兰州百合组培鳞茎发育研究
2016-06-23张进忠韦绍龙孙嘉曼韩沅杉
张进忠, 韦绍龙, 孙嘉曼, 韩沅杉, 周 维
( 1. 广西农业科学院 生物技术研究所, 南宁 530007; 2. 广西作物遗传改良生物技术重点开放实验室, 南宁 530007 )
兰州百合组培鳞茎发育研究
张进忠1, 韦绍龙1, 孙嘉曼2, 韩沅杉1, 周维1
( 1. 广西农业科学院 生物技术研究所, 南宁 530007; 2. 广西作物遗传改良生物技术重点开放实验室, 南宁 530007 )
摘要:该研究以兰州百合商品种球鳞片为外植体材料,通过组织培养诱导丛生芽萌发及高频增殖,再以丛生芽为材料诱导其发育形成小鳞茎,调节培养基对小鳞茎进行膨大发育培养,最终形成促进兰州百合组培鳞茎膨大发育的“三步”组培培养技术路线;对发育过程中形成的丛生芽、小鳞茎及膨大鳞茎进行淀粉含量测定与生长特征参数统计,分析各步培养对鳞茎形成发育过程中淀粉含量与形态变化的影响。结果表明:所建立的“三步”培养方案培育出的组培鳞茎直径、重量与鳞片数分别为1.66 cm、2.48 g和26.33片,有效地促进了鳞茎的膨大,并能诱导鳞茎主茎杆的形成发育;在培养进程中其淀粉含量呈现逐步增加的趋势,这表明与鳞茎膨大发育正相关,同时鳞茎大小、重量及鳞片数三者也表现为正相关性;当鳞茎所含鳞片数在26片以上时,其生长点易发育形成主茎杆。该文研究了兰州百合组培鳞茎的形成与膨大发育技术,所研发的“三步”培养组成的鳞茎膨大发育组培技术有效地促进了鳞茎的膨大发育,而膨大发育的鳞茎能有效地缩短田间生长周期,从时间上提高百合生产量,同时为实现兰州百合膨大的鳞茎种球规模化生产提供技术支撑。
关键词:兰州百合, 小鳞茎, 发育, 淀粉, 植物激素
Lanzhou lily, a variation ofLiliumdavidii, is the main edible and the only sweet lily in China, which has big bulb, gorgeous color, high quality and medicinal value. Lanzhou lily has broad marketing space and developing prospect because of its advantages. However, it has a long natural growth period, and the conventional propagation pattern is to sow scales that possess low-cost and handleability. The propagated bulblet can not develop main stem in the first year, which only grows some basal leaves for growth of the bulblet (Gao et al, 1986). It needs 2-3 a for formation of the big bulblet with developed main stem which can be transplanted directly as seed-bulb (Teng & Song, 2005). The commercial bulb would be harvested after the seed-bulb with main stem had transplanted and grown for another 2-3 a (Wang, 2010).
In this connection, the development of bulblet and formation of main stems by tissue culture are of great significance in commercial production of Lanzhou lily bulblet. The research about tissue culture of Lanzhou lily focuses on induction of regeneration plant, for example, inducing sprouting and plant regeneration from Lanzhou lily scale and leaf explants (Long et al, 2004; Han & Guo, 2009), inducing callus formation and shoot differentiation from petals (Liu, 2007), but there is little on the bulblet formation and development of Lanzhou lily. It has been reported that high concentrations of glucose could effectively promote the bulblet formation and development of other lilium plants (Kumar et al, 2005; Varshney et al, 2000; Rice et al, 1983; Taeb & Alderson, 1990). Nhut (2003) reported that the combination ofN6-benzyladenine (BA) and gibberellic acid (GA3) could promote main stem formation directly from theinvitroreceptacle ofLiliumlongiflorum. Up to now, there is no report about the bulblet formation and development of Lanzhou lily. To overcome low proliferation rates and long period of Lanzhou lily bulblet propagation, in this study, the propagation techniques of tissue culture were used to cultivate bulblet of Lanzhou lily at different stages, and to improve its bulblet formation and development. The correlations between starch contents and characteristic parameters of bulblet growth were investigated. The changes of characteristic parameters of bulblet swelling, especially the parameters changes of main stem formation were also examined. The proposed technology on bulblet formation and development of Lanzhou lily was establishment, which would lay the foundation for large-scale production of bulblet.
