Green coffee bean extract improves obesity by decreasing body fat in high-fat diet-induced obese m ice
2016-06-29BongKeunChoiSungBumParkDongRyungLeeHaeJinLeeYingYuJinSeungHwanYangJooonSuh
Bong-Keun Choi, Sung-Bum Park, Dong-Ryung Lee, Hae Jin Lee, Ying-Yu Jin, Seung Hwan Yang,*, Joo-W on Suh*
1Nutra Pharm Tech, Giheung-gu, Yongin, Gyeonggi 446-916, Korea2Interdisciplinary Program of Biomodulation, Myongji University, Yongin, Gyeonggi 446-728 Korea3Center for Nutraceutical and Pharmaceutical Materials, Myongji University, Yongin, Gyeonggi 446-728 Korea
Green coffee bean extract improves obesity by decreasing body fat in high-fat diet-induced obese m ice
Bong-Keun Choi1#, Sung-Bum Park2#, Dong-Ryung Lee1, Hae Jin Lee2, Ying-Yu Jin3, Seung Hwan Yang2,3*, Joo-W on Suh3*
1Nutra Pharm Tech, Giheung-gu, Yongin, Gyeonggi 446-916, Korea
2Interdisciplinary Program of Biomodulation, Myongji University, Yongin, Gyeonggi 446-728 Korea
3Center for Nutraceutical and Pharmaceutical Materials, Myongji University, Yongin, Gyeonggi 446-728 Korea
AR T ICLE IN FO
Article history:
Received 15 April 2016
Received in revised form 16 May 2016 Accepted 15 June 2016
Available online 20 July 2016
Keywords:
Green coffee bean extract
High-fat diet induced obese m ice Adipogenesis
Body fat
Dual energy X-ray absorptiometry
ABSTRACT
Ob jective: To evaluate possible lipid catabolism and body fat regulation effects of 3-caffeoylquinic acid in Green coffee bean extract (GCBE) in high-fat diet (HFD)-induced obese mice. Methods: Obesity was induced in m ice using a HFD for four weeks. Then, m ice were fed only HFD or HFD with GCBE at 50, 100, and 200 mg/kg. Fatty acid synthesis mechanism regulation of body fat was investigated through real-time PCR and Western blot assay. Body fat reduction was measured through dual- energy X-ray absorptiometry. Results: In HFD-induced obese mice, GCBE treatment significantly decreased body weight gain,liver weight and white adipose tissue weights with regulation of adipose tissue lipolysis hormones, like adiponectin and leptin. GCBE treatment decreased mRNA expression levels of adipogenesis and adipocyte metabolism related genes in adipose tissues and the liver,and decreased the corresponding protein expression. Dual energy X-ray absorptiometry measurements were used to compare body fat between mice on high-fat and those treated with GCBE. GCBE treated mice had a lower fat mass compared to HFD alone fed mice and relative body weight and fat mass were markedly decreased. Conclusions: GCBE has a potential anti-obesity effect with lowering body fat accumulation by regulating adipogenesis and lipid metabolism-related genes and proteins in WAT and liver.
#These authors contributed equally to this work.
Tel: +82-31-330-6880
Fax: +82-31-336-0870
E-mail: ym ichigan@m ju.ac.kr
Joo-Won Suh, Center for Nutraceutical and Pharmaceutical Materials, Myongji University,Yongin, Gyeonggi 446-728 Korea.
E-mail: jwsuh@m ju.ac.kr
Foundation project: This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development (No. PJ01134802).
1. Introduction
In recent decades, obesity has become a serious clinical disease that is contributed by a high-fat diet. The World Health Organization defines obesity was abnormal or excessive fat accumulation is present in many diseases. Another definition of obesity is the accumulation of body fat from the imbalance between calorie input and energy expenditure. Incremental evidence suggests obesity is related to epidem iological diseases including diabetes, heart disease, stroke, arthritis, inflammation, and cancers[1]. Therefore, the role of fat in obesity development is important to study to prevent and treat obesity. Adipocytes store energy in triglyceride form and break down lipids into free fatty acids when energy is required[2]. Furthermore, adipocytes play a major role in obesity and related disease through the secretion of w ide range of regulatory factors. Remarkably, adipocytes hormonally control metabolism through the secretion of autocrine, paracrine, and endocrine hormones and effect insulin sensitivity, immune function, eating behavior, and most importantly regulate differentiation of preadipocytes into adipocytes[3].
Polyphenols are abundant secondary metabolites in plants and are known to prevent diseases associated with oxidative stress and its related complications. The glycosylated derivate forms of polyphenol, chlorogenic acids (CGA) (ester of caffeic acid and quinic acid) are the main polyphenol in coffee[4]. There is an increase in scientific evidence that coffee affects metabolic syndromes such as obesity, type 2 diabetes, atherosclerosis, and insulin-resistance[5-9]. Green coffee is raw coffee beans that have not been roasted. Many different pharmacological studies about green coffee bean extract (GCBE) demonstrates that the CGA in green coffee regulates hypertensive, vasoreactivity, and glucose metabolism[10-12]. There are several prospective studies regarding how 5-caffeoylquinic acid (5-CQA), the major chlorogenic acid in coffee, decreases diabetes risk by decreasing glucose uptake in the small intestine. However, only the short-term effects were analyzed and more research is needed[7,13]. In particular, recent studies propose that attenuation of obesity and lipid accumulation by green coffee bean extract is derived from 5-CQA in diet-induced obesity and insulin resistance[14,15].
In the present study, we investigate a quantitative analysis of the 3-caffeoylquinic acid (3-CQA) in GCBE and examined whether it has an ameliorative effect against high-fat diet (HFD, 60% calories from fat) induced obesity in m ice. Furthermore, decreases in lipid accumulation and metabolism related genes, proteins, and body fat composition provide scientific evidence to support GCBE as a supplement to prevent obesity.
