一种有机-无机复合的硼钒酸盐[enH2]4[V6B20O50H8(H2O)]·en·8H2O
2016-07-05李海楼孙龙辉刘雅洁陈利娟
李海楼, 孙龙辉, 刘雅洁, 陈利娟
(河南大学 化学化工学院,河南省多酸化学重点实验室,河南 开封 475004)
一种有机-无机复合的硼钒酸盐[enH2]4[V6B20O50H8(H2O)]·en·8H2O
李海楼, 孙龙辉, 刘雅洁, 陈利娟*
(河南大学 化学化工学院,河南省多酸化学重点实验室,河南 开封 475004)
摘要:借助水热合成技术获得了一种新的有机-无机复合的硼钒酸盐[H2en]4[V6B20O50H8(H2O)]·en·8H2O (1) (en = 乙二胺),并对其进行了红外光谱、热重分析和单晶X射线衍射等表征. 化合物1的分子结构包含1个[V6B20O50H8(H2O)]8-多酸阴离子、4个质子化的[H2en]2+阳离子、1个游离的乙二胺分子和8个结晶水分子. 化合物1最有趣的结构特点体现在:夹心型的[V6B20O50H8(H2O)]8-多酸阴离子是由2个呈交错排列的三角形{B10O26}簇和1个六边形的{V6O18}簇通过12个三重桥氧原子连接而成的,其中两个三角形{B10O26}簇相互旋转的角度为45°.
关键词:多金属氧酸盐;硼钒酸盐;水热合成技术
Received date: 2016-03-11.
Biography: LI Hailou (1989-), male, master, majoring in poly-oxometalate-based functional materials.*Corresponding author, E-mail: ljchen@henu.edu.cn.
Polyoxometalates (POMs) are anionic metal-oxide clusters that are usually composed of early transition-metals (TMs) in their highest oxidation states such as molybdenum (Mo), tungsten (W), vanadium (V), niobium (Nb) and tantalum (Ta)[1-7]. Since their potential applications in catalysis, materials science, medicine, nanotechnology and magnetochemistry, POMs have become an outstanding class of cluster-based materials and have provoked significant interest in the past seve-ral decades[1-7].
Polyoxovanadates (POVs) as a subfamily of POMs have attracted much attention because of their intriguing and abundant architectures and conceivable applications, such as catalysis, medicine, nanotechnology and magnetism[8-10]. Square VO5pyramids in POVs display the flexible connection modes leading to the formation of diverse cage structures. In the past several decades, incorporation of heteroatoms into POV cage skeletons has become a feasible and promising strategy for ma-king a new class of heteropolyoxovanadates (HPOVs). Thus, extraneous As, Sb, Ge and Si heteroatoms also can be incorporated to the POV cage clusters to replace the V centers[11-17]. Moreover, the B atoms also can be introduced to the POV cage clusters to prepare novel polyoxovanadium borates (POVBs). Recently, some important progresses on POVBs have been made. For instance, in 1997, ZUBIETA et al. reported a novel POVB (EnH2)5[(VO)12O6{B3O6(OH)}6]·H2O that contained a [V12B18O60]10-polyoxoanion built up from a puckered {B18O36(OH)6} ring sandwiched by two triangles of six alternating cis- and trans-edge-sharing vanadium atoms[18]. In the same year, YAMASE and coworkers discovered a mixed-valence POVB H15[V12B32O84Na4]·13H2O, in which the cyclic doughnut-shaped [V12B32O84Na4]15-polyoxoanion displayed an edge-sharing dodecagonal array formed by VO5square pyramids sandwiched by two hexadecaborates and the cyclic cavity of [V12B32O84]19-polyoxoanion was occupied by the tetragonal Na44+group[19]. In 1998, a 1-D chain polymer [H2en]4[HEn]2[V6B22O53H8]·5H2O made up of {V6B20O50H6} cluster subunits linked via diborate bridges was isolated by WILLIAMS et al by means of a molten boric acid ‘flux’ method[20]. In 2012, four interesting transition-metal containing mixed-valence POVBs Na8(H3O){[Ni(H2O)5][V12B18O60H6]}·12.5H2O (11VIV/1VV), Na5(H3O)4{[Ni(H2O)3(en)][V12B18O60H6]}·9H2O (11VIV/1VV), Na9(H3O){Zn0.5[V12B18O60H6]}·11H2O (11VIV/1VV) and [HeEn][H2en]{[Zn(en)2]3[V12B18O60H6]}·3H2O (9VIV/3VV) were hydrothermally synthesized by VENEGAS-YAZIGI et al[21]. Meanwhile, CHEN and collaborators obtained a novel POVB hybrid Na[V12B16O50(OH)7(en)]2(enH2)6(enH)2(OH)(H2O)19constructed from [V12B16O50(OH)7(en)]7-cluster unitsincorporating organic amine ligands in the cluster skeletons[22]. In 2013, ZHOU’s group addressed a class of three-dimensional POVB frameworks based on [V12B18O60]16-cluster units {[Cu(dien)(H2O)]3V12B18O54(OH)6(H2O)}·4H3O·5.5H2O,{[Cd(H2O)2]3V12B18O54(OH)6(H2O)}·4H3O·9.5 H2O and {[Na(H2O)3]4Na2V12B18O56(OH)4(H2O)}(H3dien)2[23]. An unseen non-centrosymmetric decavanadoborate (NH3CH2CH2CH2NH3)5[(NH3CH2CH2CH2NH3)V10B24O66H8]·13.23H2O with an occluded 1,3-propanediammonium cation was synthesized using molten methylboronic acid flux reaction by VENEGAS-YAZIGI’s group in 2015[24]. Recently, our group investigated POVBs with aim of discovering novel inorganic-organic hybrid POVBs with novel architectures and interes-ting properties by the hydrothermal reaction. Herein, we report the synthesis, crystal structure and characterization of an organic-inorganic complicated vanadoborate [H2en]4[V6B20O50H8(H2O)]·en·8H2O (1) (CCDC 1420598) (en = ethylenediamine).
