Structure Effect on K Shell Fluorescence Parameters at Bis-4-Bromobenzyl-1,2,4-Triazol-3-Ones
2016-06-05AylkcnverKupAylkcTra
V. Aylkc,Y. Ünver,N. Kup Aylkc,E. Tra
1.Department of Metallurgical and Materials Engineering,Faculty of Technology,
Structure Effect on K Shell Fluorescence Parameters at Bis-4-Bromobenzyl-1,2,4-Triazol-3-Ones
1.Department of Metallurgical and Materials Engineering,Faculty of Technology,


In this study; the effect of the electron density over the Br atoms raising with increasing number of CH2group using the results of the K X-ray cross-sections and average fluorescence yields of bromine in quaternary-imidazole ring. In the experimental set-up,50 mCi241Am source and a collimated Ultra-LEGe detector were used. The electron density on the Br atoms raises according to the number of the CH2groups on the contrary of the inductive effect. The decreasing of the X-ray fluorescence parameters is interested with the increasing the electron density of Br atoms.
Cross-sections; Fluorescence yields; Quaternary-imidazole ring; Inductive effect
Introduction
The 1,2,4-triazole and its derivatives were reported to exhibit various pharmacological activitiessuch as antimicrobial,anti-inflammatory,anticancer and antioxidant properties[1-5]. In addition,Ribavirin (antiviral agent),Rizatriptan (antimigraine agent),Alprazolam (anxiolytic agent),Fluconazole and Itraconazole (antifungal agents) are examples of potent molecules possessing a triazole nucleus as some present day drugs[6-8].
X-ray emission spectra are known to provide data about the kind of the surroundings of the X-ray emitting atoms with changing ligands. The X-ray emission spectra are related to the kind of the surroundings of the atom and the chemical effect on the K X-ray fluorescence parameters of the 3d transition elements have been reported[9-15]. The chemical effect on the K X-ray intensity ratios for Mo and Tc compounds was investigated using the method of DV-Xα[16]. The chemical effect on the K X-ray fluorescence parameters was carried out for some silver compounds[17]. The chemical effect on the K X-ray intensity ratios of 4d transition metals[18]and Mo,Ag,Cd,Ba,La and Ce compounds and total mass attenuation coefficients[19]were determined by some researchers. The Kβ2/Kβ1,3intensity ratios of As,Se and Br were determined with chemical state analyses using X-ray method[20]. The K X-ray fluorescence cross-section values of bromide and iodide in different compounds were measured using Si(Li) X-ray detector[21]. Kβ/Kαintensity ratios were measured for Br and I in different compounds and the results were evaluated using the chemical effect[22]. The structure and anion effect on conductivity and K and L X-rays cross-sections and average fluorescence yield values of Br and I in different compounds were carried out and the relation between these values and the1H-NMR,IR,thermogravimetric analysis and conductivity were investigated by our group[23].
In this work; the effect of the electron density over the Br atoms raising with increasing number of CH2group using the results of the K X-ray cross-sections bromine in quaternary-imidazole ring.
1 Experimental procedure
1.1 Sample preparation
The1H-,and13C-Nuclear Magnetic Resonance spectra were recorded on a Varian-Mercury 200 MHz spectrometer,where TMS as an internal standard and DMSO-d6as solvent are used. IR spectrum was recorded on a Perkin-Elmer Spectrum one FT-IR spectrometer (resolution 4) in KBr pellets M.p. was measured on an electrothermal apparatus and are uncorrected.
Synthesis of the compound 2:To a solution of Ethyl 2-((4-bromophenyl)(ethoxy)methylene)hydrazine carboxylate (20 mmol ) in 50 mL water corresponding diamine (10 mmol) was added. Having refluxed this mixture for 8~12 h. The precipitate formed was filtered off. The solid obtained was washed with H2O and crystallized from appropriate solvent to afford the desired compound (Figure 1).

Fig.1 Synthetic pathway for the preparation of target compounds (2)





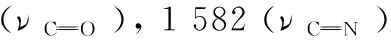
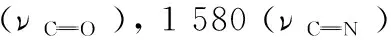
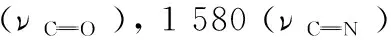
1.2 X-ray fluorescence analysis
The geometry of the experimental set-up for our annular source used in the X-ray fluorescence analysis was given earlier[23]. In this experimental set-up,50 mCi241Am source were used. The excitation energies of241Am source is 59.5 keV. The fluorescence K X-rays from the sample were detected by a collimated Ultra-LEGe detector with 5 mm thickness and an energy resolution of 150 eV at 5.96 keV[23]. In the present study,Figure 2 (a) shows the spectra of K X-rays for 2e compound. A residue spectrum with respect to X-ray spectra of 2e compound is shown in Figure 2 (b). The evaluation of the Br K X-ray spectra was described by our group earlier[23].