2. Materials and methods
2.1. Chemicals and reagents
Isopropanol, TRI reagent and protease inhibitor cocktail were purchased from Sigma-Aldrich (MO, USA). HPLC grade acetonitrile and phosphoric acid were purchased from Merck (Darmstadt,Germany). Polyclonal antibodies against FAS, SREBP-1c, PPARγ,C/EBPα, AMPK, phospho-AMPK, PPARα and β-actin were purchased from Santa Cruz Biotechnology (CA, USA). Horseradish peroxidase-linked anti rabbit IgG and HRP-linked anti mouse IgG were purchased from Bio-Rad (CA, USA). SYBR Green reaction buffer was purchased from Takara (Shiga, Japan).
2.2. Compound analysis of GCBE
GCBE was provided from KPLC group (Montagne, France). Chromatographic analysis of 3-CQA in GCBE was performed using the Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA, USA) with a UV detector. The C18 column (5 μm,4.6×250 mm; Supelco, MO, USA) was maintained at 40 ℃ for chromatographic separation. The mobile phase was mixture of 0.5% phosphoric acid in distilled water (A) and 0.5% phosphoric acid in acetonitrile (B) and delivered at 1.2 m L/min in a gradient flow as follows: 0 min 8.0%, followed by 20 min 25.0%, 35 min 100.0%,and 45 m in 8%. The injected volume was 5.0 μL and optimal detection was achieved at 325 nm. The standard samples for 3-CQA was purchased from Sigma Aldrich (MO, USA) and freshly prepared for analysis.
2.3. Animal studies
Male C57BL/6J m ice were obtained from DaeHan BioLink (Chungbuk, South Korea) at four weeks of age. The m ice were individually housed in stainless steel cages and were maintained under temperature of (23± 3) ℃ in a hum idity-controlled room with a 12-12 h light-dark cycle. All mice were given free access to water and food. After acclimatization for one week, they were fed either the normal-fat diet (NFD, n=8, certified irradiated global 18% protein diet, 2918C, Harlan Laboratories, Indiana, USA) or Highfat diet (HFD, n=40, Rodent diet with 60% Kcal from Fat, #101556;Research Diets, USA) for four weeks to induce obesity. After obesity induction, the mice were divided into five experimental groups (n=8/ group) and were matched by body weight.
The follow ing five groups were studied for six weeks: normal-fat diet, HFD, and HFD with oral adm inistration of GCBE at 50 mg/ kg of body weight (HFD+GCBE 50); HFD with GCBE at 100 mg/ kg of body weight (HFD+GCBE 100); HFD with GCBE at 200 mg/kg of body weight (HFD+GCBE 200). Food intake of the mice was recorded daily and their body weights were measured tw ice per week. At the end of the experiment, the m ice were anesthetized and the liver, kidney, and white adipose tissue (WAT) were excised immediately. Each tissue was then rinsed with phosphate-buffered saline, and stored at -80 ℃ until analysis. The experimental procedures were approved by Ethics Comm ittee of the Wonkwang University (Iksan, Korea) and the m ice w ere maintained in accordance with their guidelines.
2.4. Plasma biochemical analysis
A fter six weeks of feeding, 12 h fasted m ice were anesthetized using ether and blood was collected. Collected samples were centrifuged at 2 500×g for 15 m in at 4 ℃ for biochem ical analyses of plasma parameters. The separated plasma was stored at -80 ℃ until analysis. The levels of serum glucose, total cholesterol (T-CHO), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), Alanine transam inase (ALT), Aspartate transam inase (AST), free fatty acid (FFA), leptin, and adiponectin were measured using commercial kits (Sigma-A ldrich, MO, USA) according to the manufacturer’s instructions.
2.5. Histological analysis
Liver and adipose tissue were dissected, fixed in 10% neutral buffered formalin, and embedded in paraffin for histological exam ination. The formalin-fixed and paraffin-embedded tissue blocks were cut to a thickness of 4 μm and stained with hematoxylin and eosin (H&E). The sections were photographed under 200× magnification.
2.6. Protein extraction and Western blot analysis
Cold phosphate-buffered saline washed adipose and liver tissues were homogenized in RIPA buffer (50 mM Tris-HCl pH 7.4,150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM PMSE) and 1% protease inhibitor cocktail. The homogenates were centrifuged 8 000×g for 15 m in at 4 ℃ and the supernatants were collected. Total protein concentration was calculated by BCA protein assay (Pierce, IL,USA). Proteins were separated on 10% sodium dodecyl sulfate polyacrylam ide (SDS-PAGE) gels and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked with 5% non-fat skim m ilk in Trisbuffered saline containing 0.1% Tween 20 (TBS-T) and were incubated overnight at 4 ℃ with appropriate antibodies. Follow ing a 16 h incubation at 4 ℃, the membranes were washed with TBS-T and then incubated with anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies for 1 h. After washing,the immunocomplexes were visualized by chem ilum inescence reagents and detected by chem i-lum inometer (CLINX Science Instruments Co. Ltd., Shanghai, China).