1Experimental
1.1Physical measurements
All the chemicals used for synthesis were reagent grade and used without further purification. Elemental analyses (C, H, N) were carried out on a Perkin-Elmer 240C elemental analyzer. The IR spectrum was recorded from a sample powder palletized with KBr on a Nicolet 170 SXFT-IR spectrometer in the range of 4 000-400 cm-1. The thermogravimetric analysis was performed under nitrogen atmosphere on a Mettler-Toledo TGA/SDTA 851einstrument with a heating rate of 10 ℃ min-1from 25 to 1 000 ℃. Anal. calcd. (%) for C10H68B20N10O59V6: C, 6.69; H, 3.82; N, 7.81. Found (%): C, 6.84; H, 3,97; N, 7.69.
1.2Synthesis of 1
A mixture of H3BO3(0.930 g, 15.041 mmol), Pr(NO3)3·6H2O (0.654 g, 1.503 mmol), V2O5(0.182 g, 1.000 mmol), oxalic acid (0.063 g, 0.552 mmol) and H2O (3 mL) was stirred for 10 min. Then en (0.3 mL, 4.44 mmol) and NaOH (0.4 mL, 4 mol·L-1) were added. The resulted mixture was stirred for 100 min, sealed in a Teflon-lined steel autoclave (25 mL), kept at 110 ℃ for 7 d and then cooled to room temperature. Green stick crystals were obtained by filtering and washed with distilled water and dried in air. Yield: ca. 28% based on V2O5.
1.3X-ray crystallography
A suitable single crystal of 1 was glued to thin glass fiber with epoxy resin under an optical microscope carefully. Intensity data collections were performed on Bruker CCD Apex-II diffractometer with Mo Kα radiation (λ= 0.071 073 nm) at 296 K. Their structures were determined by the direct methods and full-matrix least-squares refinements onF2using SHELXTL-97 program package[25-26]. Routine Lorentz polarization and empirical absorption corrections were applied to intensity data. Anisotropic thermal parameters were used to refine all non-H atoms except for some C, N and O atoms. No H atoms associated with water molecules were located from the difference Fourier map. Positions of the H atoms attached to C and N atoms were geometrically placed. All H atoms were refined isotropically as a riding mode using the default SHELXTL parameters. Crystallographicl data and structure refinements for 1 are listed in Table 1.
