Study on Emission Spectrum of OH Radicals in a Combination System of Pulsed Discharge Plasma and Activated Carbon
2016-06-05GUOHeWANGHuijuanJIAYuanyuanSUNChenjingZHOUGuangshunWUQiangshunYIChengwu
GUO He、WANG Hui-juan,2*、JIA Yuan-yuan、SUN Chen-jing,ZHOU Guang-shun、WU Qiang-shun、YI Cheng-wu
1. School of the Environment and Safety Engineering,Jiangsu University,Zhenjiang 212013,China 2. Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment,Suzhou 215009,China 3. Lanzhou Petrochemical Research Center,PetroChina,Lanzhou 730060,China
Study on Emission Spectrum of OH Radicals in a Combination System of Pulsed Discharge Plasma and Activated Carbon
GUO He1、WANG Hui-juan1,2*、JIA Yuan-yuan3、SUN Chen-jing1,ZHOU Guang-shun1、WU Qiang-shun1、YI Cheng-wu1
1. School of the Environment and Safety Engineering,Jiangsu University,Zhenjiang 212013,China 2. Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment,Suzhou 215009,China 3. Lanzhou Petrochemical Research Center,PetroChina,Lanzhou 730060,China
Based on the higher oxidation potential of OH radicals (2.8 V),the synergetic effect of pulsed discharge plasma (PDP) and activated carbon (AC) and the advantages of emission spectroscopic detection,such as easy operation,high accuracy and high sensitivity,the relative emission spectra of the OH radicals generated in the PDP/AC system with oxygen flow were measured by the emission spectroscopic detection technique and the spectral intensity of the OH radicals was used to represent the relative amount of the OH radicals formed in the reaction system. The effect of additive amount of the AC,peak pulse voltage and electrode gap on the relative emission spectrum intensities of OH radicals were investigated to illustrate the crucial factors for the OH radicals formation in the PDP/AC system. In addition,the formation of OH radicals in the two liquid phases of distilled water and acid orange 7 (AO7) solution in the sole PDP system and the PDP/AC system were investigated to testify the synergetic mechanism of PDP/AC and the oxidization of OH radicals on the organic compounds in the reaction system. The obtained results showed that the catalytic effect of the AC increased with the increase of the additive amount of the AC in the PDP system,which led to the increase of the relative emission spectral intensities of the formed OH radicals in the synergistic system; higher peak pulse voltage was in favor of the energy input in the discharge system and then enhanced the formation of OH radicals; increase of the electrode gap led to the decrease of energy efficiency in the reaction system and the decrease of the formed OH radicals in the PDP/AC system; the formation of OH radicals in the PDP/AC system was higher than that in the sole PDP system both in the distilled water and in the AO7 solution; the formation of OH radicals in the distilled water was higher than those in the AO7 solution no matter the reaction system was the sole PDP system or the PDP/AC system. The two results indicated that the AC addition was beneficial to the formation of OH radicals in the PDP system and the OH radicals had an important effect on the organic compounds degradation both in the sole PDP system and in the PDP/AC system.
Pulsed discharge plasma; Activated carbon; Synergistic effect; OH radicals; Emission spectrometric analysis
Introduction
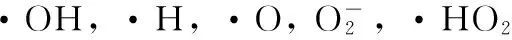
e*+H2O→H+·OH+e
(1)
O(1D)+H2O→·OH+·OH
(2)
·OH+RH→R·+H2O
(3)
Amongthem,thereaction(2)isknownashydrogenabstractionreaction.
FortheresearchontheapplicationofthePDPtechnology,theproblemoflowerenergyutilizationofthePDPhasalwaysbeenanimportantproblemrestrictingthedevelopmentofthetechnology.Researchesonfullyutilizingtheultravioletlight,ultrasonicandelectricfieldinthePDPsystem,andfurtherimprovingtheremovalefficiencyoforganiccompoundsinthePDPsystemhasbecomeahottopicforthedevelopmentofthePDPtechnology.