2.7. Isolation of total RNA and quantitative real-time PCR
Total RNA of adipose and liver tissues was isolated using the RNeasy m ini kit (Qiagen, Velno, Netherlands) according to the manufacturer’s protocol. The extracted RNA was reverse transcribed to cDNA by using Primescript first strand cDNA synthesis kit (Promega, Fitchburg, USA). Then the RNA expression levels of adipogenesis, beta-oxidation, and lipolysis related genes were analyzed by a real-time PCR LightCycler 96 system (Roche, Basel,Sw itzerland) using a SYBR Green Master PCR Kit (Takara, Shiga,Japan) according to the manufacturer’s protocols. Primer sequences were as follows (forward and reverse, respectively): β-actin, TGT CCA CCT TCC AGC AGA TGT and AGC TCA GTA ACA GTC CGC CTA GA; Peroxisome proliferator-activated receptor α (PPARα), CAG CGA GTA GCG CAT AGT CA and GGA TGT CAC ACA CAA TTC; PPARγ; TCA CAA GAG GTG ACC CAA TG and CCA TCC TTC ACA AGC ATG AA; Adiponectin, GCA CTG GCA AGT TCT ACT GCA A and GTA GGT GAA GAG AAC GGC CTT GT; Adipose triglyceride lipase (ATGL), AAC ACC AGC ATC CAG TTC AA and GGT TCA GTA GGC CAT TCC TC;Hormone-sensitive lipase (HSL), ACC GAG ACA GGC CTC AGT GTG and GAA TCG GCC ACC GGT AAA GAG; Sterol regulatory element binding protein-1c (SREBP-1c), GGC ACT AAG TGC CCT CAA CCT and GCCACA TAG ATC TCT GCC AGT GT; SREBP-2,GGC ACT AAG TGC CCT CAA CCT and CAC CAT TTA CCA GCC ACA GG; Carnitine palm itoyl transferase-1 (CPT-1), AAA GAT CAA TVG GAC CCT AGA and CAG CGA GTA GCC CAT AGT CA; and CCAAT/enhancer-binding protein α (C/EBPα), GTG TGC ACG TCT ATG CTA AAC and GCC GTT AGT GAA GAG TCT CAG. The real-time PCR cycling conditions were as follows: 2 min at 95 ℃; 45 cycles of 20 s at 95 ℃, 20 s at 60 ℃, 40 s at 72 ℃,and 30 s at 72 ℃; and a final extension for 5 min at 72 ℃ followed by a melting curve analysis. The mRNA levels of the target genes were normalized to the expression level of β-actin and the results were presented as the fold changes relative to the HFD group.
2.8. Body fat composition analysis
Dual energy X-ray Absorptiometry (DXA) measurements were used to compare body fat between C57BL6J m ice on HFD and treated with GCBE. DXA measurements were performed after six weeks of administration using a total-body scanner (InAlyzer dual X-ray absorptiometry, Medikors, Gyeonggi, Korea). DXA measures one time with low energy and one time with high energy to separate the images into bones and tissues in gram units by separating them into fat and lean before analysis. Radiography of body fat was displayed by three modes according to low density fat (blue color),medium density fat (yellow color) and high density fat (red color).
2.9. Statistical analysis
Statistical evaluations of the data w ere expressed as the mean±SEM. The statistical significance of the differences between the mean values for the treatment groups was analyzed by Student’s t-tests and one-way analysis of variance (ANOVA) using the software, Origin 7 (Microcal Software, USA). Values of P<0.05 and P<0.01 were considered statistically significant.
3. Results
3.1. CQA composition in GCBE
To determ ine the composition of 3-CQA in GCBE, we compared the chromatographic profiles of a standard on HPLC analysis (Figure 1). Total CQA content in the GCBE was 50% and the 3-CQA composition in the GCBE was approximately 27.5% and was confirmed by comparing the chromatogram of the standard. The optim ized green coffee bean was used in all subsequent experiments.
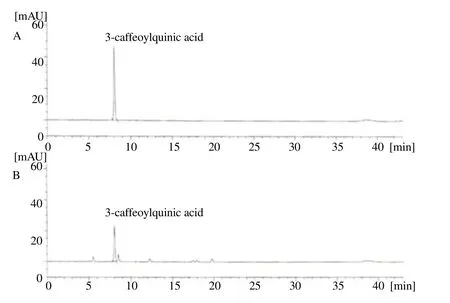
Figure 1. Representative HPLC chromatograms of 3-CQA (A) in GCBE (B)measured at a wavelength of 325 nm.
3.2. Effects of GCBE on food intake and body weight gain
We elucidated the effects of GCBE supplementation on HFD induced obese m ice. Food intake and body weight gain were compared with those of m ice fed normal diet, HFD, and HFD with GCBE (Figure 2). The initial body weights were not different among the groups. The body weight gain in HFD groups was higher than in the normal diet group. Whereas, the body weight of HFD with GCBE at 100 and 200 mg/kg were observed to significantly decrease body weight gain compared to the HFD group (P<0.05 and P<0.01,respectively).
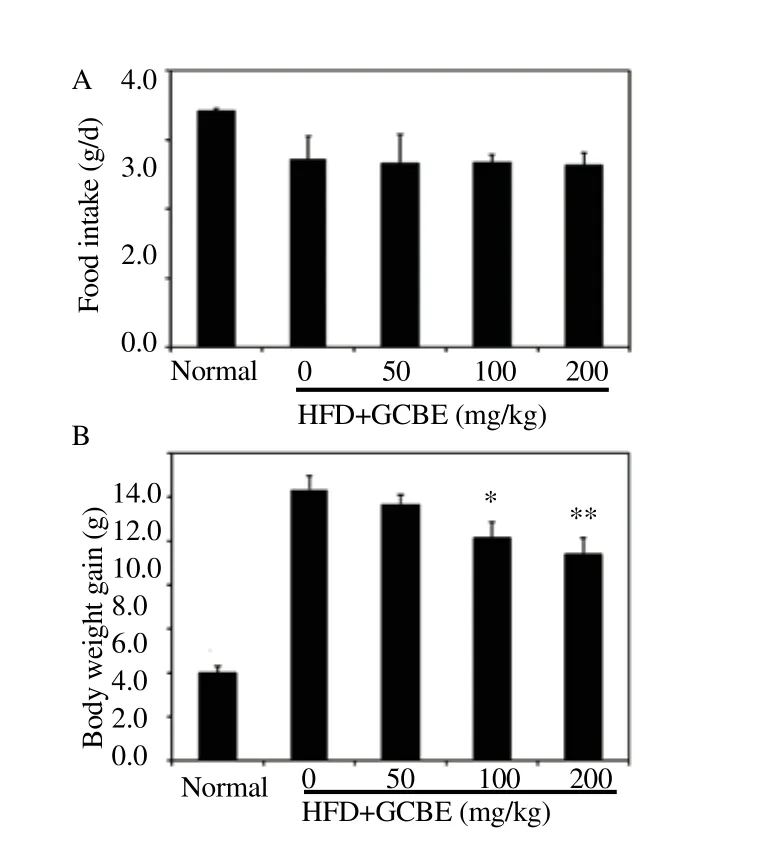
Figure 2. Effects of GCBE on body weight gain and food intake rate in HFD-induced obese mice for six weeks.