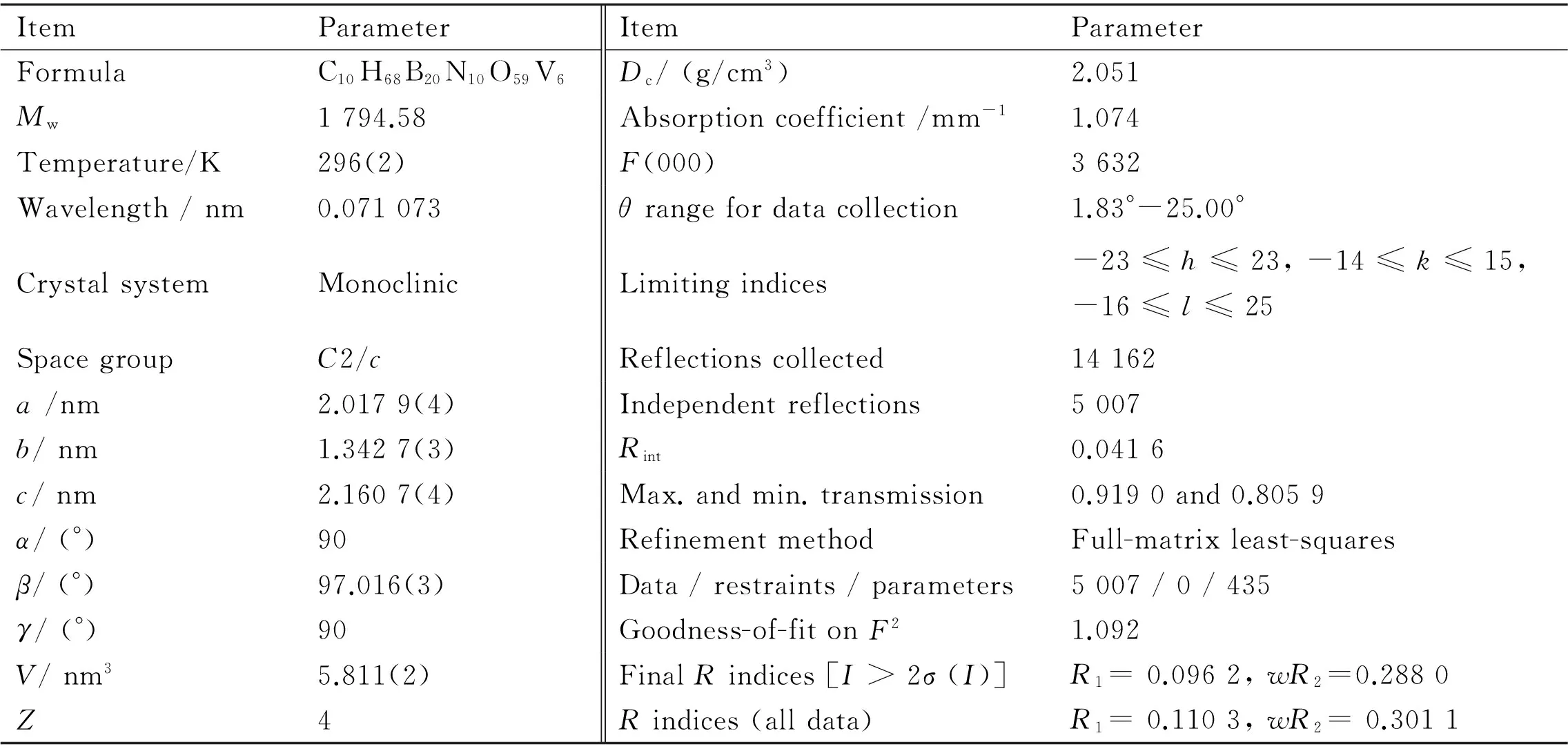
Table 1 Crystallographic data and structural refinements of 1
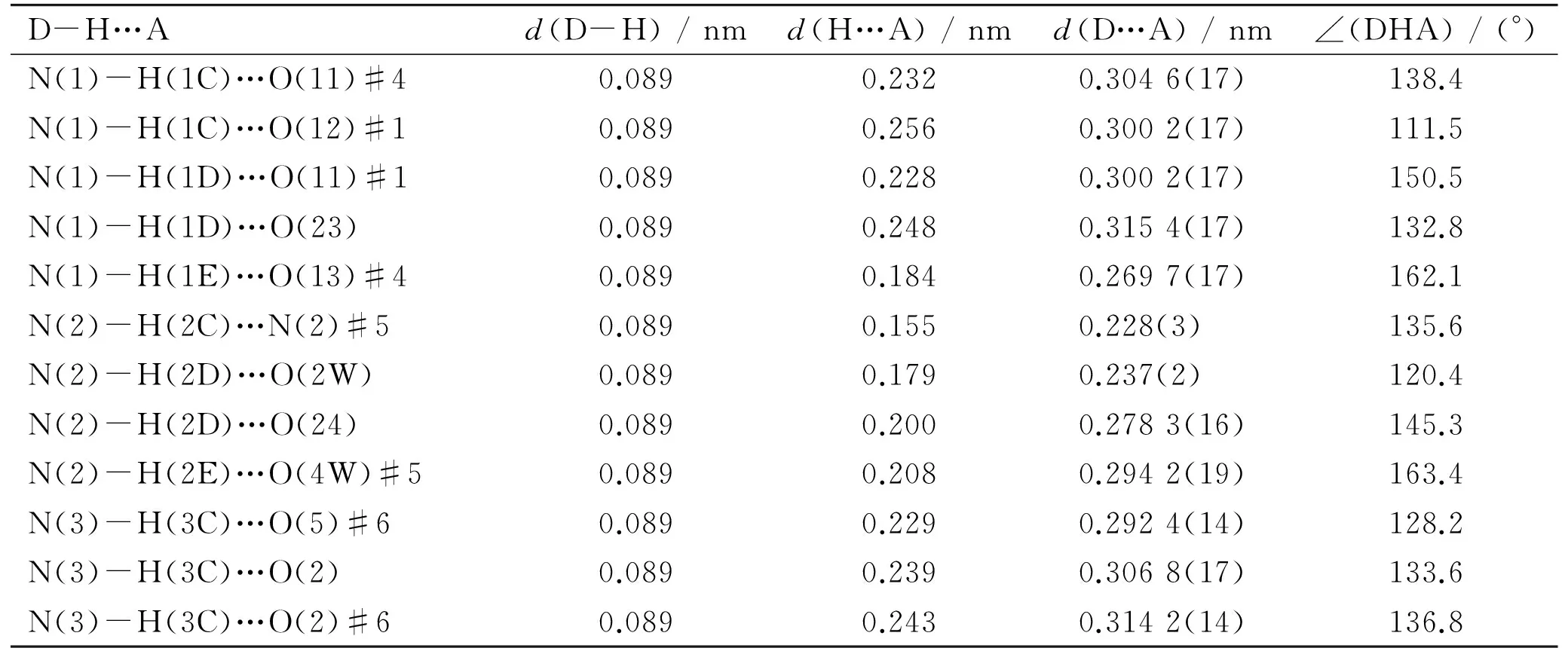
Table 2 Details of hydrogen bonds length and angle for 1, where D and A are
Continued Table 2
D-H…Ad(D-H)/nmd(H…A)/nmd(D…A)/nm∠(DHA)/(°)N(3)-H(3D)…O(2W)0.0890.2170.290(2)140.0N(3)-H(3E)…O(21)#70.0890.2200.2983(18)145.8N(4)-H(4C)…O(19)#80.0890.1830.2708(10)169.2N(4)-H(4D)…O(25)0.0890.1960.2783(10)153.4N(4)-H(4E)…O(5W)#80.0890.2080.2959(15)168.0N(5)-H(5C)…O(4W)#30.0890.1990.2838(15)160.1N(5)-H(5D)…O(1)#90.0890.1930.2821(12)174.6N(5)-H(5E)…O(11)#100.0890.1930.3040(12)161.2
Symmetry codes: #1: -x+ 1/2, -y+ 5/2, -z+ 1; #2: -x,y, -z+ 1/2; #3: -x+ 1/2, -y+ 5/2, -z; #4:x+ 1/2,y+ 1/2,z; #5: -x+ 1, -y+ 3, -z+ 1; #6: -x+ 1,y, -z+ 1/2; #7: -x+ 1, -y+ 2, -z+ 1; #8:x, -y+ 2,z- 1/2; #9: -x+ 1/2,y- 1/2, -z+ 1/2; #10:x- 1/2, -y+ 5/2,z- 1/2.
2Results and discussion
2.1Crystal structure
X-ray single-crystal diffraction indicates that 1 crystallized in the monoclinic space groupC2/cand its molecular structure consisted of one [V6B20O50H8(H2O)]8-polyoxoanion, four protonated [H2en]2+cations, a free en molecule and eight water molecules (Fig.1a). There are three crystallographically independent V atoms and ten crystallographically independent B atoms in the asymmetrical molecular unit (Fig.1b). Bond valence sum (BVS) calculations indicate that the oxidation states of V and B atoms are +4 and +3, respectively, (The BVS values of the V1, V2, V3, B1, B2, B3, B4, B5, B6, B7, B8, B9, and B10 atoms are 4.27, 4.39, 4.34, 