Enhancing the hydration reactivity ofhemi-hydrate phosphogypsum through a morphology-controlled preparation technology☆
2016-05-30LinYangJianxinCaoCaiyuLi
Lin Yang ,Jianxin Cao *,Caiyu Li
1 Schoolof Chemistry and ChemicalEngineering,Guizhou University,Guiyang 550025,China
2 College ofResource and EnvironmentalEngineering,Guizhou University,Guiyang 550025,China
3 Guizhou Key Laboratory for Green Chemicaland Clean Energy Technology,Guiyang 550025,China
1.Introduction
Wet-process phosphoric acid is an intermediate for producing phosphorus-containing fertilizers.When producing 1 ton of orthophosphoric acid in wet-process phosphoric acid,approximately 5 tons ofby-products are formed,including CaSO4·2H2O,CaSO4·0.5H2O,and CaSO4phosphogypsum[1].With the rapid development of the phosphate fertilizer industry,the output of phosphogypsum has exhibited a trend toward rapid growth.Over 22 million tons ofphosphogypsum are produced annually across China.In 2010,the totalgenerated phosphogypsum in China reached over 100 million tons[2].At present,only 15%of worldwide phosphogypsum production has been used to manufacture building materials,agriculturalfertilizers,soilstabilization amendments,Portland cement retarder,and sulfate acid[3–8].Most phosphogypsum piled up and occupied considerable land resources,which eventually led to serious environmentalcontamination.
Hemi-hydrate phosphogypsum(HPG)is obtained during the hemihydrate process ofwet-process phosphoric acid[9,10].The main mineral composition ofHPGis type-αhemi-hydrated gypsum(α-HH)thatcorresponds to the gypsumbinding material.Thus,HPGcan be used to produce building materials and gypsumproducts.However,the low hydration reactivity of HPG leads to poor gelling strength,which causes production enterprises to discard HPG.Consequently,the surrounding environment is polluted by this substance[11].Therefore,a research that aimed to enhance the hydration reactivity of HPG could provide considerable practicalvalues to realize resource utilization of HPG.The hydration reaction ofHPGis characterized by a phase transition fromα-HHto dihydrate gypsum.The description ofwater channeloccupation and space group in the crystalstructure ofα-HH has been reported in the literature[12,13].According to severalstudies,α-HHhas a honeycomb-shaped channelstructure.These honeycomb-shaped channels are partly occupied by x H2O(0<x<0.8)[14,15];hence,channels with a diameter ofapproximately 0.03(i.e.,waterchannels)are formed in the honeycomb-shaped channels,through which water molecules from the outside can enterα-HH crystal inside and then hydrate into dihydrate gypsum.In this manner,α-HHcan completely hydrate within a short period[16].
Many in fluencing factors,such as speci fic surface area and residual phosphorus acid content,play a role in the hydration reactivity of HPG.According to dissolution–crystallization theory,the hydration reactivity of HPG simultaneously increases with speci fic surface area and decreases with residualphosphorus acid content[17].However,in the hemi-hydrate process of wet-process phosphoric acid,the crystalsize of HPG should be generally big,and residualphosphorus acid content should be reduced to improve the filtration strength and recovery rate ofphosphoric acid[18,19].Accordingly,a criticalmethod thatenhanced the hydration reactivity ofHPGby increasing the number ofwaterchannels without considering the increase in its speci fic surface area was proposed.The distribution ofwater channels was eventually re flected on the crystalmorphology;hence,a study on the crystalmorphology ofα-HH was meaningfulto testits in fluence on hydration reactivity.
In the present study,we theoretically analyzed the distribution of water channels in the crystal morphology ofα-HH,compared the difference in hydration reactivity between industrial HPG and type-α high-strength gypsum,and prepared different crystalmorphologies of HPG in the experiment.Through the contrast between the crystalmorphology and hydration apparentactivation energy of HPG,we could investigate the role of water channels in hydration and provide a theoreticalbasis for the type of HPG morphology that should be controlled in the hemi-hydrate process ofwet-process phosphoric acid to enhance hydration reactivity.
2.Experimental
2.1.Materials
Industrial HPG was obtained from a phosphoric acid plant in Guizhou Province.The chemicalcomposition(calculated for the dry product)was(wt%):CaO 34.16,SO349.81,Fe2O30.05,Al2O30.35,MgO 0.59,SiO24.75,total F 0.70,water-soluble F 0.25,total P2O51.18,water-soluble P2O50.40,and crystal water 7.75.The speci fic surface area determined using the Blaine method was 330 m2·kg-1.
Type-αhigh-strength gypsum was obtained from Hubei Province.The chemicalcomposition(calculated for the dry product)was(wt%):CaO 37.12,SO351.34,SiO21.75,MgO 1.09,and crystalwater 6.51.The speci fic surface area determined using the Blaine method was 342 m2·kg-1.
Apatite powders were collected from a phosphoric acid plantin Guizhou Province.The chemicalcomposition(calculated for the dry product)was(wt%):SiO26.29,Fe2O30.31,Al2O30.23,CaO 49.63,MgO 1.04,SO30.67,P2O535.05,F 2.57,and acid non-soluble substance 6.05.The speci fic surface area determined using the Blaine method was 151 m2·kg-1.
Phosphoric acid(wt%P2O585)and sulfuric acid(wt%H2SO498)were of reagent grade and purchased from Sinopharm Chemical Reagent Co.,Ltd.
2.2.Experimentmethods
2.2.1.Theoreticalcalculation ofα-HHgrowth morphology and the distribution ofwater channels
A single-crystalstructure ofα-HH was obtained from the American Mineralogist Crystal Structural Database.The molecular mimicry software Material Studio[20]was used to build a unit cellstructure ofα-HH.The attachment energy(AE)method regards the growth rate of crystalsurface increases with the attachment energy ofcrystalsurface increase in the crystal growth process;thus,the theoretical growth morphology of the crystalcan be calculated[21].Therefore,we selected the AE morphology module of the molecular mimicry software Material Studio to forecast the growth morphology ofα-HH crystal.Different force fields such as COMPASS,pcff,and cvffwere setasinitialforce fields ofsingle-crystalstructure ofα-HH.
To investigate the distribution ofwater channels in various crystal planes,3D modeling extended the single-crystalcellstructure ofα-HH to obtain the super cellstructure based on its symmetry×,which was then carved to obtain the planes with slices measuring 2.0 nm thick.
2.2.2.Measuring hydration reactivity
Hydration reactivity was characterized by the hydration rate of the hemi-hydrate gypsum.In order to eliminate the effect of watersoluble impurities such as phosphorus and fluorine of industrial HPG on the hydration reactivity,hot water at 90°C was used to wash the industrial HPG untilthe solution was tested to be neutral.The residues were then dried and set as industrial HPG samples.
The samples were mixed with double pure water(calculated by mass ratio)and placed into a 250 ml Erlenmeyer flask.Thermostatic water bath oscillator was used to maintain the reaction temperature at 20°C.Stirring speed was 100 r·min-1.At a designated time,the samples were removed and filtered;the residues were placed into the vacuum drying oven(temperature 60°C,vacuity 60 kPa)to keep them dry after they were washed with absolute ethylalcohol.When the mass of the residues became constant,the preparation of the hydration samples was finished.The hydration rate was calculated using Eq.(1):