Duetothelargespecificsurfaceareaandthelargeporestructure,activatedcarbon(AC)hasbeenwidelyusedasanadsorbentforwastewatertreatment.ResearcheshaveconcludedthattheACcanaccelerateozonedecompositionandthenleadtotheformationofOHradicalsinthePPDsystem[6-7].TheinvestigationcarriedoutbyZhangetal.hasprovedthatthecombinedprocessofPDPandACcouldenhancetheoverallremovaloforganiccompoundsinsolutionandtheACactedasthecatalystinthesynergisticprocess[8].Baseontheaboveresearch,aPDPsystemwasestablishedintheresearchandtheemissionspectralintensitiesoftheOHradicalsweredetectedbytheemissionspectroscopicdetectiontechnologytoindicatetheformationofOHradicalsinthePDPsystem.ThechangingtrendoftherelativeemissionspectralintensitiesoftheformedOHradicalswasalsoanalyzedtoillustratethesynergeticmechanismofthePDPandtheAC.
TherearemanymethodshavebeenappliedondetectingOHradicals,suchashighperformanceliquidchromatography[9],spectroscopedetectiontechnique[10],electronspinresonancetechnique[11],fluorophotometry[12]andspectrophotometry[13].Duetotheadvantagesofgoodselectivity,highsensitivity,highaccuracyandsimpleoperation,theemissionspectroscopicdetectiontechnologyhasbeenwidelyappliedindetectiontheradicalsgeneratedintheplasmachannel.Thechangeofspectrumtakesplaceinsideoftheplasmaandmakesnodisruptiontotheplasmaitself.HenceitisaneffectivemethodforoptimizingtheoperatingparametersofthePDPandexploringtheoxidationmechanismofthePDPontheorganiccompoundsdegradationbyindicatingtheformationofOHradicalsindirectlythroughdetectingtheemissionspectralintensityoftheOHradicals.
BaseonthesynergeticeffectofPDP/ACandtheadvantageoftheemissionspectroscopicdetectiontechnology,amulti-needle-to-plateelectrodegeometryPDPsystemwasestablishedinthepapertoinvestigatetheeffectofadditiveamountoftheAC,peakpulsevoltageandelectrodegapontherelativeemissionspectralintensitiesoftheOHradicalsinthereactionsystem.Inaddition,theformationofOHradicalsgeneratedinthesolePDPsystemandthePDP/ACsystemwascomparedtotestifythesynergeticeffectofthePDPandtheAC;theformationofOHradicalsinthetwoliquidphaseofdistilledwaterandacidorange7 (AO7)solutionwereinvestigatedtoverifytheoxidizationoftheOHradicalsontheorganiccompounds.
1 Experimental
1.1 Experimental system
The schematic diagram of the experimental system used in this research is shown in Fig.1. The experimental system was composed of a pulse power supply,an electric monitoring system,a reactor and a spectroscopic detection system. The pulse power supply included a DC power supply,a storage capacitance,an adjustable trim capacitance and a rotating spark-gap switch. The frequency of the power supply was 0~150 Hz adjustable and the voltage was 0~60 kV adjustable. The electric monitoring system consisted of an oscilloscope (Tektronix TDS3032B),a voltage probe (Tektronix P6015A) and a current probe (Tektronix P6021). The inner diameter of the reactor was 80 mm and the height was 140 mm. The high voltage anodes located at the top of the discharge chamber and the anodes were seven syringe needles (type 12#,length 100 mm). One needle was in the center and the other six needles distributed uniformly around a circle of 30 mm radius. The ground cathode was a stainless steel plate (80 mm diameter,3 mm thickness),which located at the bottom of the discharge chamber. The AC added into the PDP system was put on the ground cathode,which represented by the dark spots shown in Fig.1. An oval quartz plate was embedded on the wall of the reactor for detecting the emission spectrum intensity of OH radicals generated in PDP system throng the optical fiber of the spectrometer. The gas flowing into the reactor was the oxygen,which was bubbled into the reactor through the needles and a gas flow meter (Kere LZB-6WB) was used to adjust the gas flow rate. The spectrum detection system consisted of a spectrometer (Avantes Avaspec-2048L) and a laptop (Lenovo G460).
