磁共振表观弥散系数对肝癌TACE疗效的预测价值
2016-06-17李文涛许立超
杨 欢,袁 正,李文涛,许立超,王 英
1.复旦大学附属肿瘤医院介入科,复旦大学上海医学院肿瘤学系,上海 200032;2.中国人民解放军第85医院影像科,上海 200052
磁共振表观弥散系数对肝癌TACE疗效的预测价值
杨 欢1,袁 正2,李文涛1,许立超1,王 英1
1.复旦大学附属肿瘤医院介入科,复旦大学上海医学院肿瘤学系,上海 200032;2.中国人民解放军第85医院影像科,上海 200052
[摘要]背景与目的:肝癌患者经导管动脉化疗栓塞(transcatheter arterial chemoembolization,TACE)后早期疗效评价仍是临床难点。该研究旨在探讨表观弥散系数(apparent diffusion coefficient,ADC)对肝癌患者TACE术后疾病早期进展的预测效能。方法:本研究经伦理委员会批准,所有患者均被充分告知。共入组23例肝癌患者(男性14例,女性9例,年龄21~85岁,平均年龄53.3岁),所有患者术前及术后1个月分别行MRI检查和弥散加权成像(diffusion-weighted imaging,DWI)检察(b=50、500、1 000 mm2/s)。术后3个月行MRI增强扫描检查,根据RECIST 1.1标准,把患者分为进展组和非进展组。采用配对t检验比较进展组、非进展组术前及术后1个月ADC值变化。采用非配对t检验比较进展组与非进展组之间的相关ADC参数。在23例肝癌患者中,采用受试者操作特征曲线(receiver operating characteristic curve,ROC),确定一个鉴别进展和非进展的ADC变化率(ADC%)阈值。结果:14例肝癌患者出现进展,9例肝癌患者未进展。未进展组术后1个月肿瘤ADC值明显升高,与术前肿瘤ADC值之间差异有统计学意义(P=0.01)。进展组术前、术后1个月肿瘤ADC无明显变化(P=0.221)。进展组与非进展组术前肿瘤ADC、ADC%之间差异均无统计学意义(P>0.05)。肝癌患者中,未进展组肿瘤ADC%显著高于进展组(P=0.029),用ROC分析ADC%区分进展组与非进展组的能力,以-6.455%为阈值(95%CI:0.643~1.000),曲线下面积为0.867,此时敏感度为100%,特异度为66.7%。结论:术后1个月肿瘤ADC值仅在未进展组明显增高。对于肝癌患者,ADC%能够有效预测患者经TACE治疗后是否早期发生疾病进展。
[关键词]弥散加权成像;肝肿瘤;经导管动脉化疗栓塞术;随访;表观弥散系数;进展
Correspondence to: LI Wentao E-mail: liwentao98@126.com
肝癌是常见恶性肿瘤之一,自确诊之日起,患者中位生存期为6~20个月[1]。尽管肝癌的主要治疗方法是手术切除,但超过一半的肝癌患者(约75%)由于疾病进展或肝功能下降等原因,失去了手术切除机会[2]。经导管肝动脉化疗栓塞(transcatheter arterial chemoembolization, TACE)是目前公认的治疗无法手术切除的中、晚期肝癌患者的主要方法,在抑制肿瘤生长、延长患者生存期、手术或肝移植术前的降级治疗等方面有明显效果[3]。有研究表明,术后根据患者的治疗效果决定是否再次行TACE,比按预先制定的时间点,周期性行TACE治疗效果更好[4]。在临床上,若经2次TACE治疗后患者出现疾病进展,常被作为不再单纯行TACE治疗的指标[5]。早期准确地评价肝癌患者对TACE的疗效十分重要,能够帮助临床早期制定下一步治疗方案,对于疗效好的患者,再次行TACE治疗能够帮助去除残留的肿瘤活性成分。然而,现有的疗效评价标准存在许多不足之处,TACE术后早期,由于肿瘤体积并未发生明显变化,用WHO、RECIST 1.0和RECIST 1.1标准评价疗效,尽管TACE治疗有效地延缓了疾病的进展,但常表现为较低的有效率[6]。EASL标准及mRECIST标准评价的是肿瘤的强化部分。有研究表明,首次TACE术后1个月后用EASL或mRECIST标准评价为治疗无效者中,有50%的患者在后续的TACE治疗中获益[7]。