1Materials and Methods
1.1 Experimental materials
Tissue culture plantlets of Lanzhou lily were provided by Biological Technology Institute of Guangxi Academy of Agricultural Sciences. The media were set as, Fp1: MS+0.3-0.5 mg·L-1BA+0.03 mg·L-1NAA + 30 g·L-1Sucrose+5 g·L-1Agar;Fp2: MS + 90 g·L-1Sucrose+5 g·L-1Agar;Fp3: MS+0.15 mg·L-1BA+0.15 mg·L-1GA3+ 90 g·L-1Sucrose+5 g·L-1Agar.
1.2 Experimental methods
1.2.1 Culture approachThe whole culture process was comprised of three steps. Step 1, Fp1 medium was used to culture multiple shoots of Lanzhou lily. Steps 2, the leaves of multiple shoots were excised and transferred the multiple shoots to Fp2 medium for two subcultures. Step 3, the basal leaves of bulblets on Fp2 medium for one subculture were excised and transferred the multiple bulblets to Fp3 medium for two subcultures. The growth status in every step was recorded. All culture conditions as follow, (25±2)℃ with a light intensity of 150 μmol·m-2·s-1and 14 h light/10 h dark photoperiod for 28-30 d of one subculture period.1.2.2 Trial on the change of culture stepThe multiple shoots of Lanzhou lily cultured on Fp1 transferred into Fp3 medium directly without inducing bulblet on Fp2. The growth was recorded.
1.2.3 Starch content determinationThe starch content was estimated following the spectrophotometric method according to Liu et al(2013).
1.2.4 Material preparation and electron microscopy observationObservation of cultures at different development phases through scanning electron microscope (SEM) has been done. The basal portion of adventitious shoot from step1 and scales of bulblets from other step were observed with SEM (Tescan, VEGA Ⅱ LUM, Czech), and the treatment method for specimens refer to Nhut (2003).
1.3 Data analysis
Each treatment had 30 explants and was repeated 3 times. Explants in experiments were arranged in a completely randomized design. Data were presented as mean ± standard error of three independent experiments, and was analyzed for significance by analysis of variance with the mean separation by Duncan’s multiple range test and significance was determined at 5% level (SPSS 15.0).
2Results and Analysis
2.1 Culture approach of Lanzhou lily bulblet formation and development
Multiple shoots could propagate stablely without vitrification through Step 1 (Fig. 1: a), and the proliferation coefficient got to 3-4. The bulblets formed in Step 2 and the bottom of leaves enlarged and formed small scales with significant differences in leaf morphology. The whole base of buds swelled gradually into an oval shape to achieve a change from buds to bulblet (Fig. 1: b). Step 3 was the enhancement and development of bulblets, in which the scale enlarged and the bulblet size increased rapidly (Fig. 1: c). The transformation of tissue culture plantlet of Lanzhou lily from multiple shoots to bulblets was achieved according to the three continuous culture steps.
The growth of bulblet was explored by changing the culture approach. As shown in Fig. 1: d, the multiple shoots failed to form bulblet and remained the multiple shoot status after the multiple shoots obtained from Step 1 transferred to Step 3 directly for one subculture.
The increase of bulblet size was much more through twice subculture than once subculture in Step 2 (Fig. 1: e, Table 1), with the increase rates of diameter and weight were 33.33% and 80.08%, respectively, but the increase was significant less than that in Step 3 (Table 1). Compared to the first subculture in Step 2, the increase rates of diameter and weight were up to 180.56% and 894.29% in Step 3 for one subculture. The bulblet grew more rapidly after culture for two cycles in Step 3, with the diameter and weight of bublet were up to 1.32 cm and 1.66 g (Table 1). Some of the bulblets developed main stems in this stage (Fig. 1: f).