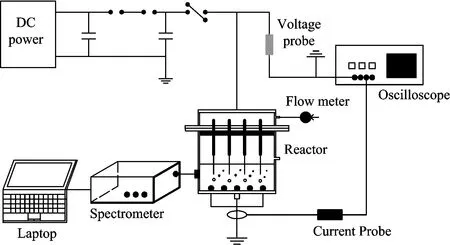
Fig.1 Schematic diagram of the experimental system
1.2 Experimental methods
AC used in the study was coal-based carbon and obtained from Shuangyuan AC Factory in Liyang,Jiangsu. In order to improve the activity of the AC,the purchased AC was firstly sieved into uniform size of 2~3 mm. Secondly,the sieved AC was boiled in 5% hydrochloric acid for 60 min. The boiled samples were then washed with distilled water to neutral and dried at 105 ℃ for 24 h,and dried samples were lastly stored in a drier before use.
In the study,the emission spectra of the OH radicals was detected when pure oxygen flew into the PDP system and the wavelength range for the detection was 250~350 nm. The integration time and the average time for the signal collection were set as 500 ms and 20,respectively. A typical emission spectrum obtained at the wavelength range of 250~350 nm was shown in Fig.2. As shown in Fig.2,characteristic peak of the OH radicals appeared at 309 nm and the corresponding transition was:A2Σ+(v′=0)→X2Ⅱ (v″=0).ForillustratingthechangeoftherelativeemissionspectralintensitiesoftheOHradicalsunderdifferentexperimentconditionsclearly,thevaluesoftherelativeemissionspectralintensitiesoftheOHradicalsat309nmwereinvestigatedandcomparedintheresearch.Inthestudy,thefixedexperimentalconditionswasasfollows:thepulsefrequencywas50Hz,theinitialconcentrationoftheAO7solutionwas50mg·L-1andthecorrespondingpHvaluewas6.9,theflowrateoftheoxygenwas2L·min-1.
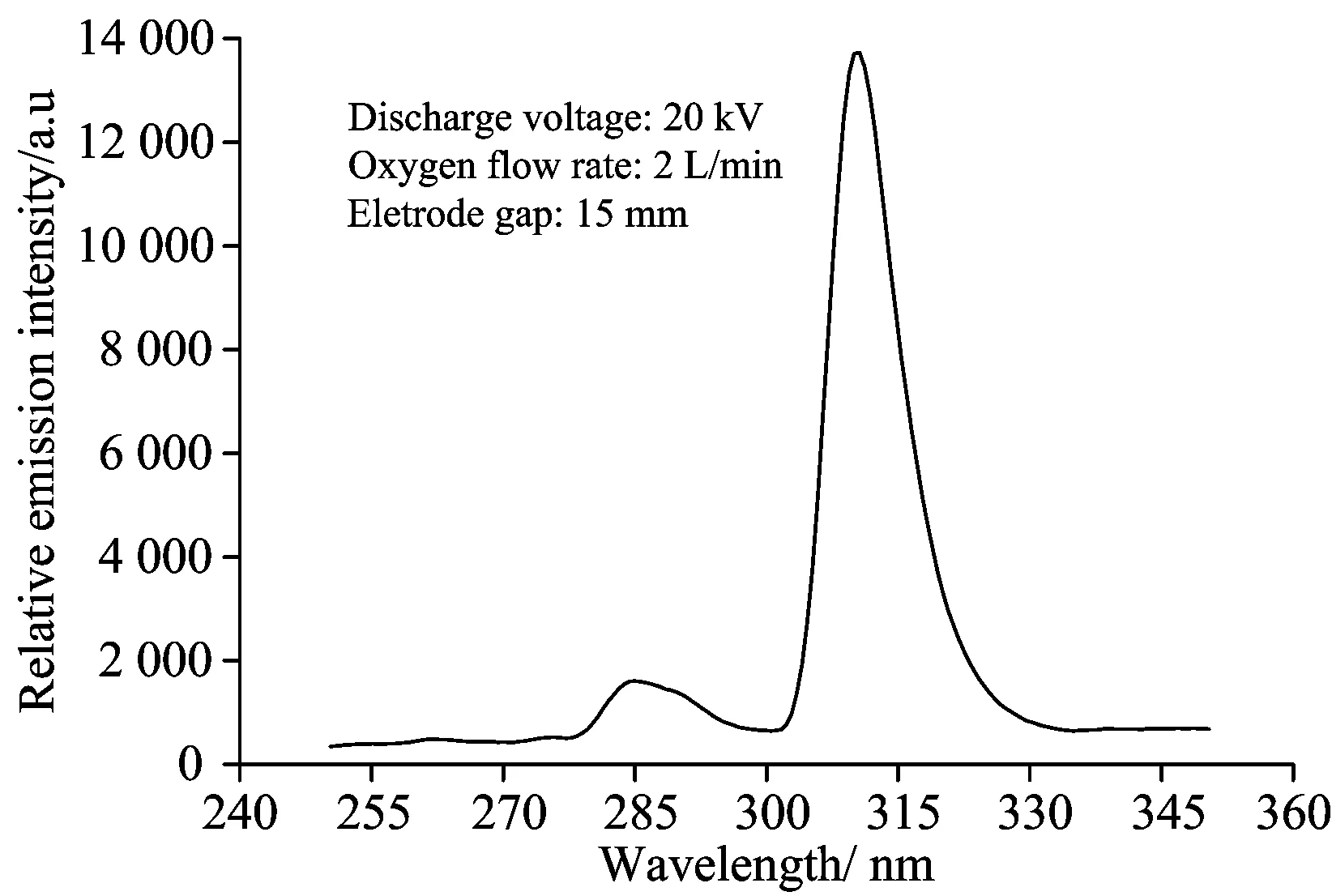
Fig.2 Emission spectrum of the OH radicals in the PDP system with distilled water
2 Results and Discussion
2.1 Effect of additive amount of the AC
For the degradation of organic compounds in the PDP/AC system,adsorption and catalysis of the AC are the two functions contributing to the synergistic effect[14]. Therefore,it is necessary to investigate the catalysis of the AC in the PDP system with different additive amount of the AC. The effect of additive amount of the AC on the emission spectral intensities of the OH radicals was studied firstly in the paper,and the investigated additive amount were 1 g,2 g,3 g and 4 g,respectively. In the experiment,besides the fixed experimental conditions the peak pulse voltage was controlled at 20 kV and the electrode gap was 15 mm. Intensities of the emission spectrum of the OH radicals at 309 nm under different additive amount og the AC were shown in Fig.3.