whereαrefers to the hydration rate,w1and w2represent the crystal water content of the samples before and after hydration,respectively(calculation method refers to GB/T 5484-2000),MH2Orefers to the molecular weight of H2O,MCaSO4×0.5H2Orefers to the molecular weight of hemi-hydrate calcium sulfate,andεindicates the hemi-hydrate gypsum content of industrial HPG or type-αhigh-strength gypsum samples(calculated in the manner of building gypsum phase distribution GB/T 9776-2008).
2.2.3.Preparing HPG
The following solutions were prepared:Solution(a):36 wt%P2O5and Solution(b):98 wt%H2SO4.Then,2055 g of Solution(a),315 g of apatite powders,and 288 g of Solution(b)were obtained.
A smallamount of Solution(b)(calculated by the concentration of liquid-phase SO42-)was poured into Solution(a).The mixed solution with different concentrations of liquid phase SO42-was then placed in a cylindricalreactor(40 cm high,25 cm inside diameter)and heated to 95°C using a thermostatically controlled oilbath.Apatite powders were then slowly and evenly added into the cylindricalreactor within 30 min and stirred at a speed of 135 r·min-1.The remaining Solution(b)was poured into the cylindrical reactor using a separating funnel at a dropping speed of 4.5 g·min-1.After 150 min of allowing crystals to grow,the slurry was filtered to obtain the filtrate,which was the finishing phosphoric acid.The residues were repeatedly washed with 90°C water untilthe washing solution was tested to be neutral.The residues were then dried in a 60°C±1°C oven untiltheir weightwas constant.HPG was produced.
2.2.4.Calculating hydration apparentactivation energy
Experimentmethod(Section 2.2.2)was adopted to test the hydration rate of HPG under different temperatures:20,30,and 40°C.The least square method of the Avramicrystallization kinetic equation was used to launch a fitting toward the relation curve between the hydration rate of HPG and the change in hydration time.The Avramicrystallization kinetic equation is shown in Eq.(2):

where t refers to the hydration time,Z refers to the composite crystallization rate constant,αrefers to the hydration rate that corresponds to the hydration time,and n refers to the Avramiparameter.
Eq.(2)was transformed into a linear equation,i.e.,Eq.(3):

Plotting ofln[-ln(1-α)]againstlntgives a straightline with slope n and intercept ln Z.
The calculation of the hydration apparent activation energy of HPG was based on the Arrhenius equation,as shown in Eq.(4):

where A refers to the pre-exponentialfactor,Earefers to the hydration apparentactivation energy,R refers to the constant8.314 J·mol-1·K-1,and T refers to the thermodynamic temperature.
Consequently plotting of ln Z against-1/T gives a straight line with slope Ea/R and interceptln A.We could determine Ea,which is the hydration apparent activation energy of HPG.
2.2.5.Characterization
The morphology and particle size of the samples were observed using a scanning electron microscope(SEM,ΣIGMA)and the image analysis software SmartTiff.The crystalstructure of the samples was analyzed via X-ray diffraction(XRD,XPert Powder),and the scanning range 2θ was from 10°to 90°.
3.Results and Discussion
3.1.Correlation betweenα-HH morphology and water channels
XRD patterns ofsingle-crystalstructure ofα-HH(0019827[13]),industrialHPGand type-αhigh-strength gypsumare shown in Fig.1.Itcan be seen that the XRD pattern of the single-crystal structure ofα-HH matches those oftype-αhigh-strength gypsumand industrialHPG.Itindicates thatthe selected single-crystalmodelofα-HHis appropriate.The cellparameters ofα-HHare as follows:space group I2,a=1.20275 nm,b=0.69312 nm,c=1.26919 nm,β =90.18°and Z=12.

