K Shell Fluorescence Parameters by Impact of 5.96 keV Photons on Ti and Its Compounds
2016-06-05O.K.KÖ
O.K. KÖ
Department of Physics,Faculty of Sciences,Karadeniz Technical University,Trabzon 61080,Turkey
K Shell Fluorescence Parameters by Impact of 5.96 keV Photons on Ti and Its Compounds
Department of Physics,Faculty of Sciences,Karadeniz Technical University,Trabzon 61080,Turkey
K shell fluorescence parameters of pure Ti and some of its compounds have been determined experimentally using an Ultra-LEGe detector with resolution 150 eV at 5.9 keV. The samples were excited 5.96 keV photons emitted from a55Fe radioisotope source with 50 mCi activity. The experimental values of the K shell fluorescence parameters have been compared with the experimental and theoretical values available in the literature for pure Ti.
K Shell fluorescence parameters;55Fe radioactive source; ED-XRF; Titanium
Introduction
The information obtained from experimental results of the X-ray production crosssections and the fluorescence yields for different elements has an important role in the study of some basic phenomena,in atomic,molecular and radiation physics and in nondestructive trace element analysis of various samples using the energy dispersive X-ray fluorescence (ED-XRF) method.
In previous studies,K shell fluorescence cross sections,fluorescence yields and intensity ratios were assessed for titanium and some of its alloys. K shell fluorescence cross sections were studied for Ti in TiB2,TiF3,TiF4TiC and TiS2. Namely,K shell fluorescence cross sections and yields have not been set for the compounds in present study. For TiS2K shell intensity ratio was studied. In this paper,K shell fluorescence cross sections and fluorescence yields have been built for Ti and its compounds by impact of 5.96 keV photons. At last,the determined values of K shell fluorescence parameters have been compared with experimental data and the theoretical values.
1 Experimental procedure