3.06, 3.04, 3.03, 3.03, 3.08, 3.02, 3.04, 3.07, 3.05 and 3.07, respectively)[27]. Taking into consideration the charge balance of the molecular structure of 1, sixteen protons were supposed to be appended. To determine the possible binding positions of these protons, BVS calculations of all the oxygen atoms on the polyoxoanion had been carried out. The results show that the BVS values of O1, O9, O17 and O21 are 0.99, 1.00, 1.01 and 0.82, respectively[27]. Obviously, the BVS values of these oxygen atoms are far lower than 2, hinting that these oxygen atoms are monoprotonated, therefore, the formula of the polyoxoanion is written as [V6B20O50H8(H2O)]8-. In addition, the remaining protons should be located on the discrete En molecules and four diprotonated [H2En]2+cations come into being. As a matter of fact, this phenomenon that organoamines are often protonated under acidic or basic conditions is very common in POM chemistry[28-30]. For instance, HÖLSCHER and coworkers communicated a Dawson-based POM microporous solid hybrid [H3N(CH2)6NH3]4[P2W18O62]·3H2O, in which four free prontonated 1,6-diaminohexane molecules are diprotonated under acidic conditions in 1995[28]. DOLBECQ et al reported two organic-inorganic complicated phosphotungstates K4(C2N2H10)12[(α-PW10Fe2O39)4]·30H2O[29]and (C2N2H10)11[{(B-α-PW9O34)Fe3(OH)3}4(PO4)4Fe]·38H2O[30]in 2008, in which ethylenediamine components are also diprotonated and form [C2N2H10]2+cations under weak basic conditions. As a result, the formula of 1 can be depicted as [H2en]4[V6B20O50H8(H2O)]·en·8H2O.