Fig.1.XRD patterns ofsingle-crystalstructure ofα-HH,industrialHPG and type-α highstrength gypsum.

Fig.2.Unit cellstructure ofα-HH.The colors indicate:green—Ca,yellow—S,red—O,and white—H.The black dash line indicates water channel.1Å=0.1 nm.
The unit cellstructure ofα-HHis shown in Fig.2.12 SO42-,12 Ca2+,and 6 H2O molecules are found in the single-crystalcell.A long chain is constructed by the tetrahedron of SO42-and Ca2+along the z-axis.A honeycombed tunnel with a diameter of 0.5517 nm is constructed using 6 SO42-–Ca2+–SO42-long chains,which is filled with H2O molecules with a length of0.1732 nm and form a channelwith a diameter of 0.3785 nm.This channelallows water molecules from the outside to pass freely;thus,α-HH can easily transform into dihydrate gypsum.
Table 1 summarizes the comparison of the optimized values ofα-HH unitcellparameters in differentforce fields with the experimentvalues.It shows thatthere are minimum deviation values ofα-HHunitcellparameters using the force field COMPASS as the initialforce field.So the force field COMPASS is a suitable force filed to forecastthe growth morphology ofα-HH crystalusing AE model.
Fig.3 shows the prediction ofα-HH growth morphology using AE model.It can be seen that the growth morphology ofα-HH is a fourfaced bipyramid short column constructed with 12 crystal planes.Among which,crystalclusters{1 0-1},{101}form the four cylinders ofα-HH crystal,whereas crystal clusters{011},{0-1 1},{110},and{1-1 0}form its conicalsurfaces.
Attachment energy and area ofgrowth surfaces ofα-HH crystalare displayed in Table 2.Crystalcluster{101}comprises the largest crystal plane area,which accounts for 35.107%.The crystal plane area of{1 0-1}accounts for 33.133%.The crystalplane area of{011}is equal to that of{0–11},which accounts for 7.467%,whereas the crystal plane area of{110}is equal to that of{1-1 0},which accounts for 8.413%.It indicates thatthe growth rates ofcrystalclusters{101}and{1 0-1}ofα-HH are litter than those of other crystalclusters.
The crystalsurface structures ofα-HH projected along the[001]direction are shown in Fig.4.Cylinders(101),(1 0-1)and conicalsurfaces(011),(0-1 1)form an angle with the water channels,and approximately three water channels are observed.Because conical surfaces(110),(1-1 0)are parallelwith the water channels,no water channel exists on these surfaces.It indicates that water molecules from the outside can only enter theα-HH crystalinside and then hydrate into dihydrate gypsum along the water channels in the four cylinders and the two conicalsurfaces{011},{0-1 1}.By contrast,water molecules from the outside cannotenterthe crystalinside from the two conicalsurfaces{110},{1-1 0}.Thus,the exposures of the cylinders ofα-HHcrystal are positive to hydration reactivity,whereas the exposures of the conical surfaces parallelto the z-axis are negative to hydration reactivity.
3.2.Comparison ofhydration reactivity and structure between industrial HPG and type-αhigh-strength gypsum
The hydration rates ofindustrialHPGand type-αhigh-strength gypsum under differenthydration times are shown in Fig.5.The hydration rate of type-αhigh-strength gypsum reaches about 74.72%within 15 min,and its complete hydration is achieved within 60 min.However,the hydration rate ofindustrial HPG is only about 2.76%within 15 min and reaches about 14.36%within 480 min.It can be concluded that the hydration reactivity oftype-αhigh-strength gypsum is considerably higher than that ofindustrial HPG.
Fig.6 shows the SEMimages ofindustrialHPG.Itcan be seen thatindustrialHPGis made ofirregular oriented crystals ofschistose shape.As shown in Position a in Fig.6,only two cylinders ofindustrialHPGare uncovered.Meanwhile,Position b in Fig.6 shows an obvious embossment of the conical surface.SEM image of type-αhigh-strength gypsum is shown in Fig.7.It can be seen that type-αhigh-strength gypsum has stumpy crystals with 20–40 μm long and length-to-diameter ratios of 2–3:1.In addition,Fig.7 shows thatcylinders of the crystalclearly appear,whereas the conicalsurfaces of the crystalare unapparent.
Analyzing the XRD pattern enables us to observe the interior structure of the crystal.As shown in Fig.1,the main mineralin both industrial HPG and type-αhigh-strength gypsum is hemi-hydrated gypsum.However,industrialHPGalso contains a smallamountofdihydrate gypsum and quartz.The rank of the diffraction peak strength of type-α high-strength gypsum is(400)>(200)>(310)>(114),which determines the more complete growth of the cylinders compared with thatof the conicalsurfaces.Nevertheless,the decrease in diffraction peak of(400)and(200)in industrial HPG,as wellas the increase in diffraction peak of(310)and(114),indicates thatthe decrease of the crystalcylinders of industrial HPG will allow the conical surfaces to grow better(shown in Fig.6).
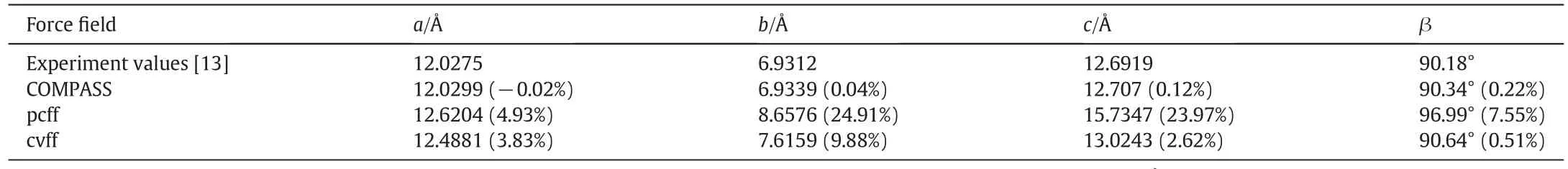
Table 1 Comparison of the optimized values ofα-HH unit cellparameters with the experiment values