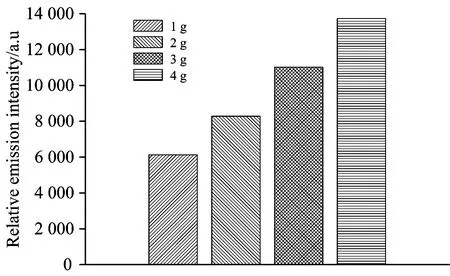
Fig.3 Emission spectral intensities of the OH radicals in the PDP system with different additive amounts of the AC
As shown in Fig.3,the emission spectral intensities of the OH radicals increased with the increase of additive amount of the AC. In other words,with the increase of additive amount of the AC,the formed amount of the OH radicals in the PDP system increased. Reason for the result could be concluded as the remarkable catalysis of the AC could be induced by the more additive amount of the AC. With the increased catalysis the AC,more ozone formed in the PDP system could decompose and more OH radicals were produced accordingly. However,formation of the OH radicals could not increase continually with the increase of the AC addition. The reason could be subscribed as follows: on one hand,the amount of ozone formed in the PDP system was limited under the fixed operating parameters of the PDP system,so the amount of ozone that could be catalyzed by the AC was limited and then the increase of the OH radicals formation was limited; on the other hand,as the reactor had the stable volume,hence more amount of the AC addition in the PDP system could make the AC gather in the limited reaction space,which could hinder the contact between the AC and the ozone and then impede the catalytic effect of the AC.
2.2 Effect of peak pulse voltage
Peak pulse voltage is one of the vital parameters which could affect the energy injection of the PDP system. To illustrate the effect of peak pulse voltage on the formation of OH radicals in the PDP/AC system,the variation rule of the emission spectral intensities of the OH radicals in distilled water was investigated under the peak pulse voltage of 16,18 and 20 kV. The additive amount of AC was 4 g. Other parameters in the reaction system were consistent with the parameters used in 2.1. The results are shown in Fig.4.