磁共振弥散加权成像(diffusion-weighted imaging, DWI)能通过检测组织内水分子运动状态间接反映组织结构及细胞功能等变化等信息,并能通过表观弥散系数(apparent diffusion coefficient, ADC)定量反映[8]。虽然DWI越来越多的被用在肝癌患者TACE术后的随访中,但常常以单个肿瘤病灶作为研究对象,对于ADC值与肝脏疾病进展相关性却鲜有研究[9]。本研究旨在探索ADC值对肝癌患者TACE术后疾病早期进展的预测效能。
1 资料和方法
1.1病例资料
选择复旦大学附属肿瘤医院2014年9月—2015年4月首次行肝癌TACE治疗的患者。患者入组标准:①所有患者均经病理确诊,其中肝细胞肝癌可根据特征性的影像学表现(增强磁共振检查:动脉期肿瘤明显强化,门脉期强化迅速撤退);② ECOG小于等于2分;③ Child-Pugh评分小于等于B期;④肝脏病灶最大直径大于等于10 mm;⑤患者大于等于18岁;⑥适合行增强MRI检查。排除标准:①患者肝脏病灶无明确病理诊断;②影像学检查示门脉癌栓、动静脉瘘。③ ECOG大于2分;④ Child-Pugh评分高于B期;⑤肝脏病灶最大直径小于10 mm;⑥不适合行MRI增强扫描的患者。
共入组肝癌患者23例,其中男性14例,女性9例,年龄21~85岁,平均年龄 53.3岁。23例患者中肝细胞肝癌11例,肝胆管细胞癌1例,肝脏转移瘤11例;其中胃肠道肿瘤肝转移7例,乳腺癌肝转移1例,喉癌肝转移1例,肺癌肝转移1例,胆囊腺癌肝转移1例。每例患者都被充分告知研究的目的和潜在的风险和受益。
1.2检查方法
采用德国西门子Verio 3.0T磁共振(Siemens MAGNETOM Verio 3.0T ),16通道相阵控腹部线圈,仰卧位检查,患者检查前均经较好的呼吸训练,以配合检查。所有患者均于术前(1周以内)及术后1个月做常规轴位T1WI和T2WI,冠状面T2WI和DWI成像。轴位T2WI采用Blade序列,其主要成像参数:TR/TE 2 000 ms/97 ms,层厚6.0 mm,层间距为层厚的20%,FOV380 mm,Averages 1。DWI检查选取3个扩散敏感梯度因子(b=50、500、1 000 mm2/s),扩散梯度场为3个方向,TR/TE 6 400 ms/75 ms,层厚6.0 mm,层间距为层厚的20%,FOV 380 mm,矩阵184×138,利用固定参数组合的自旋回波-平面回波(SE-EPI)序列进行分析。所有图像传输至后处理工作站,ADC值由1位有20年腹部影像工作经历的诊断医师在相应b值的ADC图上测量,具体测量方法为:对于肝脏多发病灶的患者,取至多5个靶病灶(直径大于10 mm),手工描绘每个层面的整个肿瘤范围并设置为感兴趣区(包括坏死部分),自动得出ADC值,每个肿瘤的ADC值为除去最上和最下层面的各个层面ADC值的平均值,取所有靶病灶ADC值的平均值为该患者肝脏肿瘤的ADC值。同时测量与肿瘤同一层面的正常肝脏实质组织的ADC值(测量3次,取其平均值),注意避开血管、胆管。按以下的公式计算患者TACE术后1个月ADC值变化的百分率:ADC%=(ADCa-ADCb)/ADCb。ADCb为患者TACE术前肿瘤ADC值,ADCa为患者TACE术后1个月肿瘤ADC值。
1.3TACE过程
在Simense旋转DSA机引导下,对所有患者常规行腹股沟区备皮、消毒、铺巾、确定局部麻醉穿刺点,以Seldinger改良穿刺法穿刺股动脉成功后,顺次置入导引导丝和导管鞘,引入5F RH管选择腹腔干或肝总动脉造影观察肿瘤染色情况,超选至肿瘤供血动脉,首先局部灌注化疗药物,然后注入碘油和化疗药物的混合乳剂。
1.4磁共振随访
术后3个月行MRI增强扫描,具体参数同前,根据RECIST 1.1标准评价患者疾病是否发生进展。疾病进展定义为:靶病灶直径之和较随访以来的最小值增加大于等于20%或者肝脏出现新发病灶。