(A) Food intake rate. (B) Body weight gain. *P<0.05 and **P<0.01 compared to the HFD group (n = 8 per group).
3.3 Effects of GCBE on organ and white adipose tissue weights
The effects of GCBE on the relative weight of liver, perirenal, retroperitoneal, and epididymal WAT were compared to the HFD group (Figure 3). The organ weights from mice in the HFD group were higher than in the normal diet group. However, the HFD with GCBE group showed a decreased liver, perirenal, retroperitoneal,epididymal, and total WAT weights. However, the relative organ weights in the HFD+GCBE 200 group exhibited a significant reduction compared to the HFD group (P<0.05).
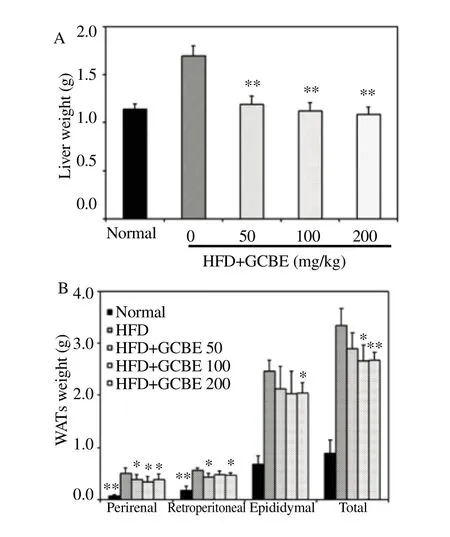
Figure 3. Effects of GCBE on organ weight in m ice fed a HFD for six weeks. *P<0.05 and ** P<0.01 compared to the HFD group (n = 8 per group).
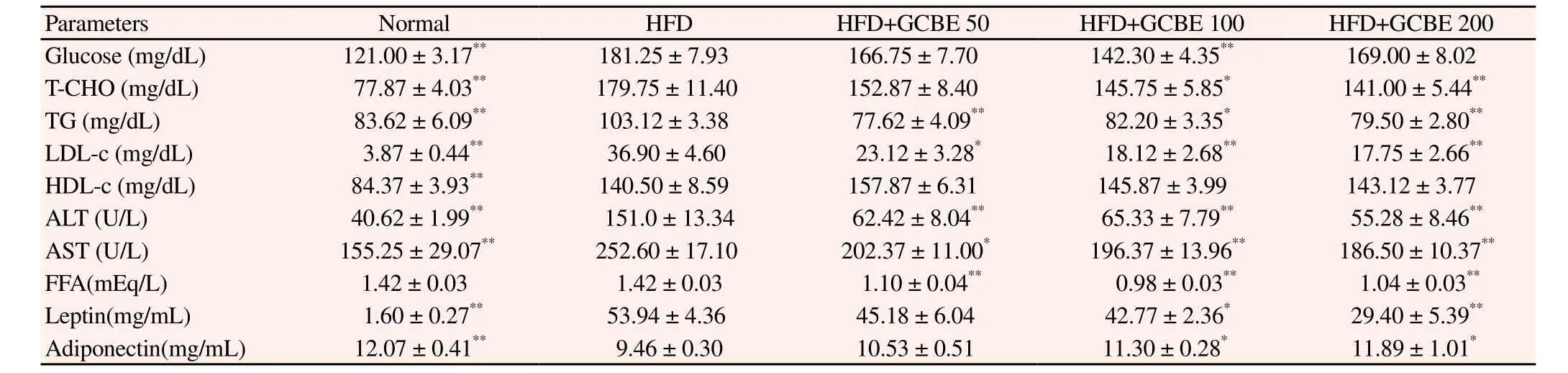
Table 1 Effects of GCBE on plasma biochem ical parameters in HFD-induced obese mice.
3.4. Effects of GCBE on plasma biochemical parameters
Table 1 shows the effects of GCBE on the plasma biochemical parameters. In the plasma biochem ical analysis glucose, T-CHO,TG, LDL-C, HDL-C, ALT, AST, and leptin levels in the normal diet group were significantly lower than in the HFD group (all,P<0.01). Analysis of lipid levels in the HFD+GCBE 200 group had significantly reduced plasma T-CHO, TG, and LDL-C levels (all, P<0.01), whereas HDL-C levels in HFD with GCBE groupsincreased when compared to the HFD group. ALT, AST, and FFA were significantly reduced in the HFD with GCBE 100 and 200 groups when compared to the normal diet group (all, P<0.01). The concentration of leptin in the HFD with GCBE 100 and 200 groups displayed a statistically significant reduction compared to the HFD group (P<0.05 and P< 0.01, respectively). However, the adiponectin levels in m ice fed GCBE showed significant increases (P<0.05).
3.5. Effects of GCBE on liver and epididymal adipose tissue morphology
Figure 4 shows microphotographic observations of hepatic tissues and eipididymal adipose tissue sections by hematoxin and eosin (H&E) staining. Experimental m ice in the figure are as follows: Normal diet, HFD, HFD with 50 mg/kg, HFD with 100 mg/kg,and HFD with 200 mg/kg groups. In the case of the liver, large macrovascular adipocyte were found in the HFD group when compared to the normal diet group. However, the HFD with GCBE 50, 100, and 200 mg/kg groups showed markedly reduced hepatic steatosis compared to the HFD induced group (Figure 4A).