The geometry of the experimental setup[11]for an annular source is illustrated in Fig.1. In this experimental set-up,5.96 keV photons emitted by an annular 50 mCi (1 850 MBq)55Fe radioactive sources were used. The fluorescence K X-rays from the sample were detected by a collimated Ultra-LEGe detector having a thickness of 5 mm and an energy resolution of 150 eV at 5.96 keV. The targets were placed at 5 cm from the source,their X-rays going through the hollow centre of the source assembly and also through a graded-Z collimator placed in front of the detector. In this experiment the counting time was set to 10 000 s for each sample. In the experimental determinations,spectral deconvolution is one of the main problems that arise when determining these parameters due to the strong peak overlapping in ED-XRF system. In the present work,a peak fitting program developed by Origin Company (OriginPro 7.0 SR0) was used to designate peak analysis. First of all required points subtracting as marking were removed thus background subtraction is done and X ray peaks are fitted as Gaussian functions with using PFM (Peak Fitting Module) in this program. In the present study,Fig.2 displays the typical K X-ray spectrum for Titanium.
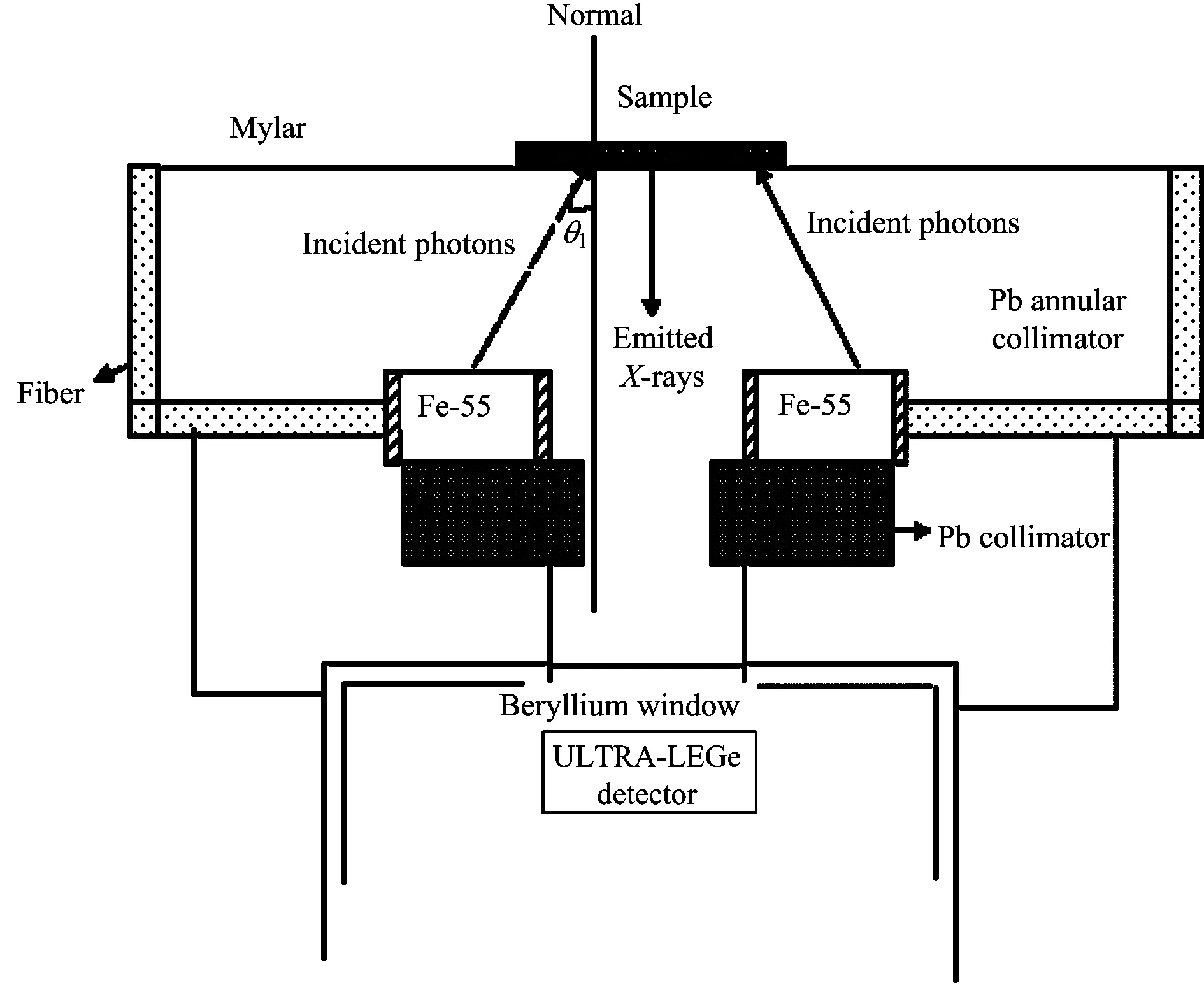
Fig.1 Geometry of the experimental set-up
The purity of commercially obtained materials is better than 99%. Powder samples were sieved using 400 mesh (~38×10-4cm) and were purchased from Sigma-Aldrich and Alfa Aesar Company.The thicknesses of samples are between 3.3 and 13.6 in mg·cm-2.
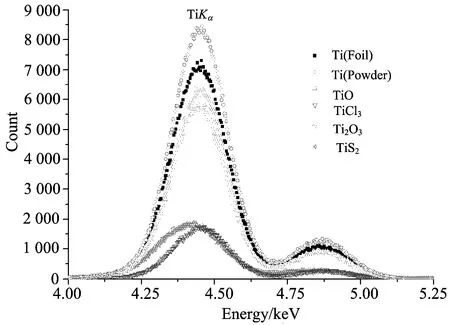
Fig.2 Typical K X-ray spectrum for Titanium and its compounds
The product I0Gε,containing the terms related to the incident photon flux,geometrical factor and intrinsic absolute efficiency of the X-ray detector,was determined by collecting the Kαand KβX-ray spectra of samples of S,Cl,K,Ca and Ti for55Fe in the same geometry using the equation
(1)
whereNKiis the measured intensity (area under the photo peak) corresponding to the Kigroup of X-rays,I0is the intensity of the incident radiation,Gis the geometric factor,εKiis the detection efficiency for the Kigroup of X-rays andβKiis the self-absorption correction factor for the target material,which accounts for the absorption in the target of the incident photons and the emitted characteristic X-rays. m is the thickness of the target in g·cm-2.
The factorI0GεKiwas fitted as a function of energy using the following equation for55Fe
(2)
whereExis the X-ray energy and,A0,A1,A2andA3are constants evaluated from a fitting polynomial. The variations of the factorsI0Gεas a function of energy are shown in Fig.3.