In the [V6B20O50H8(H2O)]8-polyoxoanion, three crystallographically independent V atoms all adopt the {VO5} square pyramid geometry, in which four μ3-O atoms constitute the basal plane of the square pyramid [V-Oμ3: 0.193 2(6)-0.197 6(6) nm] and a terminal stands on the vertex of the square pyramid [V-Ot: 0.161 8(6)-0.162 5(6) nm]. Six {VO5} square pyramids are interconnected together via the edge-sharing mode and construct a hexagonal {V6O18} alignment (Fig.1c), which is similar to the hexagonal {Cu6O12Cl6} or {Mn6O12Cl6} alignment observed in novel Cu6and Mn6hexagon sandwiched POMs [(CuCl)6(AsW9O33)2]12-or [(MnCl)6(SbW9O33)2]12-[31]. Ten crystallographically independent B atoms in the asymmetrical molecular unit are distributed in the 1∶3∶5∶7 fashion, giving rise to an equilateral triangle {B10O26} alignment (Fig.1d). More in triguingly, the three vertices of the triangle are respectively occupied by three-coordinate trigonal B1, B4 and B8 [B-O: 0.135 3(11)-0.137 6(11) nm] and the four-coordinate tetrahedral B6, B7, B2, B3, B5 and B9 locate on the three sides of the equilateral triangle [B-O: 0.142 6(10)-0.147 8(10) nm] and the four-coordinate tetrahedral B10 lies in the center of the equilateral triangle [B-O: 0.144 6(11)-0.149 8(10) nm]. The distances of B1…B4, B1…B8, and B4…B8 are 0.694 0(14), 0.698 4(13) and 0.698 3(13) nm, respectively, which indicate that the equilateral triangle {B10O26} alignment was slightly distorted. In the equilateral triangle {B10O26} alignment, each trigonal {BO3} group are connected with two {BO4} groups via two μ2-O atoms, each of tetrahedral {B2O4}, {B3O4}, {B5O4}, {B6O4}, {B7O4} and {B9O4} groups is combined with one trigonal {BO3} group and two tetrahedral {BO4} groups by virtue of two μ2-O atoms and one μ3-O atom, and the tetrahedral {B10O4} group in the center of the equilateral triangle joins three tetrahedral {BO4} groups through three μ3-O atoms. The most remarkable feature is that the [V6B20O50H8(H2O)]8-polyoxoanion can be viewed as a sandwich-type structure constructed from two triangle {B10O26} clusters encapsulating a hexagonal {V6O18} cluster through twelve μ3-O atoms (Fig.1e). It is worth mentioning that two triangle {B10O26} clusters are in the staggered fashion during the course of integrating with the hexagonal {V6O18} cluster and the rotation angle of two {B10O26} clusters is 45°. The simplified graphic of the [V6B20O50H8(H2O)]8-polyoxoanion is illustrated in Fig.1f in consideration of the {B10O26} cluster as a triangle and the {V6O18} cluster as a hexagon.
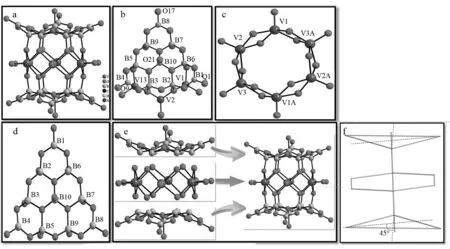
Fig.1 (a) The ball-and-stick representation of the [V6B20O50H8(H2O)]8- polyoxoanion in 1. (b) The ball-and-stick
Besides its interesting anion cluster structure, another feature of 1 is that there are the hydrogen bonding interactions between nitrogen donors of organoamine molecules and surface oxygen acceptors of [V6B20O50H8(H2O)]8-polyoxoanions. The geometric parameters of hydrogen bonding interactions are listed in Table 2. The nitrogen donors and oxygen acceptors are hydrogen-bonded together giving rise to the infinitely 3-D supramolecular architecture (Fig.2). The N-H…O distances are in the range of 0.237(2)-0.315 4(17) nm and the ∠(DHA) angles vary from 111.5 to 174.6°. As a result, the formation of these supramolecular interactions is responsible for enhancing the stability of 1.