Lipid accumulation and cellular morphology in epididymal WAT was examined by H&E staining. The adipocyte size in the HFD group showed an increase compared to the normal diet group. In the HFD with GCBE groups, we observed a marked reduction of adipocyte hypertrophy compared to m ice fed the HFD alone (Figure 4B).

Figure 4. Histological observations of liver sections and epididymal adipos tissue.
(A) Liver and (B) epididymal adipose tissue were stained with hematoxylin and eosin, and were viewed under a m icroscope (×200). Representative sections are from three mice from each treatment group.
3.6. Effects of GCBE on mRNA expression of genes in adipose tissues
We evaluated the effects of GCBE on the expression of adipogenesis and adipocyte metabolism related genes by analyzing the mRNA expression levels in the epididymal adipose tissues using quantitative real-time PCR. As shown in Figure 5, peroxisome PPARα, CPT-1,Adiponectin, ATGL, and HSL mRNA expression levels in the HFD group were decreased compared to the normal diet group. However,in the HFD with GCBE groups, the mRNA levels of PPARα,Adiponectin, ATGL, and HSL were significantly upregulated in a dose-dependent manner. In HFD plus GCBE 200 group, significantly increased mRNA levels of ATGL and HSL, whereas CPT-1 mRNA expression levels increased compared with levels in adipose tissues of HFD group. The expression levels of adipogenesis related genes displayed whether GCBE affects adipose tissues of HFD group. C/ EBPα, SREBP-1c and PPARγ were significantly downregulated in the HFD with GCBE 200 group compared with those in HFD group (P<0.01 and P<0.05, respectively). e

Figure 5. Effects of GCBE on genes expression levels in epididymal adipose tissues.
The mRNA expression levels of genes were evaluated by quantitative realtime PCR. *P<0.05 and **P<0.01 compared to the HFD group (n = 8 per group).
3.7. Effects of GCBE on mRNA expression of genes and proteins in the livers
To determ ine the mode of action of GCBE on HFD induced obesity, we exam ined the mRNA and Protein expressions in the liver. The adipogenesis and adipocyte metabolism related gene expression levels in HFD with GCBE groups, we found that PPAR , CPT-1,Adiponectin, ATGL, HSL, C/EBPα, SREBP-1c, and PPARγ were affected by GCBE in mice (Figure 6).
In the liver tissue, compared to the HFD group, mRNA expression levels of the normal group displayed reductions in C/EBPα, SREBP-1c, SREBP-2, and PPARγ; and statistically significant increases in PPAR and CPT-1 (Figure 6A) (both, P<0.05). In the HFD with GCBE groups, PPARα mRNA levels were significantly upregulated (P<0.05). The mRNA expression levels of CPT-1 also showed a significant increase in a dose-dependent manner (P<0.05). We found that in the HFD with GCBE groups, the adipogenesis related genes C/EBPα, SREBP-2, and PPARγ expression levels also significantlydecreased when compared to the m ice fed HFD (Figure 6A). The transcription factor SREBP-1c, a major lipogenic related protein,was also significantly reduced in HFD with GCBE groups (Figure 6A). Figure 6B and C show protein expression levels differences in GCBE supplemented groups. The target protein of SREBP-1c, FAS was statistically significantly reduced in the GCBE 200 treated group compared with the HFD group. Consistently, the C/ EBPα protein expression level in the HFD with GCBE groups was significantly decreased in a dose-dependent manner. Additionally,GCBE treatments decreased protein expression of PPARγ levels.
We also investigated the phosphorylation of AMPK and PPARα protein expression. In the HFD group, pAMPK and PPARα protein levels in the liver were markedly decreased compared to the normal group. In contrast, GCBE 100 and 200 treatments significantly increased pAMPK expression compared to the HFD group (P<0.01 and P<0.05, respectively), similar to the normal group. Interestingly,we observed that the PPARα protein was markedly and dose dependently increased in the mice fed HFD with GCBE groups, 5.6 times more than in the HFD alone group (Figure 6C).

Figure 6. Effects of GCBE on gene expression levels and proteins in liver tissues.
(A) The mRNA expression levels of adipogenesis related genes were evaluated by quantitative real-time PCR. (B) and (C) The protein expression levels were detected by Western blot analysis.*P<0.05 and**P<0.01,compared to the HFD group (n = 8 per group).
3.8. Distribution of fat mass and body weight measurements by DXA
To determ ine the effects of GCBE on HFD induced body composition, fat mass and body weight were determ ined by DXA. The DXA scan of the mice fed normal diet, HFD and HFD with GCBE 200 mg/kg is presented as radiography of body fat (Figure 7A). The HFD induced obese mice presented more large red color area than blue and yellow, meaning they had more high density of fat than low and medium density fats. The GCBE supplement for six weeks significantly reduced the body weight in the HFD induced obese m ice. Furthermore, the relative distribution of fat mass noticeably decreased (Figure 7B and C) (P<0.01).