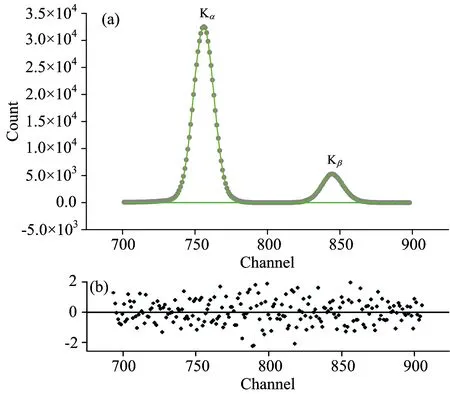
Fig.2 (a) Typical K X-rays spectra for 2e compound; (b) A residue spectrum with respect to K X-ray spectra of 2e compound
TheKiX-ray production cross-sections were measured by using the following equation
(1)
whereNKiis the measured intensity (area under the photopeak),I0is the intensity of the incident radiation,Gis the geometric factor,εKiis the detection efficiency for theKigroup of X-rays andβKiis the self-absorption correction factor for the target material,which accounts for the absorption in the target of the incident photons and the emitted characteristic X-rays. mi is the thickness of the target in g·cm-2.
The self absorption correction was calculated using the following equation
(2)
whereμincandμemtare the mass attenuation coefficients (cm2·gr-1) of incident photons and emitted characteristic X-rays respectively[24]; the angles of incident photons and emitted X-rays with respect to the surface of the samplesθ1andθ2,were equal to 45° and 90° in the present experimental set-up respectively.
The productI0GεKicontaining the terms related to the incident photon flux,geometrical factor and absolute efficiency of X-ray detector,was determined in the same geometry for present study as explained earlier[25].
The semi-empiricalKshell fluorescence yieldsωKwere calculated using the following equation
(16)
where ∑σKiisthetotalKX-ray fluorescence cross-section calculated experimentally,σK(E) is theKshell photoionization cross-section of given element for the excitation energy 59.5 keV[26].
2 Results and discussion
The measured values of K!X-ray cross-sections and average fluorescence yields of bromine in quaternary-imidazole ring excited with 59.5 keV energy are given in Table 1.
Table 1 The experimentalσKαandσKβproduction cross-section and average fluorescence yield

CompoundsσKα/(gr·cm-2)σKβ/(gr·cm-2) ωkExp.Theo.*Exp.Theo.*Exp.Theo.[28]Br(pure)1.1510.1710.6282a1.087±0.0550.153±0.0080.589±0.0302b1.103±0.0560.151±0.0080.596±0.0312c1.184±0.0600.145±0.0070.632±0.0322d1.129±0.0580.141±0.0070.604±0.0312e1.216±0.0620.138±0.0070.643±0.0332f1.081±0.0550.133±0.0070.577±0.0292h1.168±0.0600.126±0.0060.615±0.0312g1.111±0.0570.114±0.0060.582±0.030
*Calculated
The results of the measurements presented in Table 1 show that K X-ray production cross-sections are affected by the chemical surroundings of the emitting atom. The interaction between the central atom and ligands take place in outer states and their electrons for Br. These electrons specify the chemical properties of atoms taking part in a compound. In the bond formation,valance state of the atom has an important effect on the related parameters of the spectrum such as relative intensity,peak position etc. In the chemical compounds,some valance electrons is removed or transferred from the atom. Therefore,the electronic screening is reduced and binding energies of the outer shells are changed and binding energies of outer shell electrons are affected by the changed valance charge. The alteration of the binding energies of the valance charges changes the non-radiative transition probabilities. It is well known that the radiative transition rates are managed by the non-radiative transitions such as Coster-Kronig transitions. The valance electron density alters according to the electron transition and the rearrangement process. Therefore,non-radiative and radiative transitions from the valance state are affected by the changes in the valance electron density[27].
The inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction. The net polar effect exerted by a substituent is a combination of this inductive effect and the mesomeric effect. If the electronegative atom is then joined to atoms or groups,the positive charge is relayed to the other atoms in the chain. This is the electron-releasing inductive effect,also known as the +I effect such as —CH2and —CH3groups. Some groups are electron-withdrawing. This is electron-withdrawing character is indicated by the -I effect such as —NO2group. The mesomeric effect or resonance effect in chemistry is a property of substituents or functional groups in a chemical compound. The effect is used in a qualitative way and describes the electron withdrawing or releasing properties of substituents based on relevant resonance structures. Inductive effect is effective on π-bonds and mesomeric effect is effective on π-bonds[27].