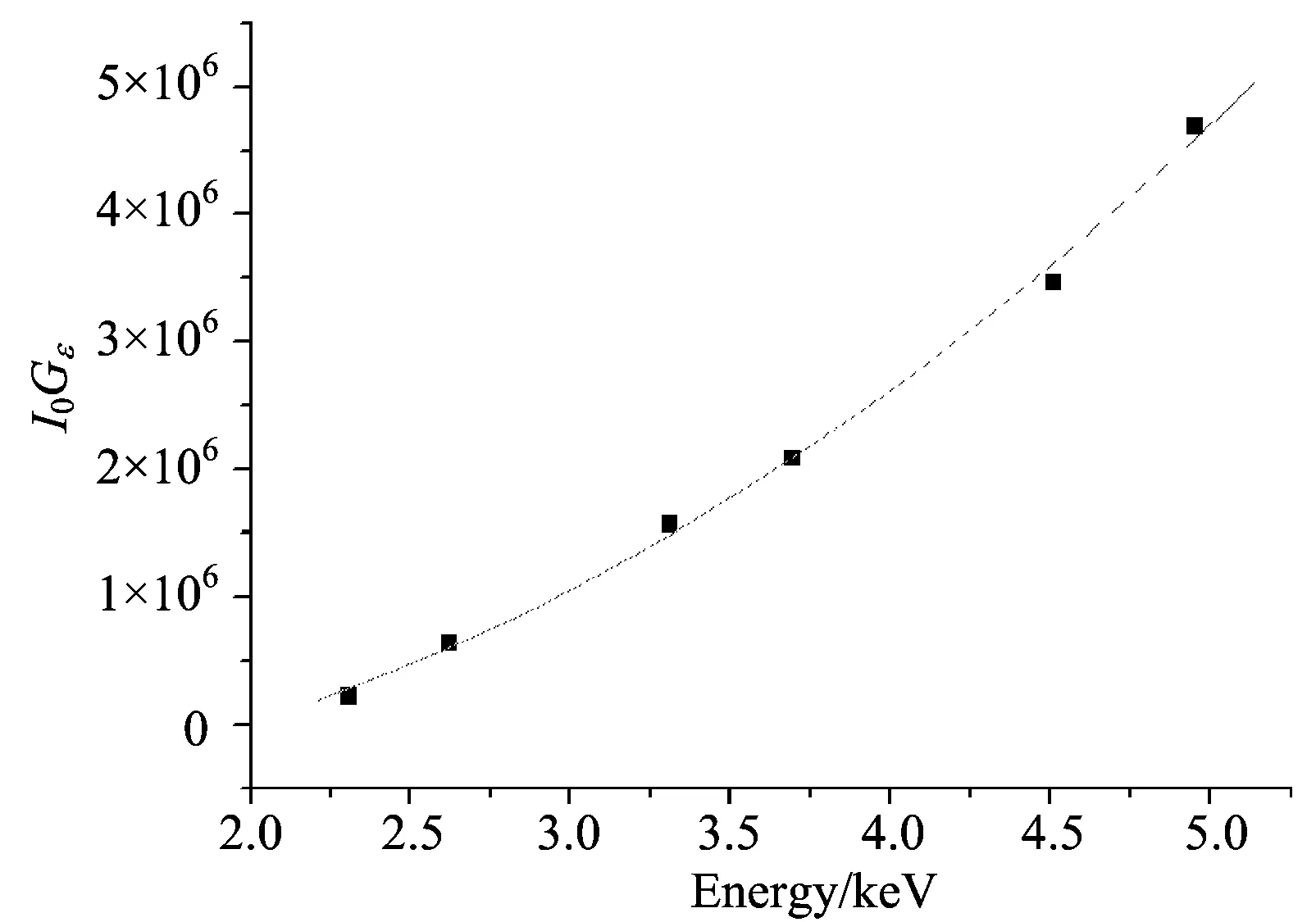
Fig.3 The variation in the factor I0Gε as a function of the mean energy for 55Fe radioisotope
The self-absorption correction was calculated using the equation[12]
(3)
whereμincandμemtare the mass attenuation coefficients[13]of incident photons and emitted characteristic X-rays,respectively;tis the thickness of the target in g·cm-2; the angles of the incident photons and the emitted X-rays with respect to the normal of surface,θ1andθ2,were equal to 45° and 0° in the present experimental set-up,respectively.