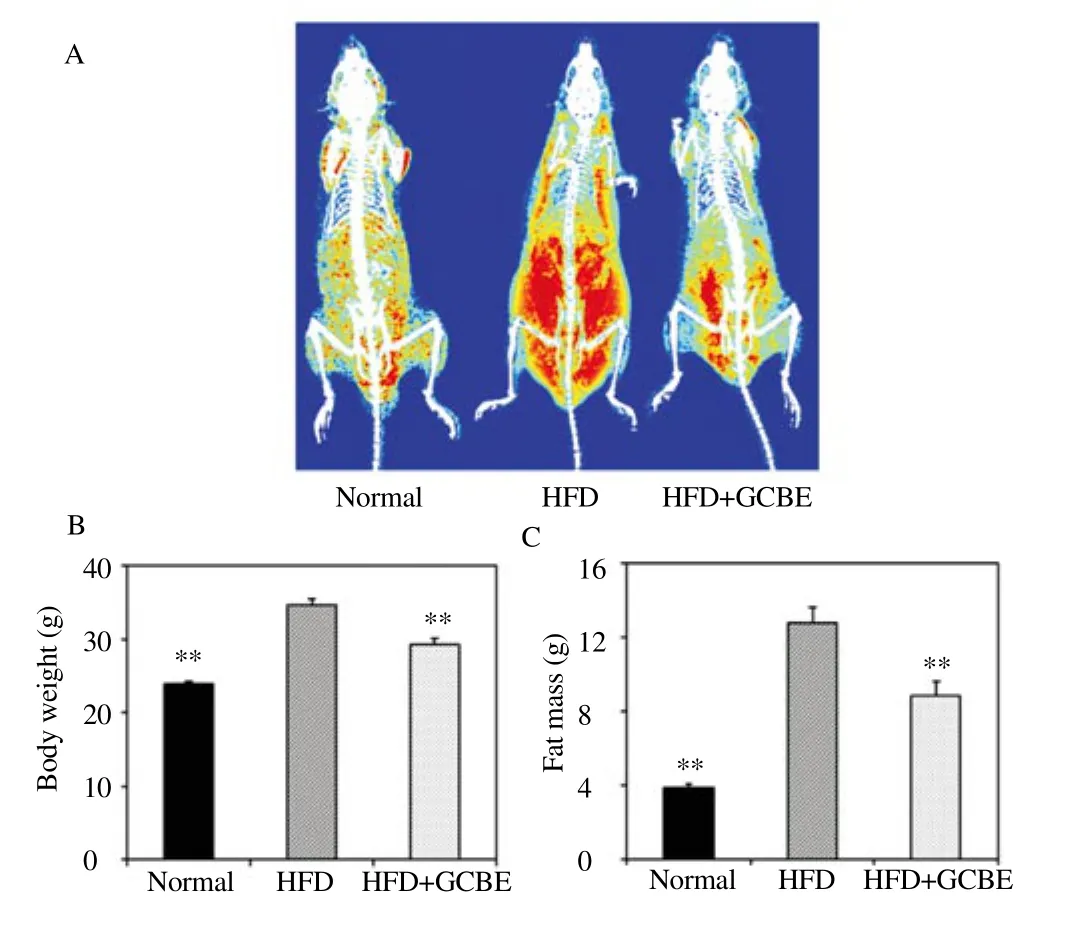
Figure 7. Distribution of fat mass and body weight measurements by DXA on m ice fed a normal fat diet, high fat diet, and high fat diet with GCBE 100 mg/kg.
(A) The radiography of body fat is displayed by three modes according to low density fat (blue), medium density fat (yellow), and high density fat (red). (B) The body weight, and (C) fat mass were measured by DXA. *P<0.05 and **P<0.01 compared to the HFD group (n = 8 per group).
4. Discussion
The chlorogenic acid was glycosy lated derivate form of polyphenol, caffeic and quinic acid combined form. Coffee has high concentrations of chlorogenic acid as most common form is 5-CQA[1]. Previous studies have indicated that chlorogenic acid has many potent biological properties[16-18]. Additionally, recent reports of chlorogenic acid provide regulatory effects of glucose and lipid concentrations in diabetes and obesity[19-22].
Song et al.[15] have reported that 5-CQA was the most abundant and active compound in decaffeinated GCBE and has weight lowering and insulin resistance effects in m ice fed the HFD at a dose of 300 mg/kg GCBE. Although previous reports show the effects of GCBE on the development of obesity, the effects of GCBE in HFD induced obese mice measuring total body fat mass has not been investigated. Thus, our study presents that feeding 3-CQA rich GCBE ameliorates and suppresses dietary-induced obesity and decreases body fat mass,directly. Our data show that GCBE has anti-obesity effects and confirms previous studies. Further, we show that GCBE effects the regulation of adipogenesis and lipid metabolism, directly effecting the distribution of fat mass.
Increased fat intake leads to obesity with body weight gain and glucose intolerant in mice[23]. We confirmed that six weeks of GCBE (Total chlorogenic acid was 50%, which was including 27.5 % of 3-CQA) supplementation in a HFD did not affect the food intake in HFD-induced obese m ice. Additionally, body weight gain in GCBE fed mice significantly decreased compared to mice fed a HFD alone. This phenomenon supports previous reports that GCBE has antiobesity effects[15].
Our results also show that GCBE supplementation significantly lowers plasma T-CHO, TG, FFA, and LDL-C levels while increasing plasma HDL-C levels in the m ice fed HFD. These results suggest that GCBE could reduce HFD induced hyperglycem ia and hyperlipidem ia.
Leptin and adiponectin regulate lipolysis through opposing activities in adipocytes[24]. Mature adipocytes produce adiponectin,which increases insulin sensitivity, glucose uptake, fatty acid oxidation, and anti-inflammatory effects in hormone-stimulated lipolysis[25]. To prevent lipid accumulation in adipose tissue,adipocytes secrete leptin to regulate food intake and fatty acid oxidation contacted with brain[26]. The plasma concentration of adipose tissues and endocrine hormones revealed that GCBE treatment reduced leptin concentrations and inversely increased adiponectin, in a dose dependent manner.
In addition, we confirmed that mice fed GCBE showed significant decreases in liver and WAT weight in HFD-induced obese m ice. Moreover, in liver and adipocytes histological analysis, GCBE treatment showed smaller adipocyte sizes and number in the m ice fed a HFD. These results indicate that GCBE could decrease adipocytes, which demonstrates lipid accumulation and un-oxidized fatty acid induces lipotoxicity and dysregulation, which then decreases stimulation of lipolysis.