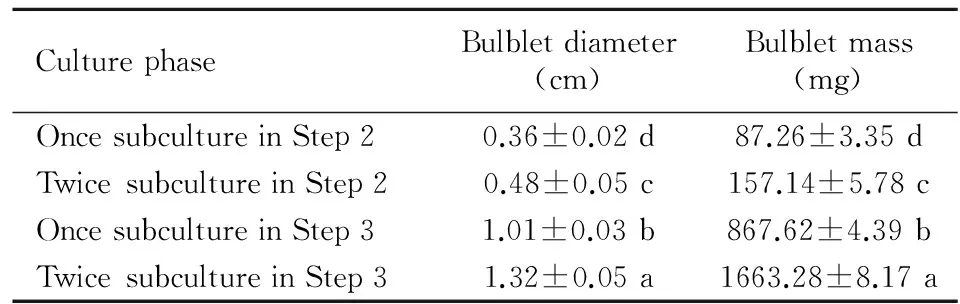
Table 1 Formation development of bulblets in Step 2 and
Note: Values are means ± SE. Means in a column followed by the same letters are not significantly different according to Duncan’s multiple range test at the 5% level. The same below.
2.2 Starch content change of Lanzhou lily during the bulblet formation and development
The starch contents of Lanzhou lily in the process of bulblets formation and development were estimated (Table 2). The starch content was the lowest with the medium containing 30 g·L-1sugar in Step 1. However, the starch content increased significantly (P<0.05) and up to 32.54% through increasing the sugar concentration to enhance the bulblets formation in the Step 2. By adding the BA, GA3and sugar to stimulate the bulblets formation, the starch content of bulblets increased to 42.68% in Step 3. Compared to Step 1 and 2, the starch contents increased 113.83%, 31.16%, respectively. The results demonstrated that the starch content increased gradually in the process of tissue culture Lanzhou lily bulbets formation and development from multiple shoots induction, and the starch content was positively correlated to the development of bulblet.
The starch grain formation during the development of multiple shoots and bulblets was oberved with scanning electron microscope (SEM), as shown in (Fig. 2: a). The bulblets starch grain increased obviously on the condition of bulblets induction and development (Fig. 2: b, c).
2.3 Correlation between main stem formation and bulblet diameter, weight and scale number
The development of main stem was promoted with the increasing of bulblet size and weight in the process of Lanzhou lily tissue culture bulblet development. However, in its natural breeding process, it needs 1-2 a from planting scales to forming bulblets with main stem (Gao et al, 1986). Therefore, it is of great importance for bulblet production that Lanzhou lily bublet development and main stem formation by tissue culture. The parameters of bublets such as diameter, weight, number of scales, were investigated in the once and twice subculture in Step 3 (Table 3). The data indicated that the bulblet diameter, weight and number of scales, which formed the main stem, were significant higher than that did not form main stems (P<0.05), with 22.96%, 48.50% and 38.36% increased rate, respectively. Meanwhile, it was easy to form main stems while the scale number of bulblets reached to 26 or more.
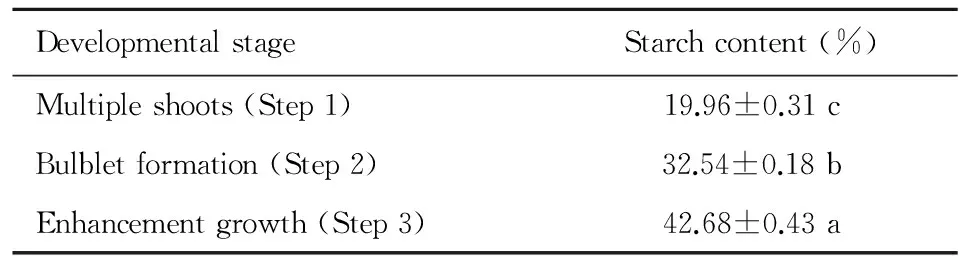
Table 2 Starch content change during the development process of Lanzhou lily

Fig. 1 Efficient approach of inducing bulblet development using bulb scale explants of Lanzhou lily a. Multiple shoots induced from callus in Step 1; b. Little bulblets formation on Fp2 in Step 2; c. Bulblets growth and development on Fp3 in Step 3; d. Shoots development come from the approach of Step1 to Step 3 directly without Step 2 of little bulblet formation culture; e. Bulblets development on Fp2 in Step 2 for twice subculture; f. Bulblet main stem formation in Step 3; g. Obtained big bulblets in Step 3.

Fig. 2 SEM allowed clear visualization of starch grain formation during Step 1-3. a. Transverse section of shoot’s base in Step 1, hardly to find starch grain; b, c. Plenty of starch grain formed on the inner of scales in turn in Step 2 and Step 3.
