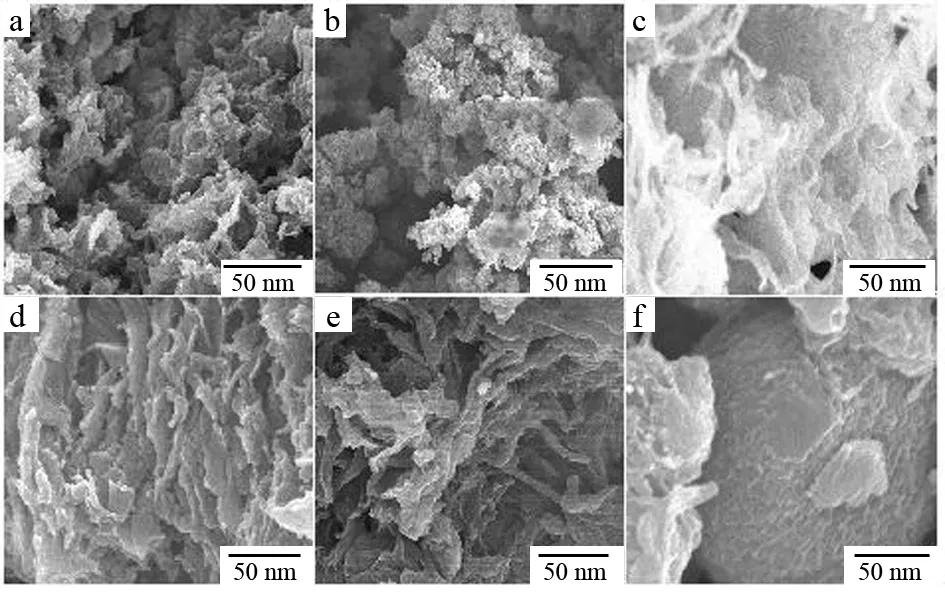
Fig.5 SEM images for the products remained for four weeks: a, S2; b, S4; c, S6; d, S8; e, S10; f, S12
3Conclusions
In this paper, some of new pure diureido hydrolyzed products were synthesized. It was found that the morphology of these products changed from flake to fibers. In addition, the original states of the hydrolyzed products were not stable with the time increased. WhenCnwas 2, the product changed into amorphous directly. WithCnincrea-sing, change rate of the product became slow, fibers disappeared slowly and changed into amorphous finally with the time passed. This interes-ting phenomena was discovered firstly. A further research on the application to these hydrolyzed products will be studied in the near future.
References:
[1] a) SHEA K J, LOY D A. Bridged polysilsesquioxanes. Molecular-engineered hybrid organic-inorganic materials [J]. Chem Mater, 2001, 13(10): 3306-3319; b) SHEA K J, LOY D A, WEBSTER O. Arylsilsesquioxane gels and related materials. New hybrids of organic and inorganic networks [J]. J Am Chem Soc, 1992, 114: 6700-6710; c) SHEA K J, LOY D A. Bridged polysilsesquioxanes. highly porous hybrid organic-inorganic materials [J]. Chem Rev, 1995, 95: 1431-1442.
[2] a) CORRIU R J P, MOREAU J J E, THEPOT P, et al. New mixed organic-inorganic polymers: hydrolysis and polycondensation of bis(trimethoxysilyl)organometallic precursors [J]. Chem Mater, 1992, 4: 1217-1224; b) CORRIU R J P, LECLERC D. Recent developments of molecular chemistry for sol-gel processes [J]. Angew Chem Int Ed Engl, 1996, 35: 1420-1436.
[3] SANCHEZ C, LEBEAU B, RIBOT F M. Molecular design of sol-gel derived hybrid organic-inorganic nanocomposites [J]. J Sol-Gel Sci Tech, 2000, 19(1): 31-38.
[4] SANCHEZ C, RIBOT F. Organic/inorganic hybrid materials [J]. New J Chem, 1994, 18(22): 1007-1047.
[5] AVNIR D. Organic chemistry within ceramic matrixes: doped sol-gel materials [J]. Acc Chem Res, 1995, 28: 328-334.
[6] a) SCHUBERT U, Catalysts made of organic-inorganic hybrid materials [J]. New J Chem, 1994, 18: 1049-1058; b) SCHUBERT U, HUSING N, LORENZ A. Hybrid inorganic-organic materials by sol-gel processing of organofunctional metal alkoxides [J]. Chem Mater, 1995, 7: 2010-2027.
[7] a) ADIMA A, MOREAU J J E, WONG C M. Chiral organic-inorganic solids as enantioselective catalytic materials [J]. J Mater Chem, 1997, 7: 2331-2333; b) HESEMANN P, MOREAU J J E, WONG C M. Immobilization of rhodium complexes in chiral organic-inorganic hybrid materials [J]. Chirality, 2000, 12: 411-420.
[8] LINDNER E, SCHNELLER T, AUER F, et al. Chemistry in interphases: a new approach to organometallic syntheses and catalysis [J]. Angew Chem Int Ed Engl, 1999, 38: 2154-2174.
[9] LEBEAU B, BRASSELET S, ZYSS J, et al. Design, characterization, and processing of hybrid organic-inorganic coatings with very high second-order optical nonlinearities [J]. Chem Mater, 1997, 9: 1012-1020.
[10] BROUDIC J C, CONOCAR O, MOREAU J J E, et al. New hybrid silica based materials for the solid-liquid extraction of actinides [J]. J Mater Chem, 1999, 9: 2283-2285.
[11] a) INAGAKI S, GUAN S, FUKUSHIMA Y, et al. Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks [J]. J Am Chem Soc, 1999, 121: 9611-9614; b) GUAN S, INAGAKI S, OHSUNA T, et al. Cubic hybrid organic-inorganic mesoporous crystal with a decaoctahedral shape [J]. J Am Chem Soc, 2000, 122: 5660-5661.
[12] MELDE B J, HOLLAND B T, BLANFORD C F, et al. Mesoporous sieves with unified hybrid inorganic/organic frameworks [J]. Chem Mater, 1999, 11: 3302-3308.
[13] LU Y, FAN H, DOKE N, et al. Evaporation-induced self-assembly of hybrid bridged silsesquioxane film and particulate mesophases with integral organic functionality [J]. J Am Chem Soc, 2000, 122: 5258-5261.
[14] a) ASEFA T, MACLACHLAN M J, COOMBS N, et al. Periodic mesoporous organosilicas with organic groups inside the channel walls [J]. Nature, 1999, 402: 867-871; b) MACLACHLAN M J, ASEFA T, OZIN G A, Writing on the wall with a new synthetic quill [J]. Chem Eur J, 2000: 2507-2511.
[15] XU Q H, MOREAU J J E, WONG C M. Influence of alkylene chain length on the morphology of chiral bridged silsesquioxanes[J]. J Sol-Gel Sci Technol, 2004, 32(1): 111-115.
[责任编辑:任铁钢]
Foundation item:国家自然科学基金项目(U1362113).