Another important finding in our study is that GCBE may improve adipogenesis and lipid metabolism in HFD-induced obese m ice. Adipogenesis is the development of adipocyte differentiation with the related gene transcription factors, C/EBPs and PPARs[27]. In the mouse models, adipose tissue regulation was related with PPARγ and C/EBPα with HFD feeding. GCBE treatment reduced C/EBPα,SREBP-1c, and PPARγ mRNA expression levels in expression[28,29]. PPARγ expression is activated in adipocytes genes[30]. The lipogenic transcription factor, SREBP-1c, is a key regulator for fatty acid and cholesterol, as well as, LDL receptor that activates lipogenesis[31].
To examine the underlying mechanisms, we induced obesity with hyperglycem ia and hyperlipidem ia epididymal adipose tissue. Additionally, we investigated lipid catabolism activity in adipose tissues. Adipocytes have excess energy and store fatty acids in the form of TG in lipid droplets. Consequently, energy regulation of the catabolic pathway, including fatty acid oxidation and glycolysis, is a strong candidate for obesity.
We also studied w hether GCBE has anti-lipolytic effects in adipocytes. PPARα is a nuclear receptor, which increases the expression of gene CPT-1 to induce m itochondrial fatty acid to dispose through β-oxidation[32]. GCBE treatment increases the mRNA expression of PPARα and CPT-1, indicating GCBE promotes energy dissipation in adipocyte tissues. The adiponectin plasma concentration regulates lipid profiles. Gene expression levels of adiponectin were also up-regulated by GCBE treatment. HFD-induced obese m ice presented a decrease of adiponectin gene expression in adipose tissues[33]. Moreover, Qiao et al.[25] proposed adiponectin inhibits lipolysis and regulates lipid metabolism suppressing catabolites of TG by inhibiting ATGL and HSL in mouse adipocytes. Our study showed that lipolysis activity of GCBE was induced by adiponectin, HSL, and ATGL mRNA expression in adipocyte tissues. Based on these mRNA expression results in adipose tissue, GCBE leads to reduced adipogenesis and lipid accumulation in the adipose tissues of HFD-induced obese mice.
Of the adipogenesis-related gene and protein expression levels in the liver, we found that the dose of GCBE treatment significantly up-regulated the lipid oxidation related genes, like PPARα and CPT-1, and down-regulated the transcription factors that regulate adipocyte differentiation, like C/EBP , SREBPs, and PPARγ. There is a possibility GCBE treatment affects the expression levels of adipogenesis-related genes and proteins. It is likely that the antiobesity effects of GCBE treatment are caused by the suppression of gene expression and proteins involved in fatty liver induced obesity. In obesity development, AMPK is a key mediator that sw itches adipocytes from anabolizing to catabolizing lipids. In the liver,AMPK altered fatty acid oxidation and glycogenesis, while switching off the synthesis of fatty acids, gluconeogenesis and ATP consumption with PPARα[34,35]. In this study, phosphorylation of AMPK and PPARα increased in GCBE treated groups in a dose dependent manner. From these results, GCBE induced AMPK activation causes anabolic stages involving fatty acid, glucose oxidation and inhibition of adipocytes proliferation in obese m ice.
Several epidem iologic studies have reported that body fat,
especially abdom inal and visceral fat, in adiposity related obesity is associated with increased risk of metabolic syndrome[36]. Excess adiposity was measured using an imaging technique, to investigate the fat tissue and fat distribution. DXA quantifies the past photon peaks through tissue-organs ex vivo with high accuracy to understand the mechanisms involved in metabolic regulation by measurement of fat distribution and composition[37,38]. Simultaneously, we measured DXA fat distribution, body weight, and total fat mass. Notably, our results show that the m ice supplemented with a HFD have a higher body weight, fat mass, and distribution of high density fat compared to m ice fed a normal diet. When we compared GCBE and HFD treatment groups, the GCBE group displayed a significant reduction of fat distribution and concentration, with less body weight and fat mass.
In conclusion, this study show s GCBE treatment markedly ameliorated body fat accumulation in HFD induced obesity and reduced body weight gain, fat mass, adipocyte sizes, WAT weight,and plasma lipid levels. We confirm that the active compound 3-CQA from GCBE, reduces body fat accumulation by the regulation of adipogenesis and lipogenesis in obesity. Thus, these findings demonstrate that GCBE represents a potential activity that prevents the developement of obesity, hyperlipidem ia and its complications in diseases.
Acknow ledgments
This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development (No. PJ01134802)of Rural Development Administration, Republic of Korea.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012; 18(3): 363-374.
[2] Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006; 83(2): 461-465.
[3] Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Bioche Mol Biol 2005; 40(4): 229-242.
[4] Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. AM J Clin Nutr 2004; 79(5): 727-747.
[5] Ding M, Bhupathiraju SN, Chen M, Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of d type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care 2014;37(2): 569-586.
[6] Dickson JC, Liese AD, Lorenzo C, Haffner SM, Watkins SM, Ham ren SJ,et al. Associations of coffee consumption with markers of liver injury in the insulin resistance atherosclerosis study. BMC Gastroenterol 2015; 15: 86-88. DOI: 10.1186/s12876-015-0321-3.
[7] Greenberg JA, Boozer CN, Geliebter A. Coffee, diabetes, and weight control. Am J Clin Nutr 2006; 84(4): 682-693.
[8] Tanaka K, Nishizono S, Tamaru S, Kondo M, Shimoda H, Tanaka J, et al. Anti-obesity and hypotriglyceridemic properties of coffee bean extract in SD rats. Food Sci Technol Res 2009; 15(2): 147-152.