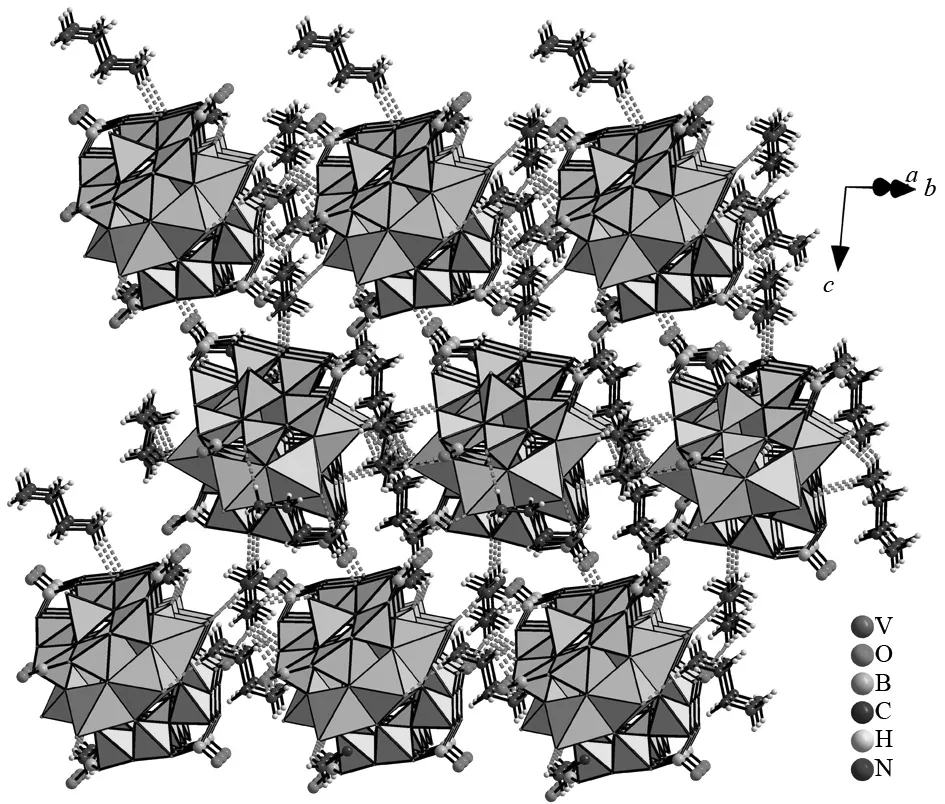
Fig.2 View of the 3-D supramolecular architecture of 1
2.2IR spectrum
As known to all, the IR spectroscopy is a good technique for confirming the structure of POM polyoxoanion and the presence of organic components. The IR spectrum of 1 had been recorded from a sample powder palletized with KBr between 4 000-400 cm-1(Fig.3). The broad absorption band centered atca. 3 420 cm-1is attributed to the O-H stretching vibration of water molecules whereas the middle intensity absorption peak at 1 631 cm-1is derived from the O-H bending vibration of water molecules. The stretching bands of -NH2and -CH2groups are observed at 3 174 and 2 912 cm-1while the bending vibration bands of -NH2and -CH2groups appear at 1 545 and 1 403 cm-1. The sharp and strong band at 1 403 cm-1can be assigned to the B-O bonds of {BO3} groups. It is noteworthy that bending vibration of -CH2group overlapps with that of the B-O bonds of {BO3} groups. The B-O stretching vibration from {BO4} groups appears at the 1 156 and 1 106 cm-1. The absorption peak at 923 cm-1results from terminal V-O stretching vibration. The absorption signals appear at 884-760 cm-1are assigned to the V-O-V stretching vibration[32]. The occurrence of these signals is in good agreement with the single-crystal structural analysis.