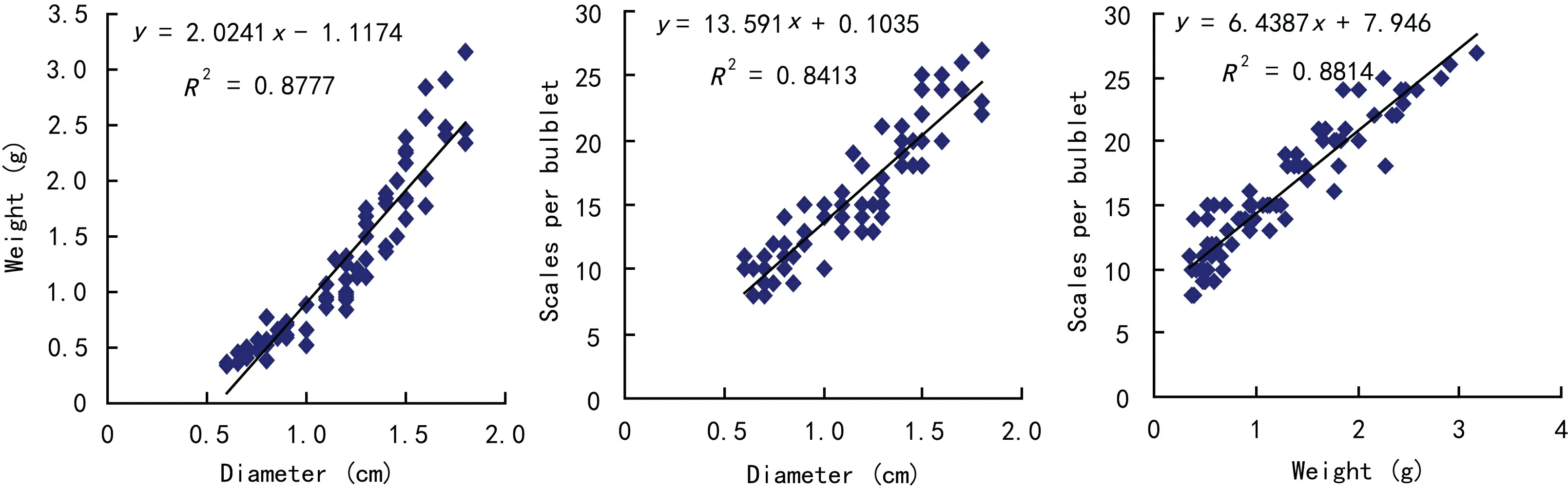
Fig. 3 Relativity of bulblet diameter, weight and scale number during the bulblet development
As shown in Fig. 3, the bulblet diameter, weight and number of scales showed a positive correlation between each other. The increasing of bulbets size accompanied with the increasing of weight and scale number during the bulblets development.

Table 3 Comparison of bulblets that formed and unformed the main stem
3Discussion
There are some related studies focused on tissue culture techniques about Lanzhou lily buds development and multiple shoots propagation inducing by the scale explants (Han & Guo, 2009; Liu et al, 2010). In this study, combination of certain concentration of BA and α-naphthaleneacetic acid (NAA) that have been reported by former studies (Lin et al, 2008; Xu et al, 2009), were used to propagate multiple shoots stably (Fig. 1: a). However, these researches were limited to the regeneration shoots by tissue culture, lack of the bulblet development research. Based on the proliferation of multiple shoots culture for the first phase of Lanzhou lily bulblet development, it provided bud materials for the formation and enlargement growth of bulblet. However, the bulbs formation and development mainly proceed in the following Step 2-3, which was difficult to proliferate. The proliferation rate of the whole tissue culture system mainly reflected in the first stage of buds proliferation rate.
The bulblet size increased significantly (Fig. 1: e) through two successive subcultures in the Step 2, which indicated that the high concentration of sugar could continuously stimulate bulblet growth to a certain extent, at the same time, the quantity of bulblet formed was related to the multiple shoot size and growth state, in general bud block of 1 cm ×1 cm size could form 10-15 bulblets (Fig. 1: b).