1.5统计学处理
数据用SPSS 19.0软件进行统计学分析。采用配对t检验分别比较进展组、非进展组术前ADC值与术后1个月ADC值。采用非配对t检验比较进展组、非进展组术前肿瘤ADC,ADC%之间差异,P<0.05为差异有统计学意义。在肝癌患者中,采用受试者操作特征曲线(receiver operating characteristic curve,ROC)得出鉴别进展组与非进展组的ADC%阈值。
2 结 果
2.1经TACE治疗后3个月疗效评价
经TACE治疗后3个月行增强MRI检察,在23例肝癌患者中,以RECIST 1.1标准评价为未进展的病例为9例,评价为进展的病例为14例。
2.2进展组、非进展组经TACE治疗后1个月ADC值变化特点
进展组治疗前肿瘤ADC值为(1.228× 10-3±0.336×10-3) mm2/s,治疗后1个月肿瘤ADC值为(1.299×10-3±0.347×10-3) mm2/s,进展组治疗前、后1个月ADC值之间差异无统计学意义(P=0.221)。未进展组前肿瘤ADC值为(1.252× 10-3±0.099×10-3) mm2/s,治疗后1个月肿瘤ADC值为(1.434×10-3±0.164×10-3) mm2/s,未进展组治疗前、后1个月肿瘤ADC值之间差异有统计学意义(P=0.01),治疗后1个月肿瘤ADC值仅在进展组表现为明显升高。
2.3进展组、非进展组治疗前ADC、ADC%比较
进展组与非进展组治疗前ADC值之间差异无统计学意义(P=0.837)。进展组ADC%为8.35%±20.99%,未进展组的ADC%为14.91%±13.77%。两者之间差异无统计学意义(P=0.418)。

图 1 肝癌患者中进展组ADC%与非进展组ADC%箱图Fig. 1 Box-and-whisker plot of ADC% in progressing hepatic cancer patients compared with that in non-progressing hepatic cancer patients
2.4肝癌患者中进展组、非进展组经TACE治疗前ADC、ADC%比较
在11例肝细胞肝癌患者中,进展6例(6/11),未进展5例(5/11)。未进展组治疗前肿瘤ADC为(1.209×10-3±0.062×10-3)mm2/s,进展组治疗前肿瘤ADC值为(1.285×10-3±0.130× 10-3)mm2/s,两者之间差异无统计学意义(P=0.26)。但未进展组的ADC%为12.53%± 17.00%,进展组的ADC%仅为-7.05%±7.02%,两组ADC%差异有统计学意义(P=0.029,图1)。在肝癌患者中,未进展组的ADC%明显较进展组的ADC%高(图2,图3)。采用受试者操作曲线(ROC曲线),求得ADC%阈值为-6.455%(95%CI:0.643~1.000),曲线下面积为0.867,此时敏感度为100%,特异度为66.7%(图4)。
2.5胃肠道肿瘤肝转移患者中进展组、非进展组术前ADC、ADC%比较
在诊断为胃肠道肿瘤肝转移的7例患者中,进展4例(4/7),未进展3例(3/7)。未进展组术前肿瘤ADC值为(1.270×10-3±0.113× 1 0- 3) m m2/ s,进展组的术前肿瘤A D C为(1.360×10-3±0.523×10-3)mm2/s,两者之间差异无统计学意义(P=0.787)。未进展组ADC%为21.47%±8.64%,进展组ADC%为15.93%±18.25%,两者之间差异无统计学意义(P=0.652)。
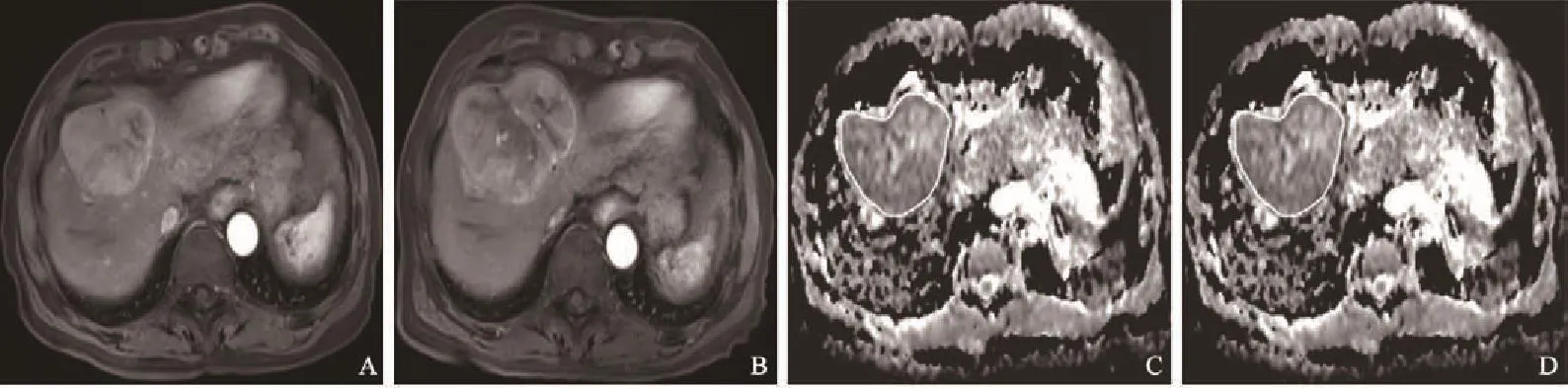
图 2 进展组患者不同时期影像学表现Fig. 2 Imagings of progressing patients in different periods
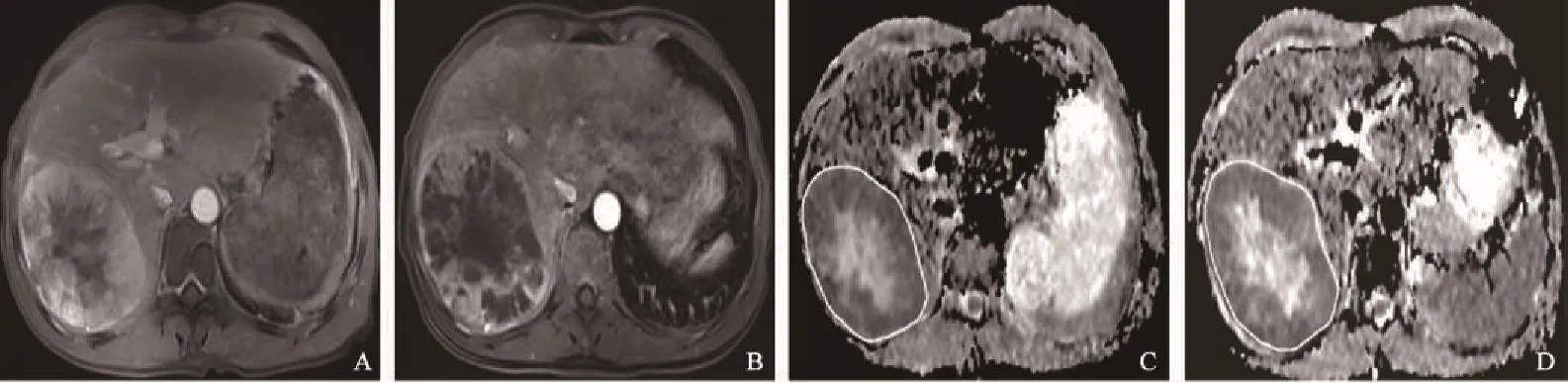