In this paper,it can be said that one of the most important factors leading to the changes in the K X-ray production cross-sections are the inductive effect in all compounds. 3d and 4p orbitals are part of the valance state of Br atom and the distribution of electron density of the valance band changes as regards to the inductive effect.
As seen from the Table 1,when the Kβproduction cross section values of Bromine have been taken into consideration,the valence state electron population has been seen the alteration of the increasing of n at —(CH2)n— group bound to the N+at position of quaternary-imidazole ring in the compounds 2a,2b,2c,2d,2e,2f,2h and 2g. This circumstance indicates the gradually increment of the electron density due to the fact that the electron releasing on the bromine anion. For these compounds,the electron releasing process comes into existence on the bromine anion as well as on the quaternary-imidazole ring.
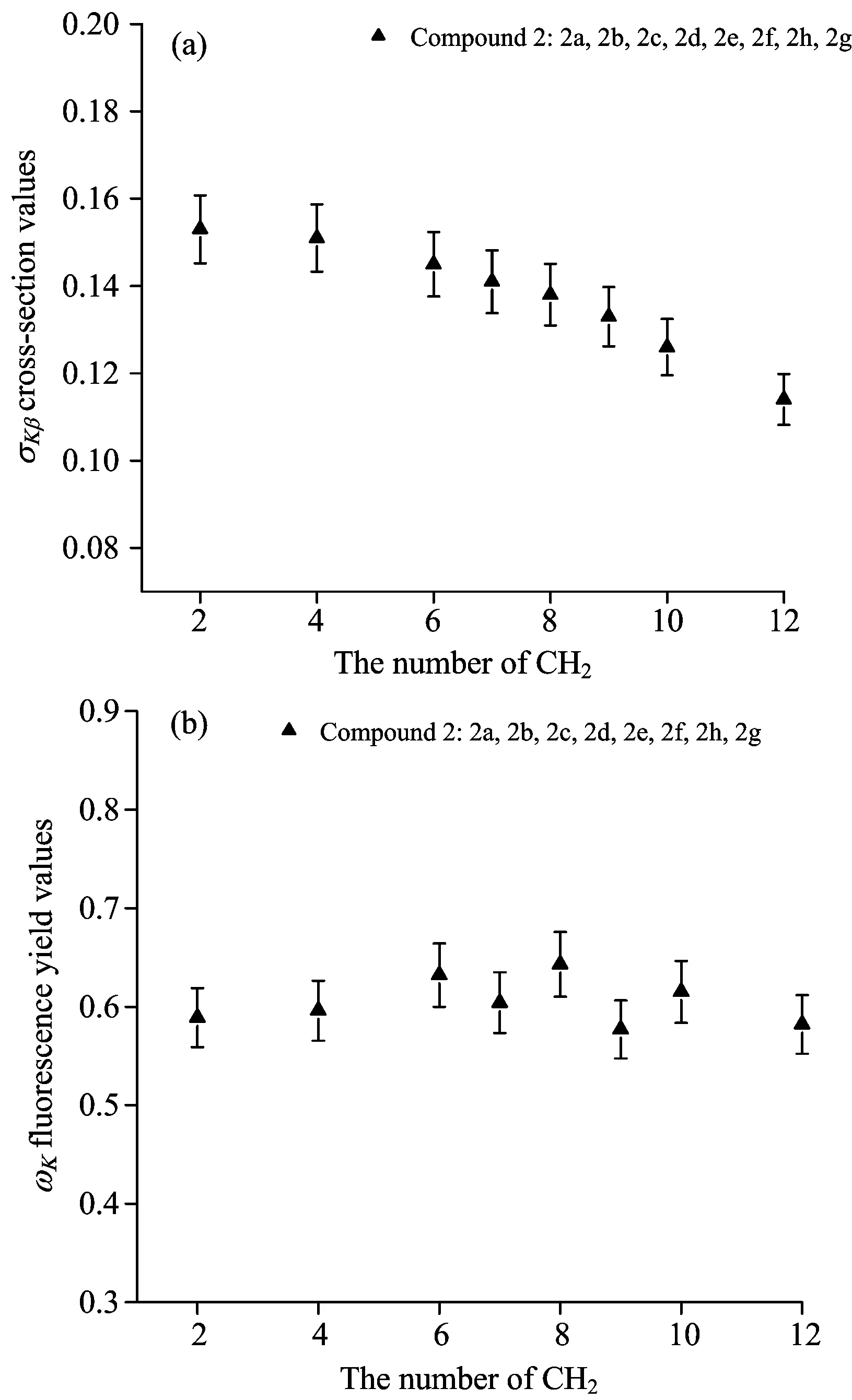
A plot of K X-ray production cross-sections and average fluorescence yields versus the number of CH2groups is shown in Figure 3.