Fig.3.The prediction ofα-HH growth morphology using AE model.
These data above indicate thatthe increase in exposure degree of the crystalcylinders makes the hydration reactivity oftype-αhigh-strength gypsum considerably above that ofindustrial HPG.It can also be certificated thatwaterchannels are mainly distributed in the crystalcylinders ofα-HH.This finding agrees with the theoreticalcalculations.Therefore,an effective means to enhance the hydration reactivity ofindustrialHPG is to change its crystalmorphology to uncover its crystalcylinders,and thus,increase the number ofits water channels.
3.3.Morphology controlofHPG
HPG1 and HPG2 with different crystalmorphologies were prepared by using the following respective concentrations ofliquid-phase SO42-in the mixed solution:20 mg·ml-1and 40 mg·ml-1[22].
The SEMimages of HPG1 and HPG2 are shown in Fig.8.When concentration ofliquid-phase SO42-is 20 mg·ml-1,HPG1 has stumpy crystals with 5–15 μm long and length-to-diameter ratios of3–5:1.When concentration of liquid-phase SO42-is increased to 40 mg·ml-1,HPG2 is made ofirregular schistose crystals which appear a smallamount of crystalcylinders and become the length-to-diameter ratios less than 1.
The XRDpatterns ofHPG1 and HPG2 are shown in Fig.9.The diffraction peak intensities of(200)and(400)in HPG2 are lowerthan those in HPG1,whereas the diffraction peak intensities of(310)and(114)in HPG2 are higher than that in HPG1.The result indicates that the length ofcrystalcylinders of HPG is reduced,and conicalsurfaces are prone to appear with the increase in liquid-phase SO42-concentration.
When preparing HPG,apatite powders were poured into the mixed solution ofphosphoric acid and sulfuric acid,which caused the reaction system to exhibitthe decomposition course ofapatite powders and the crystallization process of HPG.The reaction formulas about the reaction ofapatite powders with phosphoric acid and sulfuric acid are as follows in Eqs.(5)and(6):

Crystallization ofα-HHcan be identi fied two time regions:a period for the crystalnucleus formation and a crystalgrowth period[23].Thus when the mixed solution is saturated with respect to Ca2+and SO42-ions,this saturated solution becomes supersaturated with respect to α-HH leading to nucleation and crystal growth.After a nucleus has been formed,growth occurs via advancing monolayer steps,predominantly parallelto{1 0-1}and{101}.The step velocity of{110}and{1-1 0}steps are higher,relative to{1 0-1}and{101}steps,which is a result ofa stump shape ofα-HH crystal(seen in Table 2 and Fig.3).
During the preparation of HPG,the different concentrations of liquid-phase SO42-changed the supersaturation and crystalsurfaces growth rate ofα-HH;thus crystal morphology ofα-HH varied from each other with the change in concentration of liquid-phase SO42-.When the concentration of liquid-phase SO42-was 20 mg·ml-1in initialreaction system,SO42-ions reacted with Ca2+ions to produce a smallquantity ofα-HH,which caused supersaturation ofα-HH to decline.A small number of growth units SO42-and Ca2+ions were superimposed on the crystalclusters ofα-HH,which caused the crystal grains ofα-HH to become small.After the restof the sulfuric acid was poured into the mixed solution,the supersaturation and crystalsurfaces growth rate ofα-HH increased with the concentration ofliquid-phase SO42-increase.Many growth units SO42-and Ca2+ions constantly were superimposed on the crystalclusters ofα-HH,which provided a good opportunity for the crystals to extend along crystalplane clusters{1 0-1}and{101}.Thus the crystalcylinders ofα-HH appear and then the crystal growth morphology eventually present a stumpy shape(seen in Fig.8(a)).