The experimental KiX-ray production cross-sections were calculated by using the Eq. (1). Theoretical value of K shell cross sectionσKα,βis the probability that a vacancy in the K shell is filled through a radiative transition,is a physical quantity useful for the interpretation of a large variety data in atomic and nuclear physics. The theoretical K X-ray production cross sections were evaluated by using the relation[14].
σKi=σK(E)ωKFKi
(4)
whereσK(E) is the K-shell photoionization cross-section of the given element for the excitation energyE[15],ωKis the K-shell fluorescence yield[16],andFKiis the emission rate of the fractional X-ray for KiX-rays[17].
The semi-empirical K shell fluorescence yieldsωKwere measured according to the following equation
(5)
where ∑σKiisthetotalKX-rayfluorescencecross-sectionobtainedexperimentally,σK(E) is the theoretical K shell photoionization cross-section of a given element for the excitation energyE[15].
2 Results and discussion
The semi-empirical values of the fluorescence yields (ωK) and the fluorescence cross sections (σK) of Ti and some of its compounds (TiO,TiCl3,Ti2O3and TiS2) were respectively calculated by using equation (1) and (5). The experimental values of the K shell fluorescence yields and cross-sections have been compared with the experimental and theoretical values available in the literature for pure Ti.
The interaction between the central and ligand atoms comes into existence in valance shell. Kαtransitions are quite far from the valance state but Kβtransitions come from the valance shell and neighbor shell of the valance shell for Ti element. So Kβtransitions are affected easier by changing the valance electronic structure. The individual structure characteristics of molecules such as polarity,valence,electronegativity of atoms,coordination number and bonding type mainly affect the energy position of the K lines. The atom which is in a compound displays different characteristics when compared with to a free atom. Any participation to atom in chemical bonds changes the valence electron density that it relies on the energies of inner shell. The element,which has a greater electronegativity value than the other elements in the component,will attract the valance electrons of the neighboring element and so the element that has less electronegativity value is polarized owing to the electronegativity difference.
According to Pauli,O (3.44),Cl (3.16) and S (2.58) elements have bigger electronegativity value than Ti so the transfer of valence state electrons is supported to go from Ti to other elements in component. In this case,the screening effect in Ti element may be more different than the others. It is expected that the difference of screening will cause a changing of bonding energy of outer shell electrons and thus KβX-ray-production cross-section values will differ for Ti.
Besides,the alternation in the valance electron brings about change the other parameters as the fluorescence intensity ratio and fluorescence yields. Consequently,when the electron density of the valance shell and screening are changed,the bonding energies of the inner shells are changed,as well. The changing would be increased if the more electrons joined to the bond which affects the transition between the energy levels[18].
The K shellσKαcross-sections were compared with the previous results ofahin et al.[7]and Scofield[19]that they can be seen in Table 1. They were found in agreement within ranges 2%~10%,1%~9%,respectively. The K shellσKβcross sections were compared with the previous results ofahin et al.[7]and Scofield[19]that they can be seen in Table 1. They were found in agreement within ranges 3%~13%,3%~18%,respectively. As seen,σKβcross-sections are more different than theσKαcross-sections.
The K shell fluorescence yields were compared with the previous results of Han et al.[8],Krause[16],Broll[17]and Hubbell[20]that they can be seen in Table 2. They were found in agreement within ranges 0%~10%,0%~12%,0%~12%,3%~7%,respectively. These deviations for Han’s values may arise from a different detector type,a different exciting energy,a different detector efficiency and a different spectrometer type. In addition,the mentioned data of the other studies were done for pure elements but our present work has been performed on the pure Ti and its compounds.