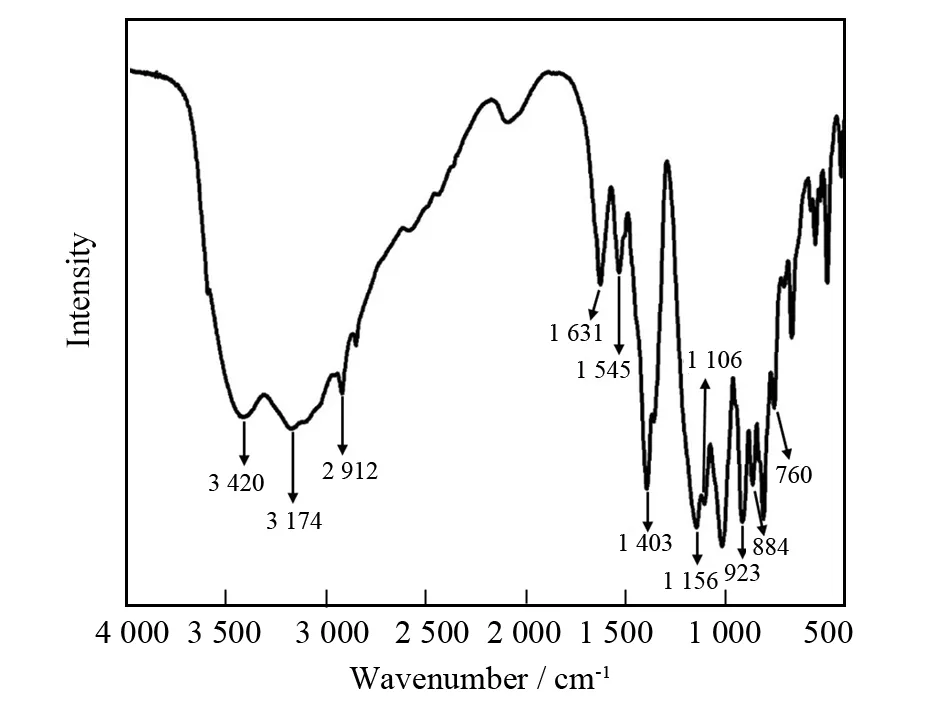
Fig.3 IR spectrum of 1
2.3Thermogravimetric analysis
The thermogravimetric analysis of 1 has been measured in flowing N2atmosphere with a heating rate of 10 ℃·min-1in the temperature region of 25-800 ℃ (Fig.4). Two distinct weight loss stages are observed in the thermogravimetric curve of 1, the first weight loss of 7.88% is comparable with the calculated value of 8.00% up to 135 ℃, which corresponds to the release of eight water molecules. The second weight loss of 25.72% occurs in the range of 135-800 ℃, which was assigned to the removal of one water molecule, the dehydration of sixteen protons and the relsease of five en molecules (calcd. 25.66%).

Fig.4 Thermogravimetric curve of 1
3Conclusion
In summary, a kind of new polyoxovanadoborate [H2en]4[V6B20O50H8(H2O)]·en·8H2O has been prepared by the hydrothermal technique and characterized by IR spectra, thermogravime-tric analysis and single-crystal X-ray diffraction. The sandwich-type [V6B20O50H8(H2O)]8-poly-oxoanion is constructed from two triangle {B10O26} cluster units in the staggered fashion sandwiching a hexagonal {V6O18} cluster via twelve μ3-O atoms. In the future, we will try to prepare lanthanide incorporated polyoxovanadoborates.
References:
[1] KATO C, NISHIHARA S, TSUNASHIMA R, et al. Quick and selective synthesis of Li6[α-P2W18O62]·28H2O soluble in various organic solvents [J]. Dalton Trans, 2013, 42: 11363-11366.
[2] RHULE J T, Hill C L, JUDD D A. Polyoxometalates in medicine [J]. Chem Rev, 1988, 98: 327-357.
[3] KATSOULIS D E. A survey of applications of polyoxometalates [J]. Chem Rev, 1998, 98: 359-388.
[4] POPE M T, MÜLLER A. Polyoxometalate chemistry: an old field with new dimensions in several disciplines [J]. Angew Chem Int Ed Engl, 1991, 30: 34-48.
[5] MÜLLER A, REUTER H, DILLINGER S. Supramolecular inorganic chemistry: small guests in small and large hosts [J]. Angew Chem Int Ed Engl, 1995, 34: 2328-2361.
[6] UCHIDA S, KAWAMOTO R, AKATSUKA T, et al. Structures and sorption properties of ionic crystals of macrocation-Dawson-type polyoxometalates with diffe-rent charges [J]. Chem Mater, 2005, 17: 1367-1375.
[7] LONG D L, STREB CARSTEN, SONG Y F, et al. Unravelling the complexities of polyoxometalates in solution using mass spectrometry: protonation versus he-teroatom inclusion [J]. J Am Chem Soc, 2008, 130: 1830-1832.
[8] GAO Y Z, XU Y Q, CAO Y, et al. Introducing organosilicone source into the synthetic system of polyoxovanadates: two novel {V15Si6O48}-based extended chains [J]. Dalton Trans, 2012, 41: 567-571.