Continuous twice subculture with a high concentration of sugar could promote bulblet growth in Step 2, but the growth rate still could not meet the requirements for the bulblet development. In order to make the bulb development more, the enhancement growth of bulblet in Step 3 has been used, the use of combination of BA and GA3promote bulblet enlargement development. As shown in Table 1, the bulblet diameter and the weight increased significantly (P<0.05) (Fig. 1: c) by the once subculture in Step 3 than by the twice subculture in Step 2, and increased at a greater rate through the twice subculture in Step 3, diameter and weight reached 1.32 cm and 1 663.28 mg, and could induce the formation of main stem (Fig. 1: f, g). The effects of continuous twice subculture in Step 2 could not achieve the effects in Step 3, so it showed the necessity of bulblet swelling growth culture in Step 3. It was reported that BA in combination with GA3could effectively promote the formation of main stem from longiflorum receptacle explants in the literature (Nhut, 2003), but the induction main stem of Lanzhou lily bulblet has not been reported. In our study, the BA and GA3with high concentration of sugar was of obviously positive significance for Lanzhou lily bulb expanded rapidly development and main stem formation.
By changing the culture approaches, the multiple shoots, which obtained from Step 1 turned to Step 3 directly, could not be induced to form bulblets but remains multiple shoots (Fig. 1: d). It showed that the materials used in Step 3 must be formed bulblets to promote its rapid enlargement and development. The bulblets development did not achieve the best through the continuous culture in Step 2. Through three steps, including reproduction by buds, inducing of small bulbs and swelling of bulbs, bulblets formation and development of Lanzhou lily by tissue culture could provide the best technical solutions to realize the scale production of Lanzhou lily bulb development. Its main features included the first buds reproduction provided a higher multiplication factor buds to achieve stable growth, the multiple shoots formed small bulblets in Step 2, and the bulblets developed quickly to form bulbs with the main stem.
A physiological and biochemical change of carbohydrate metabolism is an important process in the development of lily bulb, the metabolism of energy storage and decomposition is the basis of lily morphology (Sun et al, 2008). This study showed that the starch content increased significantly in the buds proliferation, bulbs formation and bulbs swelling stage, which was positive correlated to bulbs development. A high concentration of sugar promoted the synthesis of starch, BA and GA3plant hormones effectively improved the conversion bulb sugar to starch. Meanwhile, the accumulation of starch promoted the bulbs enlargement. Electron microscope results similar to this, multiple shoots stage of Step 1 was not observed with starch grains, bulblet formation and developmental stages of Step 2-3, a great amount of starch grains have been synthesized (Fig. 2: a-c). Studies show that sugar and starch metabolisms play an important role in the regulation during the morphogenesis of Lanzhou lily bulblet.
The formation of main stem during the development of bulblet growth development has been analyzed for the first time. During the bulblet formation and enlargement of the development process, bulb diameter, weight and the number of scales showed positive correlation, and bulblet showed main stem pumped growth when a certain number of scales (more than 26) have formed. Gao (1986) reported Lanzhou lily growth point development could be divided into three stages, scale differentiation stage, apical bud differentiation phase, and flower bud differentiation stage. The main stem differentiation showed that growth point of bulblet began to change from scale differentiation to apical bud differentiation.
References:
GAO YY, ZHANG JD, LIU DY, 1986. Observation on the characteristics of growth and development characteristics of Lanzhou lily [J]. Gansu Agric Sci Technol, 10:2-5. [高彦仪, 张金娣, 刘德义, 1986. 兰州百合生长发育特性特征观察 [J]. 甘肃农业科技, 10:2-5.]
HAN HL, GUO CJ, 2009. The tissue culture method of Lanzhou lily [J]. J Tianjin Norm Univ: Nat Sci Ed, 29(3):62-65. [韩华丽, 郭成金, 2009. 兰州百合的组织培养 [J]. 天津师范大学学报·自然科学版, 29(3):62-65.]KUMAR S,KASHYAP M, SHARMA DR, 2005.Invitroregeneration and bulblet growth from lily bulbscale explants as affected by retardants, sucrose and irradiance [J]. Biol Plant, 49(4): 629-632.