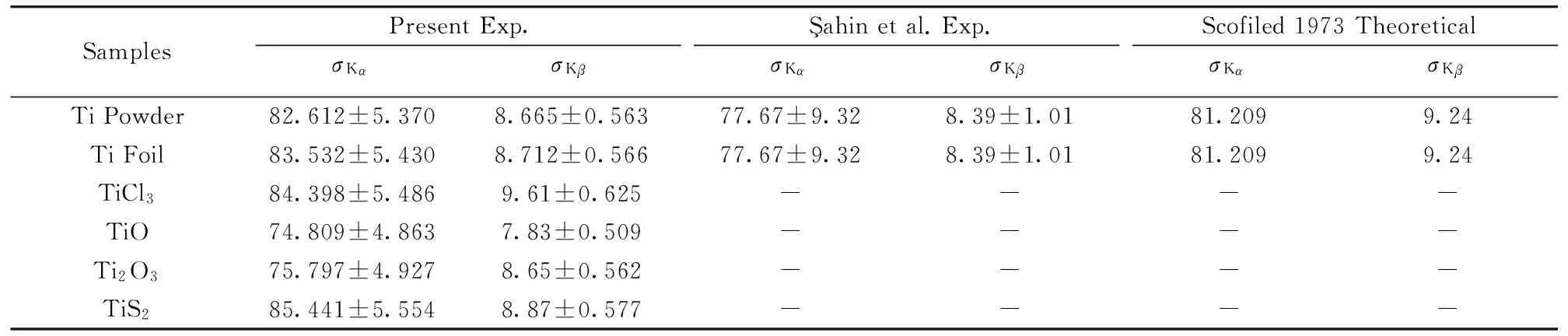
Table 1 The experimental σKα,σKβ K shell X-ray fluorescence cross-sections of Ti and some of its compounds
Table 2 Comparison of the measured values of K Shell fluorescence yield of Ti and some of its compounds with the present values and the previous studies
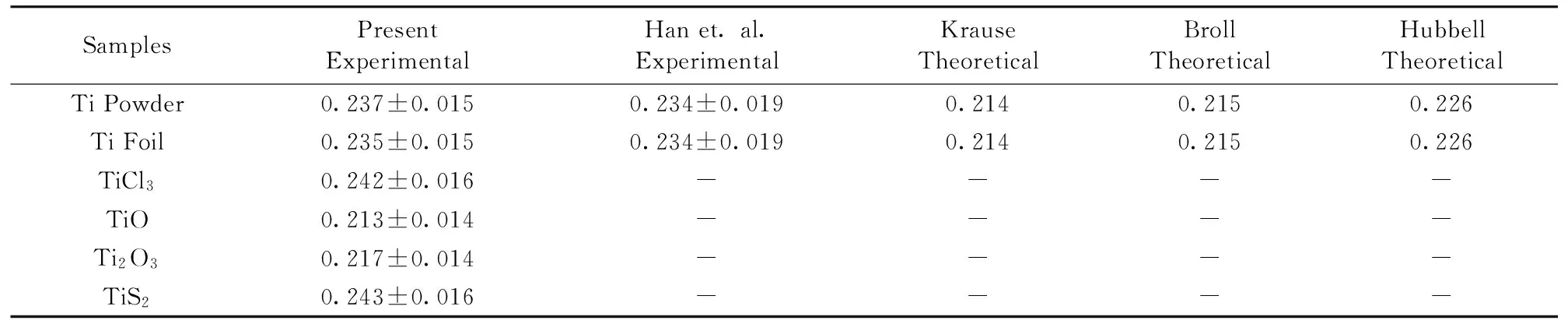
SamplesPresentExperimentalHanet.al.ExperimentalKrauseTheoreticalBrollTheoreticalHubbellTheoreticalTiPowder0.237±0.0150.234±0.0190.2140.2150.226TiFoil0.235±0.0150.234±0.0190.2140.2150.226TiCl30.242±0.016----TiO0.213±0.014----Ti2O30.217±0.014----TiS20.243±0.016----
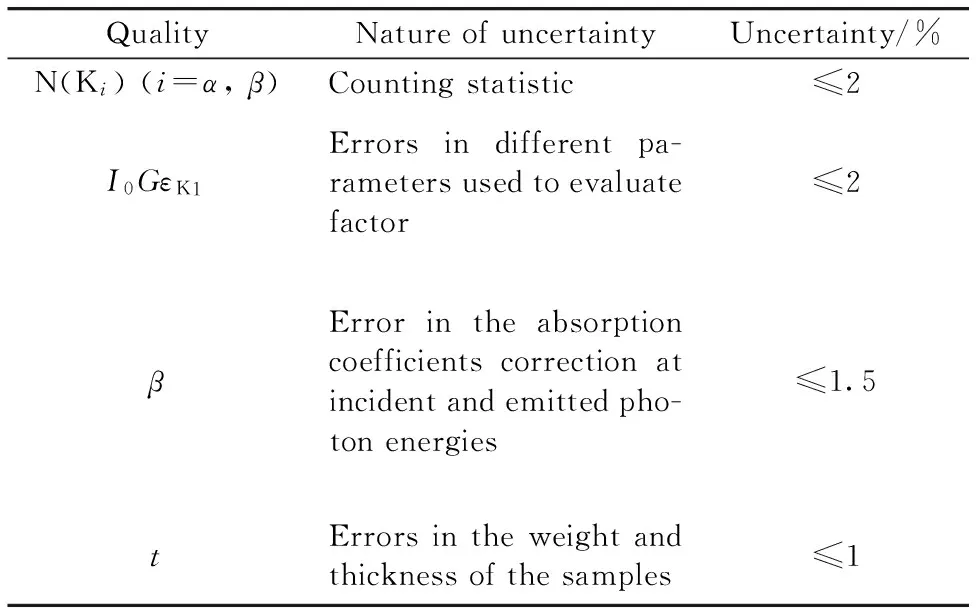
Table 3 Uncertainties in the quantities used to determine the parameters
As it can be seen from the results whileωKvalues for TiCl3and TiS2are higher than Ti elemental foil,those for TiO and Ti2O3are lower than Ti. The reason of this is the deviations on the K shell fluorescence cross section values. TheωKvalues are calculated by using Eq.(5). In this formula theωKvalues are proportional to the total K shell fluorescence cross sections. Unlike TiO and Ti2O3,the values of the fluorescence cross section for TiCl3and TiS2are higher than Ti elements,it is evidently claimed thatωKvalues for these compounds are higher than Ti elements.
The overall error in this study was estimated to be 6%. This error is the quadrature sum of uncertainties in the different parameters used to evaluate subshell fluorescence yield and cross sections,i.e. target thickness (1%),the evaluation of the peak area,(2%),I0Gεproduct (2%) and the absorption correction factor (1.5%).