Table 2 Attachment energy and area ofgrowth surfaces ofα-HH crystal
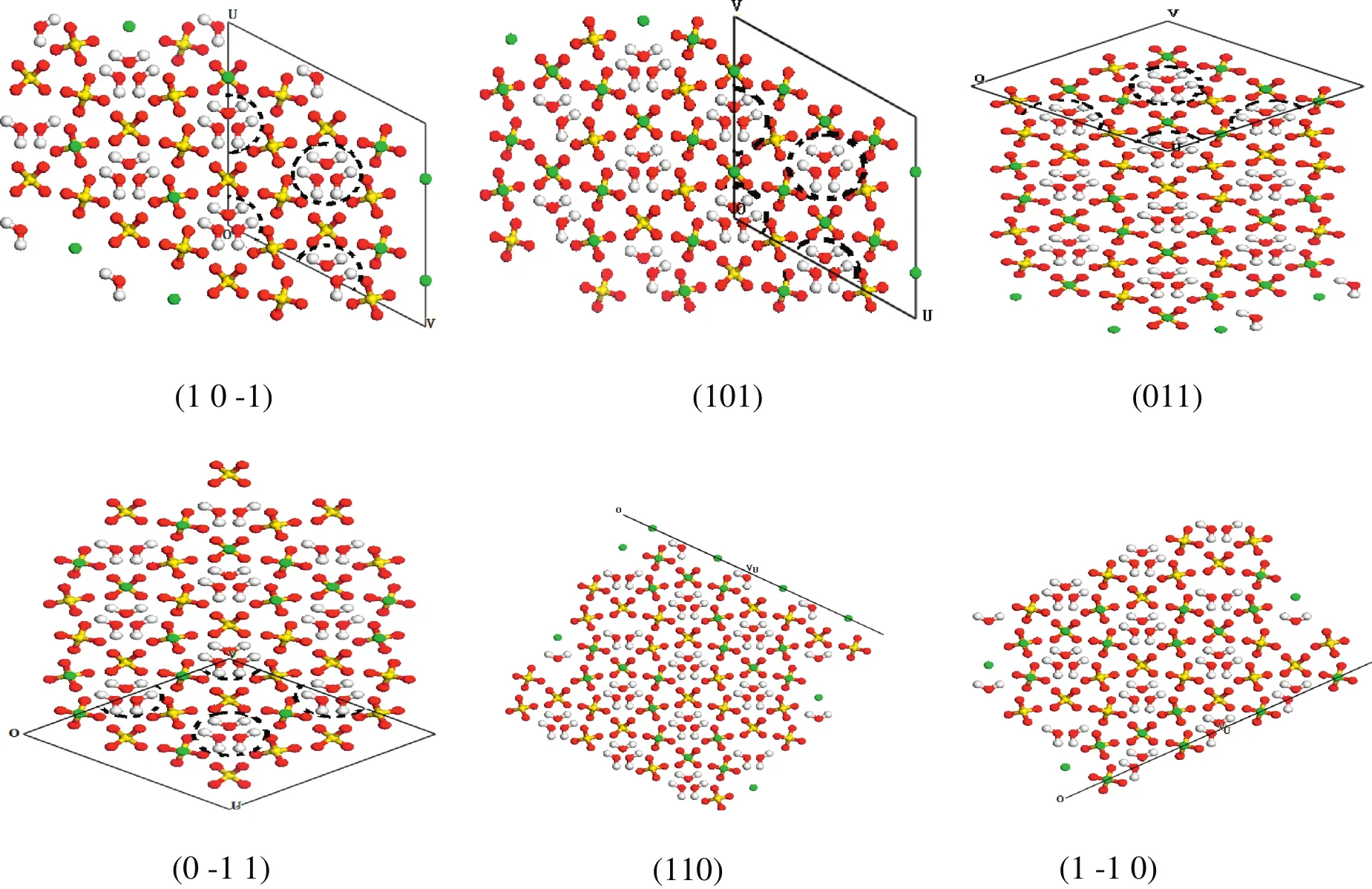
Fig.4.Crystalsurface structures ofα-HH projected along the[001]direction.The colors indicate:green—Ca,yellow—S,red—O,and white—H.The black dash line indicates water channel.
However,when the concentration of liquid-phase SO42-was increased to 40 mg·ml-1,the supersaturation ofα-HH was higher than that ofα-HH in initial reaction system with the concentration of liquid-phase SO42-at20 mg·ml-1.Thus the crystalparticles ofα-HHbecame larger and more easily deposited on the surfaces of apatite particles.The decomposition reaction rate of apatite particles was retarded byα-HH crystals,which caused the concentration ofliquid-phase Ca2+to decrease.Simultaneously,the remaining amount ofsulfuric acid was reduced.The supersaturation and crystalsurfaces growth rate ofα-HH decreased;hence,a smallnumber of growth units SO42-and Ca2+ions were superimposed on the crystalclusters{1 0-1}and{101}ofα-HH,which caused dif ficulty for the crystalcylinders to appear.Therefore,schistose crystals ofα-HH are presented(seen in Fig.8(b)).Based on the aforementioned experimental results,a schematic illustration of HPG morphologicalevolution under different concentrations ofliquidphaseis presented in Fig.10.
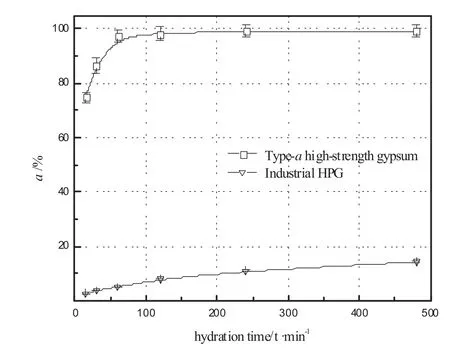
Fig.5.The hydration rates of industrial HPG and type-αhigh-strength gypsum at hydration temperature 20°C.
3.4.In fluence of crystal morphology on hydration apparent activation energy