LIN GM, LI XQ, LI CS, et al, 2008. Studies on tissue culture and high frequency propagation ofLiliumdavidiivar.unicolor(Hoog) Cotton [J]. J Agric Sci & Technol, 10(2):110-113. [林贵美, 李小泉, 李朝生, 等, 2008. 兰州百合组织培养和高频繁殖 [J]. 中国农业科技导报, 10(2):110-113.]
LIU F, 2007. Research on micropropagation from petals of Lanzhou lily [J]. Northern Hortic, 6:210-212. [刘芬, 2007. 兰州百合花瓣高频再生体系研究 [J]. 北方园艺, 6:210-212.]
LIU XH, ZHENG LX, ZHENG LM, et al, 2013. Determination of amylose and amylopectin in the commonly used starch materials by dual-wavelength spectrophotometry [J]. Guangdong Agric Sci, 40(18): 97-100. [刘襄河, 郑丽璇, 郑丽勉, 等, 2013. 双波长法测定常用淀粉原料中直链淀粉、支链淀粉及总淀粉含量 [J]. 广东农业科学, 40(18): 97-100.]
LIU YN, HUANG HY, ZHANG JW, 2010. Tissue culture ofLiliumdavidiivar.willmottiaeflakes in Lanzhou [J]. Grassl Turf, 30(4): 26-30. [刘雅楠, 黄惠英, 张金文, 2010. 兰州百合鳞片组织培养研究 [J]. 草原与草坪, 30(4): 26-30.]
LONG CL, CHENG ZY, WANG L, et al, 2004. Position effect on the propagationinvitroof different explants fromLiliumdavidiivar.unicolor[J]. Yunnan Plant Res, 26(2): 221-225. [龙春林, 程治英, 王俐, 等, 2004. 兰州百合器官离体培养外植体位置效应观察 [J]. 云南植物研究, 26(2): 221-225.]
NHUT DT, 2003. The control ofinvitrodirect main stem formation ofLiliumlongiflorumderived from receptacle culture, and rapid propagation by usinginvitrostem nodes [J]. Plant Growth Reg, 40(2): 179-184.RICE RD, ALDERSON PG, WRIGHT NA, 1983. Induction of bulbing of tulip shootsinvitro[J]. Sci Hortic, 20(4): 377-390.
SUN HM, ZHANG T, WANG CX, et al, 2008. Effect of lily bulb size on sugar and starch contents, amylase activities during bulb development [J]. J Shenyang Agric Univ, 39(5):546-550. [孙红梅, 张涛, 王春夏, 等, 2008. 百合种球大小对不同发育阶段鳞茎中糖和淀粉含量及淀粉酶活性的影响 [J]. 沈阳农业大学学报, 39(5):546-550.]TAEB AG, ALDERSON PG, 1990. Effect of low temperature and sucrose on bulb development and on the carbohydrate status of bulbing shoots of tulipinvitro[J]. J Hortic Sci, 65(2): 193-197.
TENG HW, SONG HH, 2005. High quality and efficient cultivation technology of Lanzhou lily [J]. Chin Veg, 6:46-47. [滕汉玮, 宋海慧, 2005. 兰州百合优质高效栽培技术 [J]. 中国蔬菜, 6:46-47.]
VARSHNEY A, DHAWAN V, SRIVASTAVA PS, 2000. A protocol forinvitromass propagation of asiatic hybrids of lily through liquid stationary culture [J]. In Vitro Cell Dev Biol Plant, 36(5): 383-391.
Wang HX, 2010. Lanzhou lily cultivation technology [J]. Northwest Hortic, 5:30. [王惠霞, 2010. 兰州百合栽培技术 [J]. 西北园艺, 5:30.]
XU LF, MA FW, LIANG D, 2009. Plant regeneration frominvitrocultured leaves of Lanzhou lily (Liliumdavidiivar.unicolor) [J]. Sci Hortic, 119(4): 458-461.
收稿日期:2014-09-14修回日期: 2014-12-20
基金项目:南宁市科学研究与技术开发计划项目(20133164);广西农业科学院基本科研业务专项(桂农科2014YQ15)[Supported by Scientific Research and Technology Development Program of Nanning City(20133164); Fundamental Research Project of GXAAS, China (2014Y Q15)]。
作者简介:张进忠(1979-),男,湖南澧县人,博士研究生,副研究员,主要从事作物遗传育种研究,(E-mail)jzzhang@foxmail.com。