图 3 未进展组患者不同时期影像学表现Fig. 3 Imagings of non-progressing patients in different periods

图 4 ADC%判断肝癌患者肿瘤病灶是否进展的ROC曲线Fig. 4 ROC for differentiation of progressing and nonprogressing hepatic cancer patients with ADC%
3 讨 论
经TACE治疗后,肿瘤会发生不同程度的缺血性坏死,有可能会引起肝功能损伤[10]。所以,通过DWI这一非损伤性的影像学检查,早期预测TACE疗效,帮助临床决定是否再行TACE,即可以避免不必要的肝功能损伤,也可早期结合其他治疗方法。
本研究发现,经TACE治疗后1个月,肿瘤ADC值仅在未进展组中明显增高,而在进展组,治疗前及治疗后1个月肿瘤ADC值未见明显变化,与Chan等[10]的研究是一致的,可能是由于TACE治疗后肿瘤细胞大量坏死、细胞外间隙增加、水分子弥散受限减少所致。而治疗后1个月,不管是进展组还是未进展组,肿瘤的体积均未见明显改变。此外,在乳腺癌[11]、肉瘤[12]、胶质瘤[13]及前列腺癌[14]的动物模型中,尽管治疗方法不同,但都观察到经TACE治疗后肿瘤ADC值的升高,这种治疗后ADC值的变化,普遍存在于不同肿瘤的治疗后反应中,不受肿瘤的种类和治疗方法的影响。我们认为,这种治疗后肿瘤ADC值的早期增高也许能够成为早期评价肝癌患者TACE疗效的可靠生物学标志。
在肝癌患者中,ADC%对于鉴别患者是否发生早期进展具有良好的预测效能,未进展组ADC%较进展组明显高。最近一项包括23例患者的研究中也发现,TACE治疗后24 h,ADC值升高多者较升高较少者具有更长的生存期[15]。TACE治疗后肿瘤ADC的增高往往是由于肿瘤细胞的大量坏死造成的,ADC%越大,治疗后1个月肿瘤ADC值增加相对越多,提示肿瘤坏死率越高,患者的预后越好。在Kamel等[16]的研究中也发现,治疗后肿瘤ADC值升高明显者,组织学上具有更高的坏死率。
但在胃肠道肝转移患者,进展组与未进展组之间的ADC%并未表现出显著差异,这与Cui等[17]的研究结果不一致,可能是由于本研究考虑的为单个病灶的疗效,而且未考虑肝脏出现新病灶的情况。而本研究是以肝脏整体的疗效作为评价标准,肝脏出现新病灶也考虑为肝脏疾病进展,且本研究中胃肠道肝转移患者例数相对较少。
本研究作为一个临床初步应用研究,还存在一些缺陷。首先,由于是临床研究,TACE作为对肝癌的一种姑息性治疗手段,研究中很难取得影像结果与病理结果相互对照;其次,研究的样本量相对较少,肝癌的疾病种类不统一,使得结论的可靠性尚需扩大样本量进一步证实。
总之,对于肝癌患者,不管肝癌的种类,首次TACE治疗后,ADC值仅在疗效好的患者中表现为上升。在肝癌患者中,能够利用TACE治疗后1个月ADC的变化率,早期鉴别出TACE的疗效,而在肝脏转移瘤的疗效评价中,还需要扩大样本量,以进一步研究两者之间的相关性。DWI作为一种无创性的检查方法,在肝癌患者经TACE治疗后的随访中具有重要意义,值得更深入的研究。
[参 考 文 献]
[1] MANGHISI G, ELBA S, MOSSA A, et al. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP)investigators [J]. Hepatology, 1988, 28(3): 751-755.
[2] FORNER A, LLOVER J M, BRUIX J. Hepatocellular carcinoma[J]. Lancet, 2012, 379(9822): 1245-1255.
[3] LAMMER J, MALAGARI K, VOGL T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION Ⅴ study [J]. Cardiovasc Intervent Radiol,2010, 33(1): 41-52.