Fig.4 Emission spectral intensities of the OH radicals in the PDP/AC system with different peak pulse voltages
It can be seen from Fig. 4 that the emission spectral intensities of the OH radicals increased with the increase of the peak pulse voltage. Therefore,the higher peak pulse voltage is benefit to produce the OH radicals in the PDP system. That was because the energy provided by the PDP system could be improved by the increase of the peak pulse voltage,and more high-energy electrons could be produced accordingly. These high-energy electrons could collide with the water molecules and then form more OH radicals.
2.3 Effect of electrode gap
Electrode gap in the PDP system not only affects the discharge state but also affects the status of hybrid gas-liquid because of the settled height of the solution level in the reactor. Therefore,the emission spectral intensities of the OH radicals under the selected electrode gap of 8 and 15 mm were investigated. In the research,the peak pulse voltage was 20 kV and other parameters were consistent with the parameters listed in Section 2.2. The results are subscribed in Fig.5.
From Fig.5,it can be seen that the emission spectral intensities of the OH radicals decreased with the increase of electrode gap. Hence the higher electrode gap was not in favor of the formation of the OH radicals. The main reason for the result was that the lower electrode gap was benefit for the generation of the plasma channel and the formation of the OH radicals. Furthermore,in the PDP system built in the research,the discharge state was spark discharge under the condition of the 8 mm electrode gap and the bottom of needle anodes were surrounded by water. As a consequence,there was intense effects among the discharge electric field and the gas molecules or the water molecules,which could promote the formation of the OH radicals. However,loss of the AC was greater under the condition of the 8 mm electrode gap by the direct observation of the change of the AC from granular to powder,which made the solution cloudy and also affected the further use of the AC.
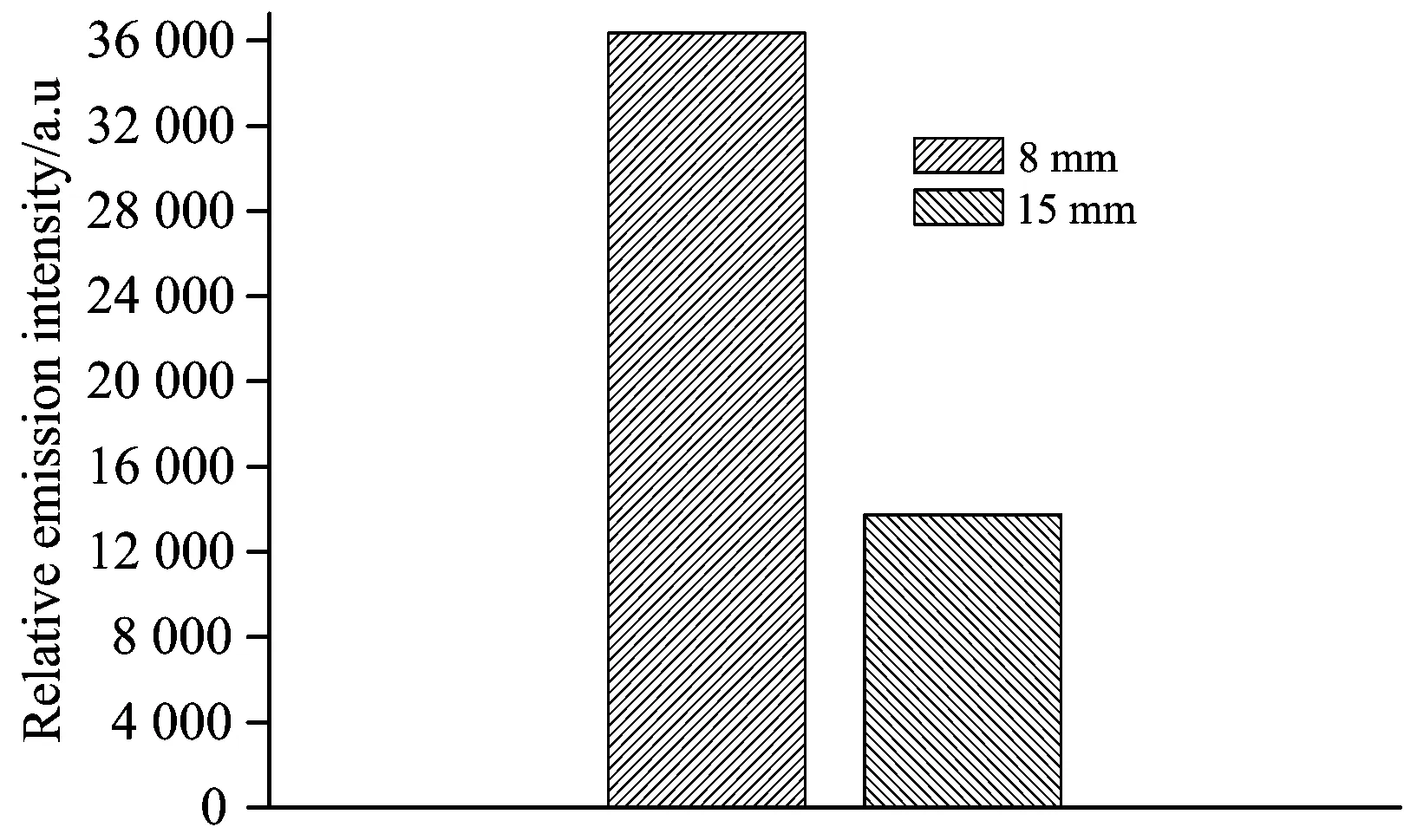
Fig.5 Emission spectral intensities of the OH radicals in the PDP/AC system with different electrode gaps
2.4 Emission spectral intensities of the OH radicals in the distilled water and the AO7 solution
In order to explain the synergetic effect of the PDP/AC and the oxidation of OH radicals on the organic compounds,emission spectral intensities of the OH radicals in the distilled water and the AO7 solution from the sole PDP system and the PDP/AC system were investigated respectively in the research. The additive amount of the AC was 4 g. The initial conductivity of the distilled water and the AO7 solution was controlled at 500 μS·cm-1. Other experimental parameters used in the experiment were consistent with the parameters used in Section 2.1. The results are shown in Fig.6.