[9] CHEN L, JIANG F L, LIN Z Z, et al. A basket tetradecavanadate cluster with blue luminescence [J]. J Am Chem Soc, 2005, 127: 8588-8589.
[10] KHAN M I, TABUSSUM S, MARSHALL C L, et al. Catalytic deNOxproperties of novel vanadium oxide based open-framework materials [J]. Catal Lett, 2006, 112: 1-12.
[11] ZHENG S T, ZHANG J, LI B, et al. The first solid composed of {As4V16O42(H2O)} clusters [J]. Dalton Trans, 2008, 37: 5584-5587.
[12] QI Y F, Li Y G, WANG E B, et al. Syntheses and structures of four organic-inorganic hybrids based on the surface restricted building unit and various zinc coordination groups [J]. Inorg Chim Acta, 2007, 360: 1841-1853.
[15] WHITFIELD T, WANG X, JACOBSON A J. Vanadogermanate cluster anions [J]. Inorg Chem, 2003, 42: 3728-3733.
[16] PITZSCHKE D, WANG J, HOFFMANN R-D, et al. Two compounds containing the mixed germanium-vanadium polyoxothioanion [V14Ge8O42S8]12-[J]. Angew Chem Int Ed, 2006, 45: 1305-1308.
[17] ZHOU J, ZHAO J W, WEI Q, et al. Two tetra-CdⅡ-substituted vanadogermanate frameworks [J]. J Am Chem Soc, 2014, 136: 5065-5071.
[18] RIJSSENBEEK J T, ROSE D J, HAUSHALTER R C, et al. Novel clusters of transition metals and main group oxides in the alkylamine/oxovanadium/borate system [J]. Angew Chem Int Ed Engl, 1997, 36: 1008-1010.
[19] YAMASE Toshihiro, SUZUKI M, OHTAKA K. Structures of photochemically prepared mixed-valence polyoxovanadate clusters: oblong [V18O44(N3)]14-, super-Keggin [V18O42(PO4)]11-and doughnut-shaped [V12B32O84Na4]15-anions [J]. J Chem Soc Dalton Trans, 1997, 2463-2472.
[20] WILLIAMS I D, WU M M, SUNG H H Y, et al. An organotemplated vanadium(Ⅳ) borate polymer from boric acid ‘flux’ synthesis, [H2en]4[Hen]2[V6B22O53H8]·5H2O [J]. Chem Commun, 1998, 2463-2464.
[22] SUN Y Q, LI G M, CHEN Y P. A novel polyoxovanadium borate incorporating an organic amine ligand: synthesis and structure of [V12B16O50(OH)7(en)]7-[J]. Dalton Trans, 2012, 41: 5774-5777.
[23] ZHOU J, LIU X, CHEN R, et al. New 3-D poly-oxovanadoborate architectures based on [V12B18O60]16-clusters [J]. CrystEngComm, 2013, 15: 5057-5063.
[25] SHELDRICK G M. SHELX-97, Program for crystal structure solution [CP]. Göttingen: University of Göttingen, 1997.
[26] SHELDRICK G M. SHELXL-97, Program for crystal structure refinement [CP]. Göttingen: University of Göttingen, 1997.
[27] BROWN I D, ALTERMATT D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database [J]. Acta Cryst, 1985, B41: 244-247.
[28] HÖLSCHER M, ZIBROWIUS B, HÖLDERICH W F, et al. (H3N(CH2)6NH3)4[W18P2O62]·3H2O, a microporous soild from Dawson anions and 1,6-diami-nohexane [J]. Angew Chem Int Ed, 1995, 33: 2491-2493.
[29] PICHON C, DOLBECQ A, MIALANE P, et al. Fe2and Fe4clusters encapsulated in vacant polyoxotungstates: hydrothermal synthesis, magnetic and electrochemical properties, and DFT calculations [J]. Chem Eur J, 2008, 14: 3189-3199.
[30] PICHON C, DOLBECQ A, MIALANE P, et al. Square versus tetrahedral iron clusters with polyoxometalate ligands [J]. Dalton Trans, 2008, 71-76.
[31] YAMASE T, FUKAYA K, NOJIRI H, et al. Ferromagnetic exchange interactions for Cu612+and Mn612+hexagons sandwiched by two B-ɑ-[XW9O33]9-(X = AsIIIand SbIII) ligands inD3d-symmetric polyoxotungstates [J]. Inorg Chem, 2006, 45: 7698-7704.
[32] ZHOU J, LIU X, HU F L, et al. One novel 3-D vanadoborate with unusual 3-D Na-O-Na network [J]. RSC Adv, 2012, 2: 10937.
[责任编辑:吴文鹏]
Foundation item:Supported by the Natural Science Foundation of China (21301049, U1304208).