[4] ERNST O, SERGENT G, MIZRAHI D, et al. Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization: comparison of planned periodic chemoembolization and chemoembolization based on tumor response[J]. AJR Am J Roentgenol, 1999, 172(1): 59-64.
[5] VILGRAIN V. Advancement in HCC imaging: diagnosis,staging and treatment efficacy assessment: hepatocellular carcinoma: imaging in assessing treatment efficacy [J]. J Hepatobiliary Pancreat Sci, 2010, 17(4): 374-379.
[6] FORNER A, AYUSO C, VARELA M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable?[J]. Cancer, 2009, 115(3): 616-623.
[7] GEORGIADES C, GESCHWIND J F, HARRISON N, et al. Lack of response after initial chemoembolization for hepatocellular carcinoma: does it predict failure of subsequent treatment?[J]. Radiology, 2012, 265(1): 115-123.
[8] VANDECAVEYE V, MICHIELSEN K, DE KEYZER F, et al. Chemoembolization for hepatocellular carcinoma: 1-month response determined with apparent diffusion coefficient is an independent predictor of outcome[J]. Radiology, 2014,270(3): 747-757.
[9] KUBOTA K, YAMANISHI T, ITOH S, et al. Role of diffusionweighted imaging in evaluating therapeutic efficacy after transcatheter arterial chemoembolization for hepatocellular carcinoma[J]. Oncol Rep, 2010, 24(3): 727-732.
[10] CHAN A O, YUEN M F, HUI C K, et al. A prospective study regarding the complication of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma[J]. Cancer, 2002, 94(6): 1747-1752.
[11] GALONS J P, ALTBACH M I, PAINE-MURRIETA G D, et al. Early increases in breast tumor xenograft water mobility in response to paclitaxel water mobility in response to paclitaxel therapy detected by non-invasive diffusion magnetic resonance imaging[J]. Neoplasia, 1999, 1(2): 113-117.