Fig.6.SEMimages ofindustrialHPG.
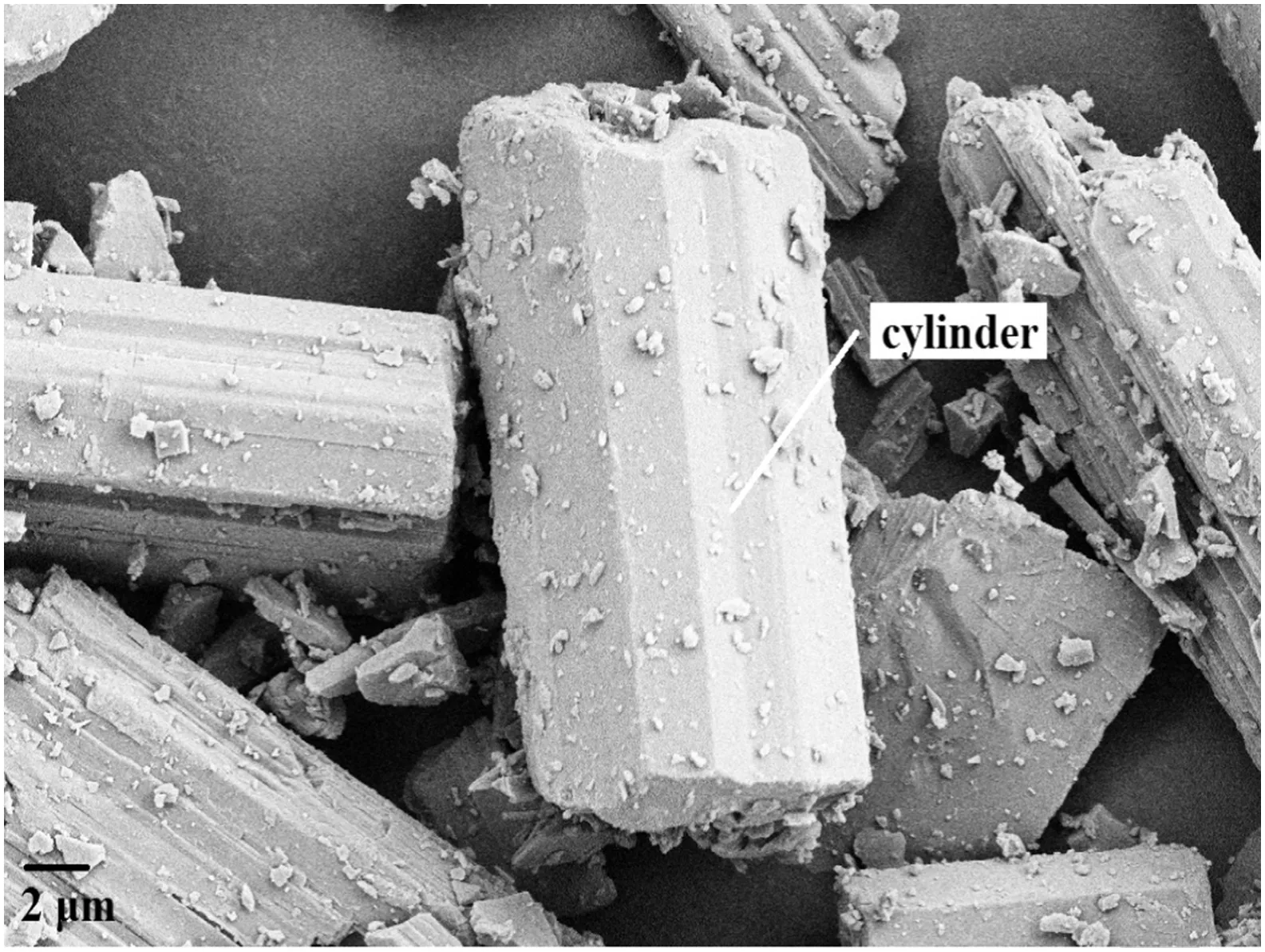
Fig.7.SEMimage oftype-αhigh-strength gypsum.
The hydration rates of HPG1 and HPG2 at different hydration temperatures are shown in Fig.11.When hydration temperature is 20°C,the hydration rate of HPG1 reaches about 67.63%within 15 min and about 86.77%after 480 min of hydration.However,the hydration rate ofHPG2 is low,i.e.,only about19.86%after480 min ofhydration in 20°C water.The results indicate the hydration reactivity of HPG1 is considerably higher than that of HPG2.Fig.11 also shows that the hydration rates of HPG1 and HPG2 decrease with the rise ofhydration temperature,which exhibits a “negative temperature effect”.This is because the hydration ofα-HHis an exothermic reaction.Meanwhile,the supersaturation of theα-HH solution subsides when temperature increases[23,24].
Hydration kinetics parameters and hydration apparentactivation energies ofHPG1 and HPG2 are displayed respectively in Tables 3 and 4.The strength rank of the hydration apparentactivation energies of HPG is as follows:|Ea(HPG2)|>|Ea(HPG1)|.Based on the activation energy criteria of chemicalreaction and diffusion control[25],E diffusion < 25.12 kJ·mol-1-<E chemical;hence,we can determine whether the hydration of HPG is controlled by chemicalreaction or diffusion.The hydration of HPG1 is controlled by diffusion,whereas the hydration of HPG2 is controlled by chemical reaction.This is because the number of water channels increases with the exposure degree of the crystalcylinders increase.Thus when the length-to-diameter ratio ofα-HH crystals is more than 1,the number of water channels in the crystal increases.Water molecules from the outside enter the crystalinside ofα-HH rapidly and hydrate into dihydrate gypsum;thus the velocity for hydration reactivity of HPG1 is higher than thatfor hydration products dihydrate gypsum diffusion.However,when the length-to-diameter ratio ofα-HHcrystals is less than 1,the numberofwater channels in the crystaldecreases.The velocity for hydration reactivity ofHPG2 is lower than thatfor hydration products dihydrate gypsum diffusion.

Fig.8.SEMimages of HPG with different crystalmorphologies(a)HPG1,(b)HPG2.