Fig.6 Emission spectral intensities of the OH radicals in different reaction systems
As illustrated in Fig.6,no matter the reaction solution was the distilled water or the AO7 solution,the emission spectral intensities of the OH radicals formed in the PDP/AC system were higher than those in the sole PDP system,which could prove the synergistic effect of the PDP and the AC for the OH radicals’ formation. The mechanism of the synergetic effect could be subscribed as follows[15]
(1)
(2)
(3)
OH-+AC→OH-AC
(4)
O3+OH-AC →·O3-AC+·OH
(5)
The above reactions illustrated that the AC could catalyze the ozone decomposition and then led to the formation of the OH radicals in the PDP system.
In addition,based on the results shown in Fig.6,it can also be concluded that the emission spectral intensities of the OH radicals in the distilled water was higher than those in the AO7 solution both in the sole PDP system and in the PDP/AC system. The result proved the oxidation of the AO7 by the OH radicals,which led to the consumption of the OH radicals.
3 Conclusion
In the paper,the emission spectral intensities of the OH radicals formed in PDP/AC system was detected by the spectroscopic detection technique. The formation of the OH radicals was illustrated under different experimental parameters. The obtained conclusions could be subscribed as follows:
(1) When the oxygen was used as the flow gas,formation of the OH radicals increased with the increase of additive amount of the AC; the higher peak pulse voltage was benefit to form the OH radicals; while the higher electrode gap was not in favor of the increase of the OH radicals’ amount.
(2) Formation of the OH radicals in the PDP/AC system was higher than that in the sole PDP system no matter the distilled water or the AO7 solution was used as the solution phrase,which could account for the synergetic effect of the PDP and the AC; both in the sole PDP system and in the PDP/AC system,the formed amount of the OH radicals in the distilled water was higher than that in the AO7 solution,which could indicate the oxidation of the OH radicals on the organic compounds.
[1] Locke B R,Sato M,Sunka P,et al. Ind. Eng. Che. Res.,2006,45(3): 882.
[2] Hsieh A,Wu K C,Hsu C. J. Taiwan Inst. Chem. Eng.,2014,45(4): 1558.
[3] Jiang N,Hu J,Li J,et al. Appl. Catal. B-Environ.,2016 (184): 355.
[4] Jiang N,Lu Na,Shang K. F,et al. Environ. Sci. Technol.,2013 (47): 9898.
[5] Sugiarto A T,Sato M. Thin Solid Films,2001,386(2): 295.
[6] Grymonpre D R,Finney W C,Locke B R. Chem. Eng. Sci.,1999,54(15): 3095.