[1] Bhuinya C R,Padhi H C. Phys. Rev.,1993,A47:4885.
[2] Raj S,Padhi H C,Polasik M. NIMB,1998,145:485.
[3] Raj S,Padhi H C,Basa D K,et al. NIMB,1999,152:417.
[10] Han I、Demir L. Physical Review A、2010,4:82.
[12] Hubbell J H、Seltzer S M. Natl. Inst. Stand. Phys. Lab. NISTIR、1995,5632.
[13] Berger M J. Hubbell J H. XCOM.1999,87-3597 (http://physics.nist.gov/PhyRefData/ Xcom/Text/XCOM.html).
[14] Seven S. Turk. J. Phys. 2002,26:483.
[15] Scofield H. Lawrence Livermore Lab、1973,(UCRL) 51326.
[16] Krause M O. J. Phys. Chem. Ref.、1979,8:329.
[17] Broll N. X-ray Spectrom.、1986,15:271.
[18] Agarwal B K. X-ray Spect.: An Introduction. Springer-Verlag,Berlin,New York,1979,220.
[19] Scofield J H. Phys. Rev.、1974,A9:1041.
[20] Hubbell J H、Trehan P N、Singh N、et al. J. Phys. Chem. Ref. Data、1994,23:339.
O433
A
2015-11-13; accepted:2016-04-08
10.3964/j.issn.1000-0593(2016)12-4125-05
*Corresponding author e-mail: okoksal@ktu.edu.tr
杂志排行
光谱学与光谱分析的其它文章
- Studies on the Interaction of Perfluorononanoic Acid with Human Serum Albumin by Multi-Spectroscopic,Molecular Docking and Isothermal Titration Calorimetry Techniques
- Study on Emission Spectrum of OH Radicals in a Combination System of Pulsed Discharge Plasma and Activated Carbon
- Simulating the Three-Dimensional Image of Cold Atomic Cloud
- Structure Effect on K Shell Fluorescence Parameters at Bis-4-Bromobenzyl-1,2,4-Triazol-3-Ones
- 动态应变场下相移光栅光谱特性及实验研究
- 基于Wollaston棱镜的图像复分快照式成像光谱仪设计