Fig.9.XRD patterns of HPG1 and HPG2.
4.Conclusions
Analysisofgrowth morphology ofα-HHhas shown thatwaterchannels are mainly distributed in the crystalcylinders,whereas no water channelexists in the conicalsurfaces parallelto the z-axis.
The investigation ofmicro-morphology and hydration reactivity has proved thatthe exposure degree of the crystalcylinders ofα-HHdirectly affects the hydration reactivity of both industrial HPG and type-α high-strength gypsum.The exposures of the crystalcylinders ofα-HH are positive to hydration reactivity,whereas the exposures of the conicalsurfaces are negative to hydration reactivity.By adjusting the concentration ofliquid-phase SO42-makes possible to controlthe growth rate of the crystal cylinders ofα-HH and to obtain HPG having the stumpy crystals ofα-HHand high hydration reactivity.The appropriate concentration ofliquid-phase SO42-is 20 mg·ml-1for the crystallization process of HPG.
Nomenclature


Subscripts


Table 3 Hydration kinetics parameters of HPG1and HPG2

Table 4 Hydration apparent activation energies of HPG1and HPG2

Fig.10.Schematic illustration of the HPG morphologicalevolution under different concentrations ofliquid-phase SO4 2-.
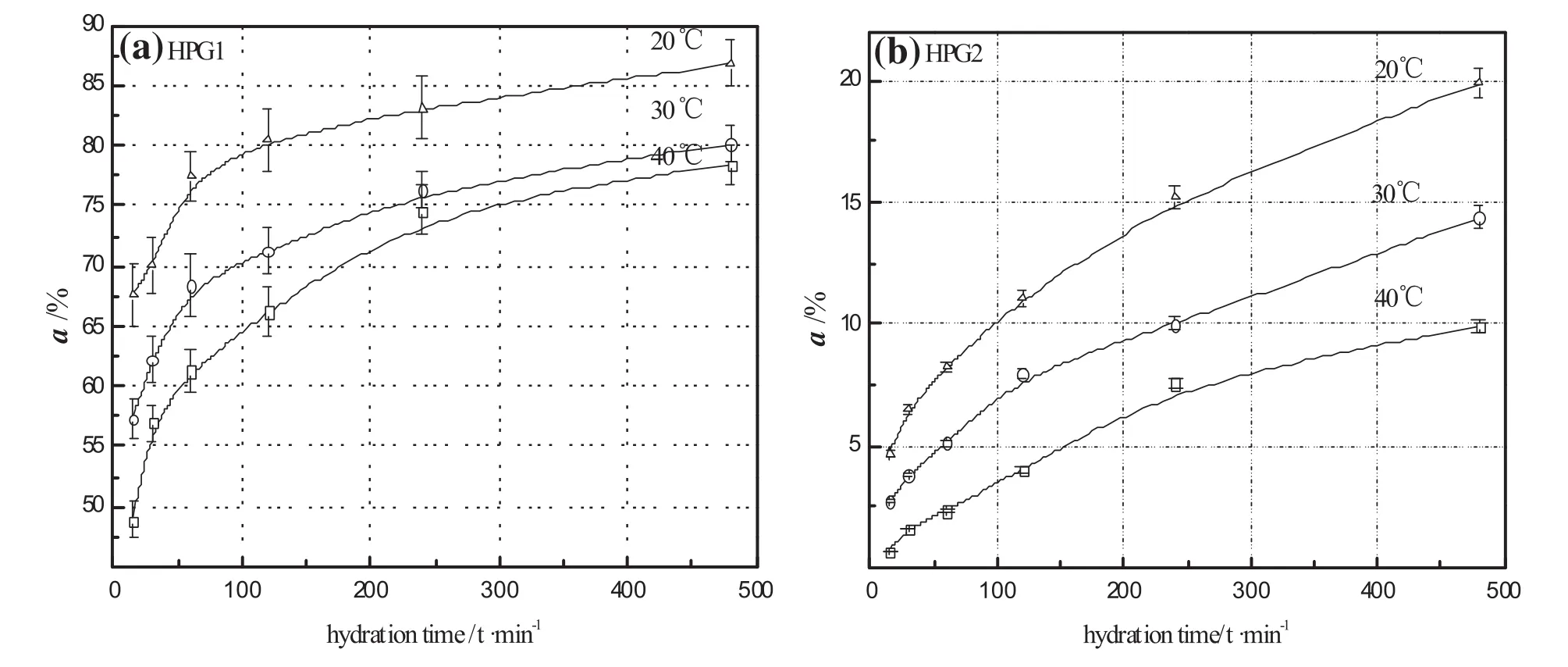
Fig.11.The hydration rates of HPG at different hydration temperatures(a)HPG1,(b)HPG2.
[1]S.V.Dorozhkin,Fundamentals of the wet-process phosphoric acid production.2.Kinetics and mechanism of CaSO4·0.5H2O surface crystallization and coating formation,Ind.Eng.Chem.Res.36(2)(1997)467–473.
[2]J.Zhou,H.Gao,Z.Shu,Y.X.Wang,C.J.Yan,Utilization of waste phosphogypsum to prepare non-fired bricks by a novel hydration–recrystallization process,Constr.Build.34(3)(2012)114–119.
[3]L.P.Ma,P.Ning,S.C.Zheng,X.K.Niu,W.Zhang,Y.L.Du,Reaction mechanism and kinetic analysis of the decomposition of phosphogypsum via a solid-state reaction,Ind.Eng.Chem.Res.49(8)(2010)3597–3602.
[4]K.Sunil,A perspective study on fly ash-lime-gypsum bricks and hollow blocks for low cost housing development,Constr.Build.16(8)(2002)519–525.
[5]W.G.Shen,G.J.Gan,R.Dong,H.Chen,Y.Tan,M.K.Zhou,Utilization of solidi fied phosphogypsum as Portland cement retarder,J.Mater.Cycles Waste 14(3)(2012)228–233.
[6]S.Manjit,Effect of phosphatic and fluoride impurities of phosphogypsum on the properties of selenite plaster,Cem.Concr.Res.33(9)(2003)1363–1369.
[7]A.Samia,M.Tahar,S.Sami,Sulfate reduction from phosphogypsum using a mixed cultue of sulfate-reducing bacteria,Int.Biodeterior.Biodegrad.56(4)(2005)236–242.
[8]O.Hentatia,N.Abrantesbd,A.L.Caetanocd,S.Bouguerraaef,F.Gongalvescd,J.Römbkeg,R.Pereiraef,Phosphogypsum as a soilfertilizer:ecotoxicity of amended soiland elutriates to bacteria,invertebrates,algae and plants,J.Hazard.Mater.294(3)(2015)80–89.
[9]T.T.Tjioe,H.V.D.Woude,J.Verbiest,P.F.M.Durvilie,G.M.V.Rosmalen,Cadmium incorporation in the crystallization of calcium sulfate hemihydrates from phosphoric acid,Cryst.Res.Technol.21(10)(1986)1287–1297.
[10]S.V.Dorozhkin,Process ofepitaxialcrystalgrowth for CaSO4·0.5H2O on a surface of dissolving fluorapatite crystals studied scanning electron microscopy,Scanning 18(2)(1996)119–124.