[12] THOENY H C, DE KEYZER F, CHEN F, et al. Diffusionweighted MR imaging in monitoring the effect of a vascular targeting agent on rhabdomyosarcoma in rats[J]. Radiology,2005, 234(3): 756-764.
[13] HALL D E, MOFFAT B A, STOJANOVSKA J, et al. Therapeutic efficacy of DTI-015 using diffusion magnetic resonance imaging as an early surrogate marker[J]. Clin Cancer Res, 2004, 10(23): 7852-7859.
[14] JENNINGS D, HATTON B N, GUO J, et al. Early response of prostate carcinoma xenografts to docetaxel chemotherapy monitored with diffusion MRI[J]. Neoplasia, 2002, 4(3):255-262.
[15] DONG S, YE X D, YUAN Z, et al. Relationship for patients with unresectable primary hepatocellular carcinoma after chemoembolization [J]. Eur J Radiol, 2012, 81(3): 472-477.
[16] KAMEL I R, BLUEMKE D A, RAMSEY D, et al. Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma [J]. AJR Am J Roentgenol, 2003, 181(3): 708-710.
[17] CUI Y, ZHANG X P, SUN Y S, et al. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases [J]. Radiology, 2008, 248(3): 894-900.
Role of the apparent diffusion coefficient of MRI in evaluating therapeutic efficacy after transcatheter arterial chemoembolization in hepatic cancer patients
YANG Huan1, YUAN Zheng2, LI Wentao1, XU Lichao1, WANG Yin1(1.Department of Interventional Radiology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; 2. Department of Radiology, PLA 85th Hospital, Shanghai 200052, China)
[Key words]Diffusion-weighted imaging; Hepatic cancer; Transcatheter arterial chemoembolization; Follow-up;Apparent diffusion coefficient; Progress
[Abstract]Background and purpose: Early evaluating the therapeutic efficacy of transcatheter arterial chemoembolization (TACE) in patients with hepatic cancer is still a difficult clinical problem. The purpose of this study was to evaluate the ability of the apparent diffusion coefficient (ADC) to help predict early disease progression after TACE. Methods: Institutional review board approval was obtained, and all patients signed informed consent. Magnetic resonance imaging (MRI) and diffusion-weighted imaging (DWI) (b=50, 500, 1 000 mm2/s) were performed before and 1 month after initiating TACE for 23 patients with hepatic cancer (14 were male, 9 were female; mean age: 53.3 years; range: 21-85 years). Contrast-enhanced MRI was performed 3 months after initiating TACE. Patients were classified as either progressing or non-progressing according to RECIST 1.1. The preoperative ADC values of tumor and the ADC values of tumor 1 month after TACE were analyzed by paired t-test in both progressing and non-progressing group. Unpaired t-test was used to compare ADC parameters between progressing and non-progressing group. In all the 23hepatic cancer patients, receiver operating characteristic (ROC) curve analysis was performed to determine a threshold ADC ratio (ADC%) to differentiate progressing from non-progressing patients. Results: Thirteen progressing and 9 non-progressing patients were evaluated. Increase in ADCs of tumor was observed in non-progressing patients at 1 month after TACE compared with preoperative ADCs. There was a significant difference between the 2 groups (P=0.01). In progressing group, preoperative ADCs of tumor were similar to those at 1 month after TACE (P=0.221). There was no significant difference in preoperative ADCs of tumor and ADC% between the progressing and non-progressing groups. In patients with hepatic cancer, 1 month ADC ratio in non-progressing patients were significantly higher than those of progressing patients (P=0.029). Using ROC to evaluate the ability of ADC% could predict early disease progression after TACE. Using -6.455% as the threshold, the area under the ROC curve was 0.867 (95%CI: 0.643-1.000). The sensitivity was 100%, and the specificity was 66.7%. Conclusion: One month after TACE, the increases in ADCs of tumor were observed only in the non-progressing group; and the ADC ratio seems to be a promising tool for helping predict the early disease progression after TACE in patients with hepatic cancer.
DOI:10.3969/j.issn.1007-3969.2016.03.009
中图分类号:R735.7
文献标志码:A
文章编号:1007-3639(2016)03-0257-06
通信作者:李文涛 E-mail:liwentao98@126.com
收稿日期:(2015-05-25 修回日期:2015-09-30)