[9] Ho L, Varghese M, Wang J, Zhao W, Chen F, Knable LA, et al. Dietary supplementation with decaffeinated green coffee improves diet-induced insulin resistance and brain energy metabolism in m ice. Nutr Neurosci 2012; 15(1): 37-45.
[10] Kozuma K, Tsuchiya S, Kohori J, Hase T, Tokimitsu I. Antihypertensive effect of green coffee bean extract on m ildly hypertensive subjects. Hypertens Res 2005; 28(9): 711-718.
[11] Ochiai R, Jokura H, Suzuki A, Tokimitsy I, Ohishi M, Kokai N, et al. Green coffee bean extract improves human vasoreactivity. Hypertens Res 2004; 27: 731-737.
[12] Blum J, Lemaire B, Lafay S. Effect of a green decaffeinated coffee extract on glycemia. Nutra Food Res 2007; 6(3): 13-17.
[13] Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycem ic effects of chlorogenic acid and caffeine. AM J Clin Nutr 2003;78(4): 728-733.
[14] Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese m ice. Food Chem Toxicol 2010; 48(3): 937-943.
[15] Song SJ, Choi S, Park T. Decaffeinated green coffee bean extract attenuates diet-induced obesity and insulin resistance in m ice. Evid Based Complement Alternat Med 2014; DOI: 10.1155/2014/718379.
[16] Meng S, Cao J, Feng Q, Peng J, Hu Y. Ro les o f ch lorogenic acid on regu lating g lucose and lipids m etabo lism: a review. Evid Based Complement Alternat Med 2013; 2013: 801457. DOI: 10.1155/2013/801457.
[17] Santos MD, A lmedia MC, Lopes NP, Souza GEP. Evaluation of the antiinflammatory, analgesic and antypiretic activity of the natural polyphenol chlorogenic acid. Biol Pharm Bull 2006; 29: 2236-2240.
[18] Lindsay J, Laurin D, Verreault R, Herbert R, Heliwell B, Hill GB, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol 2002; 156(5): 445-453.
[19] Rodriguez de Sotillo DV, Hadley M. Chlorogenic acid modifies plasma and liver concentrations of: cholesterol, triacylglycerol, and m inerals in (fa/fa) Zucker rats. J Nutr Biochem 2002; 13(12): 717-726.
[20] Ong KW, Hsu A, Tan BK. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One 2012; 7(3): e32718.
[21] Ong KW, Hsu A, Tan BK. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem Pharmacol 2013; 85(9): 1341-1351.
[22] Jin S, Chang C, Zhang L, Liu Y, Huang X, Chen Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db m ice. PLoS One 2015; 10(4): e0120842.
[23] Rossmeisl M, Ri JS, Koza RA, Kozak LP. Variation in type 2 diabetesrelated traits in mouse strains susceptible to diet-induced obesity. Diabetes 2003; 52(8): 1958-1966.
[24] Wedellová Z1, Dietrich J, Sik lová-Vítková M, Kološtová K,Ková iková M, Dušková M, et al. Adiponectin inhibits spontaneous and catecholamine-induced lipolysis in human adipocytes of non-obese subjects through AMPK-dependent mechanisms. Physiol Res 2011;60(1): 139-148.
[25] Qiao L, Kinney B, Schaack J, Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes 2011; 60(5): 1519-1527.
[26] Arch JR. Central regulation of energy balance: inputs, outputs and leptin resistance. Proc Nutr Soc 2005; 64: 39-46.
[27] Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev 2000; 14: 1293-1307. DOI: 10.1101/gad.14.11.1293.
[28] Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 1999; 4(4): 585-595.
[29] Linhart HG, Ishimura-Oka K, DeMayo F, Kibe T, Repka D, Poindexter B, et al. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc Natl Acad Sci USA 2001; 98(22): 12532-12537.
[30] Farmer SR. Regulation of PPARγ activity during adipogenesis. Int J Obes 2005; 29: S13-16.
[31] Xiaoping Z, Fajun Y. Regulation of SREBP-mediated gene expression. Sheng Wu Wu Li Hsueh Bao 2012; 28: 287-294.
[32] Song S, Attia RR, Connaughton S, Niesen MI, Ness GC, Elam MB, et al. Peroxisome proliferator activated receptor alpha (PPAR alpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol Cell Endocrinol 2010;325(1-2): 54-63.
[33] Kadawaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005; 26(3): 439-451.
[34] Hardi DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obesity 2008; 32(Suppl 4): S7-12.
[35] Daval M, Foufelle F, Ferre P. Function of AMP-activated protein kinase in adipose tissue. J Physiol 2006; 574(1): 55-62.
[36] Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med 2005; 56: 45-62.
[37] Petrobelli A, Boner AL, Tatò L. Adipose tissue and metabolic effects: new insight into measurements. Int J Obes 2005; 29: S97-100.
[38] Chen W, Wilson JL, Khaksari M, Cow ley MA, Enriori PJ. Abdom inal fat analyzed by DEXA scan reflects visceral body fat and improves the phonotype description and the assessment of metabolic risk in mice. Am J Physiol Endocrinol Metab 2012; 303(5): E635-643.
doi:Document heading 10.1016/j.apjtm.2016.05.017
*Corresponding authors: Seung Hwan Yang, Center for Nutraceutical and Pharmaceutical Materials, Myongji University, Cheoin-gu, Yongin, Gyeonggi , Korea.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effects o f arteannuin B, arteannuic acid and scopo letin on pharmacokinetics of artem isinin in m ice
- Predicted pattern of Zika virus infection distribution with reference to rainfall in Thailand
- Effects of scallop shell extract on scopolam ine-induced memory impairment and MK801-induced locomotor activity
- H igh seroprevalence of asymptomatic viral haemoparasites among prospective blood donors in N igeria
- Molecular epidem iology and phylogeny of N ipah virus infection: A m ini review
- Hepatoprotective properties of oleanolic and ursolic acids in antitubercular drug-induced liver damage