[7] Valdés H,Zaror C A. J. Hazard. Mater.,2006,137(2): 1042.
[8] Zhang Y Z,Zheng J T,Qu X F,et al. J. Colloid. Interf. Sci.,2007,316(2): 523.
[9] Fukui S,Hanasaki Y,Ogawa S. J. Chromatogr. A,1993,630(1): 187.
[10] Hong Y J,Nam C J,Song K B,et al. J. Instrum.,2012,7(3): 3046.
[11] Hiramoto K,Ryuno Y,Kikugawa K. Mutat. Res-Gen. Tox. En.,2002,520(1): 103.
[12] Ou B,Hampsch-Woodill M,Prior R L. J. Agr. Food Chem.,2001,49(10): 4619.
[14] Zhang X W,Zhou M H I,Lei L C. Carbon,2005,43(8): 1700.
[15] Feng Y,Shi W J,T D J,et al. Acta Sci. Circumst.,2013,33(10): 2664.
*通讯联系人
O657.3
A
脉冲放电等离子体/活性炭联合体系中OH自由基的发射光谱研究
郭 贺1、王慧娟1,2*、贾媛媛3、孙晨静1、周广顺1、吴强顺1、依成武1
1. 江苏大学环境与安全工程学院、江苏 镇江 212013 2. 江苏高校水处理技术与材料协同创新中心,江苏 苏州 215009 3. 中国石油兰州化工研究中心、甘肃 兰州 730060
基于OH自由基的强氧化性(2.8 V)及脉冲放电等离子体(pulsed discharge plasma,PDP)与活性炭(activated carbon,AC)联合体系的协同作用、依托于光谱检测技术简单、准确性高、灵敏度高等优点、利用发射光谱技术测量了以O2作为载气的PDP/AC联合体系中产生的OH自由基的相对发射光谱、用以表征体系中OH自由基相对生成量的变化。通过考察PDP水处理体系中不同AC添加量、脉冲峰值电压、电极间距对OH自由基相对发射光谱强度的影响、分析了影响OH自由基生成量的因素; 通过比较、分析去离子水和酸性橙II(acid orange,AO7)溶液中OH自由基发射光谱强度的变化规律、表征了OH自由基生成量的变化、以说明PDP/AC的协同作用机理及OH自由基对有机物的氧化作用。研究结果表明、增加AC的添加量可以增强其在PDP体系中的催化效果、导致PDP/AC联合体系中OH自由基的相对发射光谱强度的增加; 随着脉冲峰值电压的升高、注入PDP体系中的能量增加、从而增加了体系中OH自由基的产量; 电极间距增加导致PDP体系能量效率降低、降低了OH自由基的产生量; 无论是以去离子水还是以AO7溶液为溶液相、PDP/AC联合体系中OH自由基的产量均高于其在单独PDP体系中的生成量、且在PDP/AC联合体系和单独的PDP作用体系中、去离子水中OH自由基的相对发射光谱强度均高于其在AO7溶液中的强度值、这证明了AC对PDP体系中OH自由基生成的协同作用和有机物对OH自由基的消耗。
脉冲放电等离子体; 活性炭; 协同作用; OH自由基; 发射光谱分析
2015-12-03、
2016-04-07)
2015-12-03; accepted:2016-04-07
National Natural Science Foundation Committee of China (21207052)
10.3964/j.issn.1000-0593(2016)12-4135-06
*Corresponding author e-mail: wendyjuaner@126.com,hjwang@ujs.edu.cn
猜你喜欢
杂志排行
光谱学与光谱分析的其它文章
- Studies on the Interaction of Perfluorononanoic Acid with Human Serum Albumin by Multi-Spectroscopic,Molecular Docking and Isothermal Titration Calorimetry Techniques
- Simulating the Three-Dimensional Image of Cold Atomic Cloud
- K Shell Fluorescence Parameters by Impact of 5.96 keV Photons on Ti and Its Compounds
- Structure Effect on K Shell Fluorescence Parameters at Bis-4-Bromobenzyl-1,2,4-Triazol-3-Ones
- 动态应变场下相移光栅光谱特性及实验研究
- 基于Wollaston棱镜的图像复分快照式成像光谱仪设计