As shown in Fig.3(a),there are variations from linearity in some experimental results. The reason of these variations is the inductive electron-releasing process. In the Figure 3 (b),this case has not been beholded because the contribution of the Kβproduction cross-section values on the total K shell production cross-section values is small than the Kα production cross-section values. Therefore,there is no substantial difference between the present experimental K shell fluorescence yield values and theoretical value and this parameter is not adequate to explain the alloying effect.
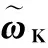
The uncertainties in the present measurements are estimated to be less than 6% and are found due to propagating the errors in various parameters used for determination of X-ray parameters. The uncertainties in these parameters are listed in Table 2.

Table 2 Uncertainties in the quantities used to determine the parameters
[1] Isloor A M,Kalluraya B,Rao M,et al. Chem. Soc.,2000、4:265.
[2] Amir M,Kumar H,Javed S A. Eur. J. Med. Chem.、2008、43(10):2056.
[3] Sztanke K,Tuzimski T,Rzymowska J,et al. Eur. J. Med. Chem.、2008、43(2):404.
[5] Padmavathi V,Thriveni P,Sudhakar G、et al. Eur. J. Med. Chem.、2008、43(5):917.
[6] Kalluraya B,Isloor A M,Shenoy S. Indian J. Heterocycl. Chem.、2001、11:159.
[7] Kalluraya B,Isloor A M,Priya F V,et al. Indian J. Heterocycl. Chem.、2004、13(3):245.
[8] Koparir M,Orek C,Parlak A E,et al. European Journal of Medicinal Chemistry、2013、63:340.
[9] Brunner G,Nagel M,Hartmann E. E. Arnt,J. Phys. B: At. Mol. Phys.、1982、15:4517.
[10] Folkmann F. Nucl. Instr. and Meth. B、1996、109/110:39.
[11] Raj S,Padhi H C,Polasik M. Nucl. Instr. and Meth. B,1998,145: 485.
[12] Mukoyama T,Taniguchi K,Adachi H. X-Ray Spectrom.,2000、29:426.
[13] Raj S,Padhi H C,Polasik M. Nucl. Instr. and Meth. B,2000,160: 443.
[14] Mukoyama T,Taniguchi K,Adachi H. Phys. Rev. A,2001,63: 042514.
[15] Kawai J,Ohta M,Konishi T. Analytical Sciences,2005,21: 865.
[16] Mukoyama T,Kaji H,Taniguchi K,et al. X-Ray Spectrom.、1997、26:269.
[17] Kulshreshtha S K,Wagh D N,Bajpei H N. X-Ray Spectrom.,2005,34: 200.
[19] Sogut O,Seven S,Baydas E,et al. Spectrochim. Acta,Part B、2001、56:1367.
[20] Iwatsuki M,Fukasawa T. X-Ray Spectrom.、1987、16:73.
[21] Kucukonder A. Eur. Phys. J. D、2001、17:293.
[22] Kucukonder A,Sogut O,Buyukkasap E,et al. X-Ray Spectrom.、2003、32:60.
[23] Aylikci V,Unver Y,Dugdu E,et al. Chem. Phys. Lett.、2013、556:365.
[24] Berger M J,Hubbell J H. XCOM: Photon Cross-Sections on a Personnel Computer (version 1.2),NBSIR85-3597,National Bureau of Standarts,Gaithersburg,MD,USA,for version 3.1,1999,see http://physics.nist.gov/.
[25] Apaydin G,Aylikci V,Biyiklioglu Z,et al. Chinese J. Chem. Phys.、2008、21:591.
[26] Scofield H. Lawrence Livermore Laboratory (UCRL),1973. 51326.
[27] Aylikci V,Cengiz E,Apaydin G,et al. Chem. Phys. Lett.、2008、461:332.
[28] Krause M O. J. Phys. Chem. Ref.,1979,Data 8,307.
O433
A
2015-04-10; accepted:2015-07-29
10.3964/j.issn.1000-0593(2016)12-4120-05
*Corresponding author e-mail: vaylikci@mku.edu.tr
杂志排行
光谱学与光谱分析的其它文章
- Studies on the Interaction of Perfluorononanoic Acid with Human Serum Albumin by Multi-Spectroscopic,Molecular Docking and Isothermal Titration Calorimetry Techniques
- Study on Emission Spectrum of OH Radicals in a Combination System of Pulsed Discharge Plasma and Activated Carbon
- Simulating the Three-Dimensional Image of Cold Atomic Cloud
- K Shell Fluorescence Parameters by Impact of 5.96 keV Photons on Ti and Its Compounds
- 动态应变场下相移光栅光谱特性及实验研究
- 基于Wollaston棱镜的图像复分快照式成像光谱仪设计