[11]Z.Valancius,D.Nizeviciene,V.Leskeviviene,N.Kybartiene,In fluence of the technological parameters on the structure and properties of hemi-hydrate phosphogypsum,Ceramics-Silikáty 49(2)(2005)120–125.
[12]H.J.Kuzel,M.Hauner,Chemicaland crystallographic properties of calcium sulfate hemihydrate and anhydrite III,Zement-Kalp-Gips 40(12)(1987)628–632.
[13]W.Abriel,R.Nesper,Bestimmung der kristallstruktur von CaSO4(H2O)0.5 mit röntgenbeugungsmethoden und mit potentialpro fil-rechnungen,Z.Kristallogr.-New Cryst.Struct.205(1–2)(1993)99–113(in German).
[14]S.Follner,A.Wolter,A.Preusser,S.Indris,C.Silber,H.Follner,The setting behaviour ofα-andβ-CaSO4·0,5 H2O as a function ofcrystalstructure and morphology,Cryst.Res.Technol.37(10)(2002)1075–1087.
[15]H.Schmidt,I.Paschke,D.Freyer,W.Voigt,Water channel structure of bassanite at high air humidity:Crystal structure of CaSO4·0.625H2O,Acta Crystallogr.B 67(6)(2011)467–475.
[16]M.Triollier,B.Guilhot,The hydrate of calcium sulphate hemihydrate,Cem.Concr.Res.6(4)(1976)507–514.
[17]N.B.Singh,B.Middendorf,Calcium sulphate hemihydrate hydration leading to gypsum crystallization,Prog.Cryst.Growth Charact.53(1)(2007)57–77.
[18]I.V.Melikhov,I.E.Mikheeva,V.N.Rudin,Mechanism ofcrystallization ofcalcium sulfate hemihydrate under conditions modeling the production of phosphoric acid by the hemihydrate procedure,Theor.Found.Chem.Eng.19(6)(1985)467–473.
[19]P.Z.Wu,The Wet-Process Phosphoric Acid,first ed.Chemical Industry Press,China,1987.
[20]Material studio 6.5,Discover/Accelrys Softwrae Inc.,San Diego,California,USA,2007.
[21]P.Hartman,P.Bennema,The attachment energy as a habit controlling factor theoreticalconsiderations,J.Cryst.Growth 49(1)(1980)145–156.
[22]L.Yang,J.X.Cao,Y.M.Liu,Study on in fluence of the crystalline,morphology and cementitious properties of hemi-hydrate phosphogypsum,J.Synth.Cryst.44(9)(2015)2460–2467(in chinese).
[23]X.Q.Wu,S.T.Tong,B.H.Guan,Z.B.Wu,Transformation of flue-gas-desulfurization gypsum toα-hemihydrated gypsum in salt solution at atmospheric pressure,Chin.J.Chem.Eng.19(2)(2011)349–355.
[24]R.Z.Yuan,Cementitious Materials Science,second ed.Wuhan University ofTechnology Press,China,1996.
[25]H.Y.Qian,S.Y.Li,M.Deng,S.M.Zhang,Hydration dynamic oflight-burned magnesia,Ind.Miner.Process.36(12)(2007)1–4(in Chinese).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Heat transfer ofnano fluidics in hydrophilic pores:Insights from molecular dynamics simulations☆
- Numericalsimulation ofstirred tanks using a hybrid immersed-boundary method☆
- Numericalsimulation ofmicromixing effect on the reactive flow in a co-rotating twin screw extruder☆
- Coalescence behaviour ofwater droplets in water-oilinterface under pulsatile electric fields
- Effects of Sn residue on the high temperature stability of the H2-permeable palladium membranes prepared by electroless plating on Al2O3 substrate after SnCl2–PdCl2 process:A case study☆
- Application ofdiffusive transport modelfor better insight into retardation mechanisms involved in ion-imprinted membrane transport
